Effect of Nitrogen Hydrogen Ratio on The Characteristics and Combined Properties of Plasma Nitriding Layer for 38Crmoal
Lu Song 1, Tiantian Peng1, Xiaobin Zhao1,2 and Jing Hu1,2*
1 School of Materials Science and Engineering, Jiangsu Key Laboratory of Materials Surface Science and Technology, Changzhou University, Changzhou 213164, China
2National Experimental Demonstration Center for Materials Science and Engineering Changzhou University, Changzhou, 213164, PR China
Submitted: August 20, 2018; Published: September 11, 2018
*Corresponding author: Jing Hu, School of Materials Science and Engineering, Jiangsu Key Laboratory of Materials Surface Science and Technology, National Experimental Demonstration Center for Materials Science and Engineering Changzhou University, Changzhou 213164, China.
How to cite this article: Lu Song, Tiantian P, Xiaobin Z,Jing H. Effect of Nitrogen Hydrogen Ratio on The Characteristics and Combined Properties of Plasma Nitriding Layer for 38Crmoal. JOJ Material Sci. 2018; 5(2): 555656. DOI: 10.19080/JOJMS.2018.05.555656
Abstract
Critical nitrogen hydrogen ratio in plasma nitriding was primarily investigated to get enhanced performance for 38CrMoAl steel. The modified surface layer was characterized by optical microscopy (OM), X-ray diffraction (XRD), micro-hardness tester and wear tester. The results showed that the critical nitrogen hydrogen ratio was 1:5 while plasma nitriding at 540℃ for 6h. Under this condition, no compound layer was formed, and more importantly, brittleness was significantly decreased, accompanied with high surface hardness, while the compound layer was formed accompanied with lower surface hardness and serious brittleness upon nitriding with nitrogen hydrogen ratio higher than the critical value.
Keywords: 38crmoal Steel; Plasma Nitriding; XRD; Compound Layer
Abbrevations: OM: Optical Microscopy; XRD: X-Ray Diffraction; TAPP: Top-Notch Academic Programs Project
Introduction
38CrMoAl steel is a kind of metal material commonly used for producing high-pressure valve due to its excellent wear resistance [1,2]. And a high-pressure valve is generally subjected to severe impact and wear in real applications. Therefore, optimal combination of hardness, wear resistance and toughness are required for 38CrMoAl high-pressure valve. To meet the technical requirements, surface modification of high-pressure valves are necessary [3-5], and plasma nitriding is one of the most widely used surface modification techniques in industry, since a modified layer with high surface hardness and excellent wear resistance can be obtained [6-9]. Unfortunately, during conventional plasma nitriding, a hard and brittle compound layer (also called white layer) is generally produced, which is easy to crack and peel off from the surface during the service life of 38CrMoAl steel high-pressure valve upon subjecting the severe impact and cyclic load [10-12], and thus leading to premature failure of the components. Hence, it is of significant value to control the formation of compound layer during plasma nitriding for high-pressure valve.
It has been reported that compound layer can be avoided through low temperature plasma nitriding [13-15]. However, the combined properties of the nitriding layer obtained by low temperature plasma nitriding can’t meet the advanced technical specifications in some real applications, such as surface hardness and wear resistance [16]. In this study, plasma nitriding at different nitrogen hydrogen ratio was carried out for 38CrMoAl steel to effectively control the formation of compound layer, and it was found that there existed a critical nitrogen hydrogen ratio for avoiding the formation of compound layer. More importantly, enhanced combined properties of hardness, wear resistance and toughness could be obtained by plasma nitriding with the critical ratio.
Experimental Procedures
38CrMoAl steel was selected as the substrate material with the chemical composition (wt. %) of: 0.37 C, 1.5 Cr, 0.45 Mn, 0.17 Mo, 0.95 Al and balance Fe. The specimens were machined into a size of 10mm×10mm×5mm, followed by quenching at 930℃ and tempering at 600℃ to get a substrate with uniform microstructure. All the surfaces of specimens were treated by silicon carbide emery papers of different granulometry (240, 500, 1500and 2000 mesh) to achieve a fine finish, then ultrasonically cleaned in anhydrous ethanol prior for 15 min and dried before nitriding.
After cleaning with ethanol, the specimens were placed into plasma nitriding equipment (LD-8CL) and evacuated to 18 Pa by a rotary pump. All specimens were sputtered of 30 min by hydrogen with a flow of 500 mL/min at a pressure of 300 Pa. After sputtering process, a mixture gas of N2 and H2 with its ratio of 1:3, 1:4 and 1:5 was supplied at a gas pressure of 400 Pa to run plasma nitriding process at the same temperature of 540℃for the same duration of 6h. The cross-sectional microstructure was observed by optical microcopy. The phase constituents were determined by X-ray diffraction (XRD) with Cu-Ka (λ=1.54 Å) radiation. Hardness measurements were made in a HXD- 1000TMC micro-hardness tester, with the test load of 10 g and holding duration of 15 s. Each hardness value was determined by averaging at least 5 measurements. Meanwhile, the surface brittleness was evaluated by checking the morphology of the indentation with the test load of 50 g and 200g.
Results and Discussion
Microstructure Characterization
The cross-sectional microstructures of 38CrMoAl steel nitriding with different nitrogen hydrogen ratio are presented in Figures 1(a-c). It can be seen that the compound layer thickness decreases with the decrease of nitrogen hydrogen ratio, and no compound layer is formed at the ratio of 1:5 in Figure 1(c), which implies that the compound layer thickness is dependent on nitrogen hydrogen ratio and there exists a critical ratio to avoid the formation of compound layer. Here in this research, the critical nitrogen hydrogen ratio is 1:5 for avoiding the formation of compound layer.

XRD Analysis
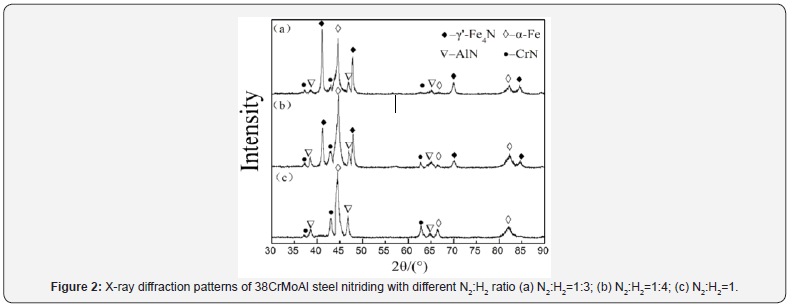
Figure 2 shows XRD patterns of 38CrMoAl specimens nitrided with different nitrogen hydrogen ratio. It can be seen that the main phase is α-Fe along with CrN and AlN for specimen (c) with nitrogen hydrogen ratio of 1:5, while γ’-Fe4N is dominant for the other two specimens. Therefore, XRD patterns confirm that no compound layer is formed on the surface of specimens (c), and with compound layer on the other two specimens, which is in good agreement with the results in Figure1.
Micro-Hardness Profile
Figure 3 shows the micro-hardness profiles of 38CrMoAl steel nitrided with different nitrogen hydrogen ratio. It clearly indicates that the surface hardness of specimen with nitrogen hydrogen ratio of 1:5 is the highest, though the thickness of effective hardening layer is a little thinner.
Brittleness Analysis
The indentation morphologies of 38CrMoAl steel nitrided with different nitrogen hydrogen ratio are shown in Figure 4. It can be seen that no crack appears around the indentation of specimen with the nitrogen hydrogen ratio of 1:5 under any test load. However, there exist severe cracks around the indentation with the ratio of 1:4, and the indentation is almost totally broken for specimen with the ratio of 1:3 under any test load. Thus, it can be concluded that the brittleness is significantly decreased by nitriding with the ratio of 1:5 due to no compound layer existed on the surface, which is of significant value for high pressure valve in real applications.


Mechanism Discussions
Generally, a nitriding layer formed during plasma nitriding is composed of compound layer and nitrogen diffusion layer. And a compound layer is mainly composed of γ’-Fe4N or ε-Fe2-3N, which is much more brittle than the underneath diffusion layer due to the different characteristics, including different crystal lattices, therefore, the compound layer is easy to crack upon subjecting impact loads [10]. Moreover, the big difference of elasticity modulus between compound layer and diffusion layer results in uncoordinated deformation, especially upon subjecting cyclic loads [10-11], which makes the compound layer have a tendency to crack and peel off from the surface, and thus result in premature failure. Therefore, though the compound layer can provide high surface hardness and excellent wear resistance, it is of significant value for 38CrMoAl high-pressure valve to get a nitriding layer without compound layer for preventing its premature failure in real applications [12-16].
As was reported that there exists a critical nitrogen potential, i.e. the lowest nitrogen potential for controlling the formation of a compound layer, in other words, the compound layer cannot be formed when the nitrogen potential is lower than the critical value[17-19]. Since in real applications there is no efficient way to evaluate the nitrogen potential of plasma nitriding, nitrogen hydrogen ratio is used to represent nitrogen potential in this study, and it is found that the critical nitrogen hydrogen ratio to control the formation of compound layer is 1:5 for 38CrMoAl steel at 540℃.Therefore, the results can provide valuable guide for designing plasma nitriding process in real applications.
Conclusion
In summary, critical nitrogen hydrogen ratio was primarily investigated for 38CrMoAl. The results show that the specimen nitrided at N2:H2=1:5 owns the highest surface hardness and optimal toughness due to no compound layer formed on the surface, in other words, enhanced combined properties can be obtained for 38CrMoAl steel. Meanwhile, it is found that there exists a critical Nitrogen hydrogen ratio for avoiding the formation of compound layer, and the critical Nitrogen hydrogen ratio for 38CrMoAl steel is 1:5 corresponding to the nitriding temperature of 540 ℃.
Acknowledgement
The research was supported by National Natural Science Foundation of China (51774052) and Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
References
- CE Pinedo (2003) The use of selective plasma nitriding on piston rings for performance improvement. Mater Des 24(2): 131-135.
- XL Liu, H Zheng, MH Yang (2011) Heat Treat, pp. 2643-2645.
- MY Dai, CY Li, J Hu, J Alloy (2016) Compd 688: 350-356.
- WP Tong, NR Tao, ZB Wang, J Lu, K Lu (2003) Nitriding Iron at Lower Temperatures. Sci 299(5607): 686-688.
- SK Wang, W Cai, JC Li, J Hu (2013) A novel rapid D.C. plasma nitriding at low gas pressure for 304 austenitic stainless steel. Mater Lett 105: 47-49.
- M Pellizzari, A Molinari, G Straffelin (2001) Surf Coat Tech 142: 1109- 1115.
- F Bottoli, MS Jellesen, TL Christiansen (2017) High temperature solution-nitriding and low-temperature nitriding of AISI 316: Effect on pitting potential and crevice corrosion performance. Appl Surf Sci 431: 24-31.
- JQ Wu, H Liu, JC Li, J Alloy (2016) Compd 680: 642-645.
- F Mahboubi, K Abdolvahabi (2006) The effect of temperature on plasma nitriding behaviour of DIN 1.6959 low alloy steel. Vacuum 81(3): 239-243.
- J Alphonsa, VS Raja, S Mukherjee (2015) Development of highly hard and corrosion resistant A286 stainless steel through plasma nitrocarburizing process. Surf Coat Tech 280: 268-276.
- YC Chen, CL Lin, CG Chao (2015) J Alloy Compd 633: 137-144
- HM Chen, YC Lin, YC Chen (2012) Advanced Materials Research 579: 278-286.
- Y Chen, L Song, CK Zhang, XM Xie, J Hu (2017) Vacuum 143: 98-101.
- AN Allenstein, CM Lepienski, AJA Buschinelli Appl Surf Sci 277(277): 15-24.
- WP Tong, Z Han, LM Wang (2008) Surf Coat Tech 201: 4957-4963.
- YT Xi, DX Liu, H Dong (2008) Appl Surf Sci 254: 5953-5958.
- W Grafen, B Edenhofer (2005) Surf Coat Tech 200: 1830-1836.
- B.Y. Jeong, M.Kim (2001) Surf Coat Tech 14: 182-186.
- SY Zuo, W Zhou, X Kuang (2015) Die & Mould Manufacture 1: 76.






























