Study on Prevalence and Associated Risk Factors of Poultry Coccidiosis in and Around Alage at vet College, Southwestern Ethiopia
Eshetu Ketema and Nigussu Fasil*
Hawassa university, Faculty of veterinary Medicine, Ethiopia
Submission: February 28, 2019; Published: March 26, 2019
*Corresponding author: Nigussu Fasil, Hawassa university, Faculty of veterinary Medicine, Ethiopia
How to cite this article: Eshetu Ketema, Nigussu Fasil. Study on Prevalence and Associated Risk Factors of Poultry Coccidiosis in and Around Alage at vet College, Southwestern Ethiopia. Dairy and Vet Sci J. 2019; 11(1): 555805. DOI: 10.19080/JDVS.2019.11.555805
Abstract
A cross-sectional study was conducted from November 2016 to March 2017 in and around Alage ATVET College, southwestern Ethiopia, with the objective of determining the prevalence of poultry coccidiosis and its associated risk factors in intensively and backyard managed chickens. Systematic random sampling technique was used to select the study samples. Fresh fecal samples were collected from a total of 451 chickens. Poultry fecal droppings floatation technique was used to detect coccidian oocyst. The result revealed that out of 451 chickens examined, 88 were positive for coccidiosis and the overall prevalence was 19.5% (88/451). According to the study 363 of chickens were found to be negative with a prevalence of 80.5% (363/451). The frequency of detection of oocyst in the fecal samples from exotic and local breed chicken were 20.7% and 17.7%, respectively. It showed statistically non-significant difference between the two breed groups (P=0.421, X2=0.646). The result also showed that the prevalence of coccidiosis between the sexes was 20.1% and 18.5 % in female and male chickens respectively.
This result is statistically not significant between the two sexes (P=0.684, X2=0.1661). The prevalence rates of 22.5%, 10.9% and 9.3% were recorded in chicken grouped under the age category of 1-3 weeks, 4-6 weeks and of greater than 6 weeks, respectively. The prevalence among the age showed statistically significant difference (P=0.017, X2=8.1696) which was being higher in chickens up to 1-3 weeks of age (22.5%). The prevalence rates of 3.7% and 28.3% were also recorded in post-treated and non-treated chickens respectively and this difference in prevalence was statistically significant (P=0.000, X2=39.7269). Furthermore, coccidiosis occurrence in intensive farm was 20.6% and in backyard management system was 17.9% and this difference in prevalence was not statistically significant (P=0.477, X2=0.5053). The study showed that coccidiosis is important disease of poultry in and around Alage ATVETC south western Ethiopia and this is an indication for intervention to tackle the disease without any priority within the risk factors.
Keywords: Alage; Coccidiosis; Floatation; Oocyst; Prevalence
Lists of Abbreviations: EARO: Ethiopian agricultural research organization; FAO: Food and agriculture and organization; ILRI: International livestock research institutes; AATVETC: Alage Agricultural Technical Vocational Education Technical College; CSA: Center for Statistical Agency
Introduction
In developing countries poultry production offers an opportunity to feed the fast-growing human population and to provide income for resource poor farmers. Moreover, poultry in many parts of the modern world is considered the chief source of not only cheaper protein of animal origin but also of high-quality human food [1]. The total poultry population in Ethiopia is estimated to be 56.5 million [2]. The total national egg and poultry meat production is estimated to be 78000 and 723000.Three types of poultry production systems metric tons respectively, of which local birds, kept under the traditional systems of production, contribute 98.5% and are identified in Ethiopia [3]. These are backyard poultry production system, small scale and large-scale intensive poultry production systems. The main objective of rearing chicken in all production systems is concerned with egg and meat production, for income generation and home consumption [4]. In the past coccidiosis was one of the diseases most feared by commercial poultry growers in the U.S.A. Death losses of 20% or more were common. “Backyard” growers are usually so small that coccidiosis was not a problem, but as the size of free-range flock increases, coccidiosis becomes a threat [5].
Poultry coccidiosis, caused by several distinct species of Eimeria, and remains the most economically significant parasitic infection of the poultry industry, worldwide. The disease is endemic in most of the tropical and subtropical regions where ecological and management conditions favor an all-year round development and propagation of the causal agent [6]. Substantial work on coccidiosis based on experimental infections and drug and vaccine trials has been presented over many years. However, reports on infection prevalence, infection levels and frequencies of the different Eimeria species in commercial poultry productions are few and sporadic. Often the reports are not comparable due to the difference in management and production systems, sample materials, sampling periods, sampling methods and prophylactic measures applied. More knowledge of the etiology and population dynamics of mixed coccidial infections in commercial poultry production is therefore needed. Moreover, with the increasing interest in poultry production evidenced by the proliferation of poultry farms, it is pertinent to continually evaluate the prevalence and management issues [7].
Among the infectious diseases of poultry, cocccidiosis is the major parasitic disease. Poultry coccidiosis is an economically important disease in chicken caused by the intracellular protozoa parasite of Eimeria species in the genus Eimeria family Eimeridae order Eucoccidiorida and phylum Apicomplexa [8]. Infection by coccidia in sufficient number to produce clinical manifestations of disease is called coccidiosis [9]. Though nine species of Eimeria have been identified as causative agents of poultry coccidiosis, only seven of them have been reported to be pathogenic. Eimeria tenella (E. tenella) and Eimeria necatrix (E. necatrix) are the most pathogenic species. Eimeria arcevulina (E. acervulina), Eimeria maxima (E. maxima) and Eimeria mivati (E. mivati) are common and slightly too moderately pathogenic while Eimeria brunetti (E. brunetti) is uncommon but pathogenic when it does occur. Eimeria mitis (E. mitis), Eimeria praecox (E. praecox) and Eimeria hagani (E. hagani) are relatively non-pathogenic species [10]. The disease is endemic in most of the tropical and subtropical regions where ecological and management conditions favor an all-year round development and propagation of the causal agent. In Ethiopia, poultry coccidiosis is caused by E. acervulina, E. necatrix, E. maxima and E. tenella, is endemic in all parts of the country and affects mainly young growing birds [11].
Coccidiosis is a disease of major economic importance in the poultry industry. It is strictly host-specific and the different species parasitize specific parts of the intestine. The disease is characterized by droopiness, paleness of the comb, diarrhea and occasional appearance of blood in droppings. The oocysts exist in the litter, premises and are distributed by clothes, shoes, dust and others [12]. Several factors influence the severity of infection like age and the number of ocysts eaten [13]. Infection with coccidia parasites costs the poultry industry in the USA more than USD 1.5 billion in annual losses. It is a widespread disease in growing chickens around the world that can seriously restrict the development of poultry production. In all parts of the world poultry coccidiosis represents a major disease problem. With increasing interest in poultry production evidenced by the proliferation of poultry farms, it is pertinent to continually evaluate the prevalence, frequencies of the different Eimeria species and management issues associated with common poultry diseases such as coccidiosis in any given zone and in Ethiopia despite the immense research works done by several outstanding researchers in the area of poultry coccidiosis in different parts of the country [14]. In Ethiopia, some reports indicated coccidiosis loss from 8.4 % and11.86% profit in largeand small-scale farms, respectively [15]. The disease is still continued being a major problem demanding much research and investigation. Factors contributing to outbreaks of clinical Coccidiosis include litter moisture exceeding 30%, immune suppression, suboptimal inclusion of anticoccidials in feed and environmental and managemental stress such as overstocking, poor feeding systems, and inadequate ventilation. Subclinical coccidiosis manifests mainly by poor weight gain and reduced efficiency of feed conversion contributing to big economic losses [16].
Coccidiosis can be controlled by good management including good ventilation, dry and clean litter, cleaning and decontamination of drinkers and feeders, and proper stocking density in poultry farms [17].
Therefore, the objectives of this study were:
a. To determine the prevalence of poultry coccidiosis in and around Alage ATVET College.
b. The associated risk factors causing tremendous loses in poultry production due to poultry coccidiosis.
Materials and Methods
Description of the Study Area
The A.A.T.V.E.T.C. is located at longitude of about 380 30’ East and latitude of 070 30’ North with a total area of 4200 hectares with an altitude of 1600 meters above sea level. The area is situated at 217 kilometers southwest of Addis Ababa and 32km west of Bulbula town along Addis Ababa-Hawassa highway road. Agro-ecologically it is dry plateau of the southwestern part of the Ethiopian central rift valleys. The area has three distinct seasons, namely main rainy (June to September), short rainy (March to May), and dry (October to February), the mean annual rainfall of the area is 800mm, with minimum and maximum temperatures of 11 and 29 °C, respectively based on data. The dominant soils types are vertisols with sand-slit clay with a pH of 7.9. The area is suitable for livestock production. According to livestock unit there were about 254 cattle, 300 pigs and 9000 poultry in the College [18].
Study Population
The study was performed in A.A.T.V.E.T.C. on intensively managed poultry farm which found in the College and extensively managed backyard system nearby, which were exotic and indigenous respectively. The poultry in the A.A.T.V.E.T. College were brought from Hawassa poultry farm enterprise and they were of the same breed (Bovans brown) types. Poultry in the College were kept at in door; feeding and the management system of the farm can be considered as intensive with deep litter system while those around were of extensive backyard system. This study was performed in layer type breeds and the study birds were grouped in to exotic and indigenous.
Study Design
A cross-sectional study involving a systematic random sampling of intensive poultry farm and individual farmhouse hold were carried out from November 2016 to March 2017, to determine the prevalence and economic impact of coccidiosis in these poultry farms. For this purpose, qualitative analysis of fecal examination to investigate oocyst discharge has been done. In this study, ages, sex, breed, management system and history of treatment given were taken as potential risk factors. Based on age, the study populations were divided in three groups from the age category of 1-3 weeks, 4-6 weeks and above 6 weeks, respectively. Based on history of treatment as regularly treated and not regularly treated while by their breed classified as exotic and local. According to management classified as intensive and backyard. Based on sex grouped as male and female [19].
Sample Size Determination and Sampling Method
This study was conducted to select poultry used for the study of prevalence of coccidiosis in the intensive farm and individual farm household in and around A.A.T.V.E.T.C. respectively. 450 of the total population sizes of the study, from the above-mentioned site, each chicken was selected based on systematic random sampling. Sample size was determined based on expected prevalence of 20.57 % in Ambo, West Shewa, Ethiopia, by Oljira [20] and sample size was calculated according to the following formula by using 95% confidence level and 5% absolute precision [21].
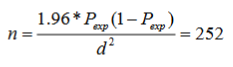
Where,
Pexp=expected prevalence
n= the required sample size
d = Desired absolute precision (5%)
Where Z (a multiplier for 95% confidence interval based on the normal distribution) =1.96, p=20.57%% and d=5%. Therefore, a minimum required sample size was 252. However, 451 chickens were sampled to increase precision.
Study Methodology
Fecal sample collection: Based on the difference of their different risk factors, freshly deposited fecal samples were collected from the upper surface of the litter immediately after dropping of the feces and collected in a screw capped glass bottles (universal bottles) by using spatula which was cleaned after each collection and packed in ice box and transported to the A.A.T.V.E.T.C. Parasitological laboratory as much as it was in fresh state. While collecting of fecal samples; date, management system, sex, age group, breeds and treatments given for sampled poultry was recorded. A 5 gram of poultry fecal droppings were taken and floatation fluid (50ml of saturated sodium chloride solution or saturated sugar solution) was added and after thoroughly mixed and processed, then prepared for examination under the microscope by putting the slides covered by cover slips containing the oocysts directly [22]. The fecal droppings were examined by compound microscope under the magnifications of 10x and 40xs. The Examination of fecal contents showed oval, thick walled oocyst and large round in significant numbers was considered as coccidian oocyst [23].
Data Entry and Analysis
Data collected from the study sites were coded and entered in to a Microsoft excel spread sheet program for analysis. Statistical analysis was done on STATA software. Descriptive statistics like percentage was used to express prevalence while chi-square (X2) test was used to compare the association between variables and a statistically significant association between variables was considered at p- value less than 0.05.
Results
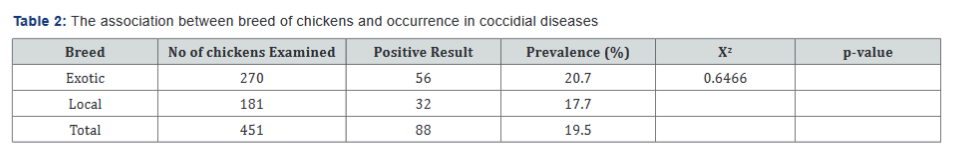
The Overall Prevalence: Out of the total 451 fecal droppings samples of chickens examined, infections at different sex, breed, ages, management systems and post prophylactic treatments given, 88 samples were positive for coccidial oocyst giving the overall prevalence rate of 19.5%, whereas the rest 363 (80.5%) were negative up on fecal examination for oocysts of coccidial parasite. Prevalence of Coccidiosis Between sexes: Out of 294 female chickens examined, 59 (20.1%) were infected with Eimeria oocysts whereas from 157 males examined, 29 (18.5%) were positive for coccidian oocysts. The prevalence of coccidiosis was higher in females than the males in study area. The association was statistically not significant (P=0.684, X2=0.1661) (Table 1). Prevalence of Coccidiosis Between Breeds: Out of 270 exotic chicken breeds examined, 56 (20.7%) were infected by the Eimeria oocysts and from 181 local breed, 32 (17.7%) were positive. This shows that there was higher prevalence of Eimeria infection in exotic breed than locals according to this study. The association was statistically not significant (P=0.421, X2=0.6466) (Table 2).

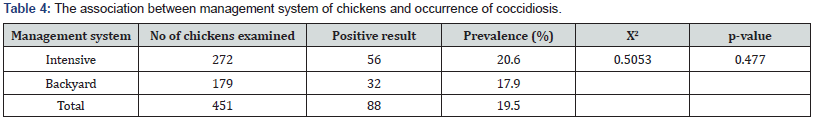
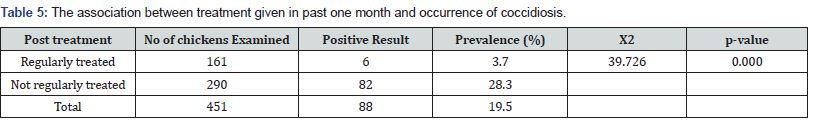
Prevalence of Coccidiosis between Age Groups: Out of 88 positive chickens, 77 (22.5 %) were in 1-3 weeks, 6 (10.9%) were 4-6 weeks and 5 (9.3 %) were of greater than 6 weeks old. The highest number of clinical coccidiosis cases (22.5%) was recorded at age of 1-3weeks old. There was a statistically significant difference (P=0.017, X2=8.1696) in the prevalence of coccidiosis at different age of chickens (Table 3). Prevalence of Coccidiosis between Management Systems: In the deep litter farms (intensive) out of 272 fecal samples of chickens examined, 56 samples were found positive for coccidial oocyst with a prevalence rate of 20.6%, whereas from backyard home rearing system 179 fecal samples were examined and 32 samples were positive for chicken’s coccidial oocysts with a prevalence rate of 17.9%. There was a statistically no significant difference (P=0.477, X2=0.5953) in the prevalence of coccidiosis at different management systems (Table 4). Prevalence of Coccidiosis Between post- treated and non-treated groups: Out of 451, 290 were received post treatment while 161 were not received in the past one month. The prevalence rates of 3.7% (82/290) and 28.3% (6/161) were recorded in post-treated and non-treated chickens, respectively. There was a statistically significant difference (P= 0.000. X2=39.7269) in the prevalence of coccidiosis in the treated and non-treated chickens (Table 5).

Discussion
Coccidiosis is the most prevalent intestinal parasitic disease of poultry and its prevalence and economic significance has been reviewed by different workers in different production system [24]. The results of this present study show that in dry season chickens could be infected by Eimeria species. In this study an overall prevalence of chicken coccidiosis was found to be 19.5% (88/451).This result is in agreement with the finding in Ambo [20], who reported a prevalence of 20.57%.The present result support the previous finding in central Ethiopia [17], Addis Ababa [25] and in Kombolcha [19] with prevalence rate of 25.8%, 23.1% and 22.3%, respectively. Moreover, this result was in support with the finding in Arsi Tiyo [26] who reported a prevalence of 22.58%. This finding was lower than the report in Gonder, North west Ethiopia,) with the [27] revalence of 43%. However, this prevalence of Coccidiosis result is much lesser than the findings of Dinka & Tolossa [28] in Debre Zeit, Ethiopia (71.1%) and Alemargot [29] in Addis Ababa (80%). This variation in prevalence of the disease may be due to epidemiology of coccidian infection, application of preventive measures which basically sanitary measures and use of anti coccidial drugs that were given at early stages of age. Other point may be due to breed difference, improvement of management system and bio security measures when compared to the setup in the previous study.
In this current study, coccidian infection was found to occur more in females (20.1%) than in male chickens (18.5%). The association between the sexes was statistically non-significant (P= 0.684). This finding agrees with those of Oljira [20] who also reported higher frequency of avian coccidiosis in female chickens (21.43%) as compared to male ones (19.38%). However, the present result disagrees with the finding of Alemayehu [25] in Addis Ababa and Gebretensae [27] in Gondar who reported that a higher prevalence of poultry coccidiosis in male chickens than female chickens (54.9%). Absence of statistically significant difference between female and male might be due to the equal chance of exposure for the coccidiosis infection. The prevalence rate of the disease was non- significantly (P>0.05) higher in exotic, (20.7%) breed than local chickens, (17.7%). This result agreed with the most previous research work done in different parts of the world, who reported higher prevalence of coccidiosis in exotic breed than local chickens [30].
Higher incidence of coccidiosis in exotic chickens compared to local ones observed could be linked to the fact that the exotic chickens were reared in confinement where there is higher oocyst accumulation in the litter and were likely to be most exposed to the infective stages of the organism in litters and feeds while the local breeds of chickens were usually found roaming and scavenging around the surroundings. They may not come into contact with the infection or may not ingest the infective stages of the organism. This agrees with the findings of Jatau, Oljira & Garbi [20,31,32] who also reported high prevalence of coccidian infection in exotic breed chickens as compared to the free-range local chickens. However, the findings of this current study did not corroborate previous reports by Benisheikh [33] who reported higher coccidiosis rate in local chickens (38.8%) than in exotic breeds (22.8%). Ashenafi [17] in Ethiopia and Hadipour [34] in Iran have reported high incidence of avian coccidiosis in indigenous scavenging chickens.
Age difference plays a significant role in prevalence distribution of coccidia oocyst shedding. In this study, the prevalence of coccidiosis was 22.5% in 1-3 weeks chickens, 10.9% in 4-6 weeks and 9.3% in 7-8 weeks. Indeed, strong statistical association (P=0.017) was observed between the prevalence of coccidian oocyst shedding of age groups. This agreed with the report of McDougald & Reid [35] who also found that most Eimeria species affect birds between 2 and 6 weeks of age. Resistance to the disease usually increases with age of birds. As the age of the birds increases, they develop immunity against the disease. This may be the reason why the disease rate decreases with increasing age of birds [36]. The difference in age variation among birds could be due to the effect of age susceptibility. Adult birds could have developed acquired immunity to infection due to previous repeated contacts with several coccidia species in the litter, that can enable them to harbor the infection without showing clinical signs whereas young birds may not have developed full immunity and can be more vulnerable and experience great mortality where outbreak of the disease occur [37]. This finding is in concurrence with previous report of Muazu [38] who reported 36.7% prevalence of coccidial infection among adult birds and 52.9% among the younger birds.
Moreover, Lawal [39] has also reported the predominance of coccidial infection among young birds as compared to adult birds. The finding of this present study was not consistent with those of Dakpogan & Salifou [40] who reported higher rate in adult chickens (40.70%) as compared to the young ones (28.20%). The highest frequency occurred in the age group between 1-6 weeks. This finding is in congruent with the findings of other authors and researchers regarding the frequency occurrence of clinical coccidiosis with respect to the age of birds. This was because most coccidian infections occur at the age of 2-4 weeks but clinical diseases develop one or more weeks later. As a result, the clinical diseases appear to reach climax at 5-7 weeks of age and as the age exceeded 7 weeks, most birds will develop immunity against the diseases. However, this period may be prolonged by mistaken /defective use of anticoccidial drugs; hence a slight change was observed in the frequency of occurrence of clinical Coccidiosis in that the peak of the disease was observed at the age of 41-50 days (6-7 weeks) unlike the findings of [41].
In this study, it found that there was statistically no significant difference with the occurrence of poultry Coccidiosis between different management system (intensive and backyard) (p=0.505). No significantly higher infection rate was detected in birds under intensive management system (20.6%) as compared to birds in backyard (17.9%). However, the current result was disagreement with the previous report in Gondar, Ethiopia by Gebretensae [27] who recorded higher prevalence in chickens which are managed in backyard production system (45.7%) than floor (49.1%) and cage (25.6%) production systems. The observed higher prevalence of coccidiosis in intensive management system might be explained in terms of the rearing systems where all of the poultry farms included in the study practiced deep litter rearing system. Deep litter poultry houses further exacerbate the risk of coccidial infection as it provides optimal condition of temperature and humidity for oocyst sporulation. On other hand poor poultry management where there is overcrowding, leaking water troughs and accumulation of feces are factors that contributed to the high prevalence rate [42].
Previous chemo prophylactic treatment and vaccination of chickens against various poultry diseases and coccidian infection has high significance difference between the treated groups and non –treated groups (p=0.00). Among the 451 total studied chickens, 161 of them were regularly treated and vaccinated and were from the intensive farm whereas 290 of them were collected both from the intensive and extensive backyard system and were not get prophylactic treatment. From 161 those which were regularly treated and vaccinated only 6 of them were infected with the Eimeria oocysts with a prevalence of 3.7%. This is lower than the prevalence of 28.3% in non-treated and vaccinated groups and this is because they may develop immunity against the development of the resistance by the Eimeria species. The basic prophylactic method is to make it impossible for the coccidian oocysts to develop and spread. The second extremely significant element of coccidiosis prophylaxis is the effective disinfection of farm buildings before placing the birds there. Vaccination of other predisposing diseases given was may also played a great role in the development of immunity in treated groups. Live vaccines containing attenuated and non-attenuated strains or were used. The vaccines were administered in the breeding unit (large drop method) or on the farm (with drinking water or in the feed spray). It is also possible to administer the vaccine to the eye on the 18th day of incubation. In case of prophylactic vaccination, do not administer the coccidiostat drug with the feed, sulphonamides and tetracyclines and the vaccinated birds must be in a good condition and have appropriate environmental conditions.
This high reduction of prevalence of coccidiosis observed in the current study may be ascribed mainly to the application of preventive measures which basically rely on the use of anticoccidial drugs that were given at early ages starting from the second weeks of age for duration of 7 to 14 days of the growing periods. Another reason may be due to the slight improvement of the management system and bio security measures when compared to the setup in the previous study [41]. The probable reasons for this discrepancy could be the differences in virulence of the Eimeria species at different management system and /or due to the prophylaxis use of anti coccidial drugs in feed and water or may be due to breed differences. It is likely that resistance has not developed to more recent anticoccidial drugs [43] and very few drugs are equally efficacious against all Eimeria species [44]. The occurrence and incidence of coccidiosis is also, to a great extent affected by the type of chick reared and breed sensitivities to infection. Many coccidiostatics drugs have been directed against Eimeria tenella, with the result that other species are increasingly incriminated as a cause of poultry coccidiosis [45- 47].
To minimize the effects of resistance, poultry producers rotate the use of various anticoccidials with successive flocks, combine chemical and ionophore treatments, or employ shuttle programs during a flock grow out. Rotation of coccidiostat drug from one group (chemical or ionophoric one) into another one, from the other group, limits the resistance. In the present study, percentage prevalence of infection in backyard chickens may be high due to poor managemental practices, malnutrition and non-use of coccidiostat as preventive measures. Strategic prophylaxes and treatment against Eimeria should be developed and implemented in order to reduce the economic losses due to the disease in area. Furthermore, efforts needed to be done to develop economical and sustainable prevention and control strategies as coccidiosis remains a major challenge to poultry producers in country wide.
Conclusion and Recommendations
Despite the reduction in the prevalence of coccidiosis in the present study, coccidiosis is a major burden to poultry producers and veterinary health professionals. The occurrence of coccidiosis in different sex categories was not statistically significant differences and may be due to the equal chance of exposure for the coccidiosis infection even though it was slightly higher in females. The occurrence of coccidiosis in different age categories was statistically significant differences. However, prevalence of coccidiosis was slightly higher in young age group than adult birds. This might be the adult birds develop immunity to the trickle infections acquired from the environment and maintain the state of balance to the infection. Prevalence of coccidiosis in local breed is slightly lower than the exotic breed. This result indicated may be due management practice of local breed to cope up the effect of the disease.
Furthermore, prevalence of coccidiosis in treated and nontreated groups were highly statistically significant differences and were lower in the treated chickens. This result may be due to the development of immunity obtained from previous prophylaxis. In general terms, differences in resistance to coccidiosis in relation to sex, breed and management were not significant. Slightly higher prevalence of the infection in studied exotic chicken in the current study indicates the maintenance of oocyst in the environment, improper cleaning and disinfections methods chicken house and indiscriminate and improper use of water in the house. To conclude that coccidiosis in local chickens in the present study is slightly less than the exotic breed. The infection rate detected in this study may suggest for the presence of favorable condition for biology and transmission of the pathogen. The effect of coccidiosis on the production ability of chicken and its economic importance should be further studied.
Therefore, the following recommendations are forwarded:
Maintenance of good hygiene and sanitation in the farm is necessary because disinfectants are not effective against coccidian
Waterers and feeders should be put at height level with backs of the birds, so they cannot defecate or scratch litter into them
a. The pens should be cleaned as well as removed off dirty droppings regularly
b. Access of infected droppings should be prevented from the non-infected birds
c. Drinkers should be cleaned and sanitized
d. Avoid continuous use of anticoccidial drugs as they lead to emergence of drug resistant population of coccidian
e. The use of anticoccidial drugs by the shuttle (two or more drugs employed within a single flock) and rotation (rotation of different compounds between flocks) programs is recommended.
f. The mass of poultry populations should be timely vaccinated.
g. Keep older birds away from young (chick), since old birds are carriers.
h. Avoid moisture and humidity in litters.
i. Keep the litter dry by frequent turning of the litter to reduce the sporulation of the oocyst.
j. Awareness should be a created among the local chicken farmers through training on general knowledge of coccidiosis occurrence, medication procedures and prevention and control.
k. Stress conditions such as overcrowding should be minimized by reducing the number of chickens in intensive housing which triggers the disease occurrence.
l. There should be regular treatments and vaccination of the chicks especially in backyard systems
m. Strict biosecurity measures should be taken.
Acknowledgements
Above all Glory to GOD! The almighty creator and Lord of the Universe for His innumerable favor’s benevolence op on me through all work of my life. I would like to express my deepest gratitude to my advisor Dr. Nugussu Fasil, for his intellectual guidance. Finally, my deeper feelings go to my family and to all my classmate students.
References
- EARO (2000) Animal Science Research Strategy Directorate. Poultry Research Strategy; Addis Ababa, Ethiopia, p. 1-33.
- FAO/ILRI (1995) Livestock development strategy for low income Countries. In proceeding of the Joint ILRI/FAO round table on countries, Addis Ababa Ethiopia, p. 22.
- Yami A, Tadelle D (1997) The Status of Poultry Research and Development in Ethiopia. D.Z.A.R.C. Research Bulletin 4: 40-46.
- Nasser M, Lohr J, Mebratu GY, Zessin K, Baumann MPO, et al. (2000) Oral Newcastle disease vaccination trials in Ethiopia. Avian Pathology 29: 27-34.
- Fantico A (2005) Parasite Management for Natural and Organic Poultry Coccidiosis.
- Obasi OL, Ifut OJ, Offiong EA (2006) An outbreak of caecal Coccidiosis in a broiler flock post Newcastle disease vaccination. Journal of Animal and Veterinary Advances 5: 1239-1241.
- Charlton B (2006) Coccidiosis in: Avian disease manual, (5th edn), India: International book distributing company in association with American association of avian pathologist, USA, pp. 153-156.
- Taylor MA, Coop RL, Wall RL (2007) Veterinary Parasitology. (3rd edn), Oxford, Blackwell Publishing, UK, pp. 475-483.
- Conway D, Mckenzie M (2007) Poultry Coccidiosis, Diagnostic and Testing Procedures. (3rd edn), Ames, Iowa. Blackwell publishing, USA, p. 37-40.
- Lillehoj H, Trout J (1993) Coccidia: A Review of Recent Advances on Immunity and Vaccine Development. Avian Pathology 22: 3-31.
- Safari M (2004) Studies on prevalence and economic impact of poultry Coccidiosis in different production systems in Debre Zeit and Addis Ababa, Ethiopia: Faculty of Veterinary Medicine, Addis Ababa University, Ethiopia.
- Radiostitis O, Gay C, Constable P, Hinchliff K (2007) Disease associated with protozoa, veterinary medicine a text book of the disease of horse, sheep, pig, and goat. Harcout publishers Ltd, London, UK.
- Vegad J (2008) Poultry Coccidiosis. In: Poultry Diseases, a guide for farmers and poultry professionals. India International Book Distributing Company, India.
- Dinksew T, Girma A (2000) Socioeconomic aspect and husbandry practices of poultry kept in Hawassa city: The opportunities and challenges of enhancing Poultry production in East Africa. Proceedings of a conference held at Debub University, Hawassa, Ethiopia, 12: 175- 181.
- Kinung’hi S, Tilahun G, Hafez Woldemeskel M, Kyule Grainer H, Baumann M (2004) Assessment of Economic Impact Caused by Poultry Coccidiosis in Small and Large Poultry Farms in Debre Zeit, Ethiopia. International Journal of Poultry Science 3: 715-718.
- Razmi G, Kalideri A (2000) Prevalence of subclinical Coccidiosis in broiler chicken farms in the municipality of Mashhad, Khorasan, Iran. Preventive Veterinary Medicine 44: 3-4.
- Ashenafi H, Tadesse S, Medhin G, Tibbo M (2004) Study on Coccidiosis of scavenging indigenous chickens in Central Ethiopia. Tropical Animal Health Production 36: 693-701.
- Central Statistics Agency (CSA) (2011) Federal Democratic Republic of Ethiopia, Central Statistical Agency. Agricultural Sample Survey Report on Livestock and Livestock Characteristics, Addis Ababa Statistical Bulletin 2: 505.
- Abadi A, Netsanet W, Haileleul N (2012) Coccidiosis Prevailing in Parent Stocks: A Comparative Study Between Growers and Adult Layers in Kombolcha Poultry Breeding and Multiplication Center, Ethiopia. Global Veterinaria 8: 285-291.
- Oljira D, Melaku A, Bogale B (2012) Prevalence and Risk Factors of Coccidiosis in Poultry Farms in and Around Ambo Town, Western Ethiopia. American-Eurasian Journal of Science and Research 7: 146- 149.
- Thrusfield M (2005) Veterinary Epidemiology UK Blackwell Science Ltd, A Blackwell publishing company, UK.
- Soulsby L (1982) Helminths, Arthropods and Protozoan’s of Domesticated Animals, (7th edn), Bailliere Tindall, London, UK, pp. 630.
- Chauhan H, Roy S (2007) Poultry Diseases Diagnosis and Treatment (3rd edn), New Delhi, New Age International (P) Ltd. Publishing, India, pp. 152-157.
- Luu L, Bettridge J, Christley R, Melese K, Blake D, et al. (2013) Prevalence and molecular characterization of Eimeria species in Ethiopian village chickens. BMC Veterinary Research 9: 208.
- Alemayehua T, Tekeselassieb A, Kassac SA (2012) Prevalence study of poultry Coccidiosis in small- and large-scale farms in Addis Ababa, Ethiopia. Science Journal of Crop Science 1: 26-31.
- Gari G, Getachew T, Dorchies PH (2008) International Journal of Poultry Science, Asian Network for Scientific Information. Study on Poultry Coccidiosis in Tiyo District, Arsi Zone, Ethiopia, 7: 251-256.
- Gebretnsae H, Gebreyohannes M, Tesfaye A (2014) Prevalence of poultry Coccidiosis in Gondar town North West Ethiopia. American -Eurasian Journal of Science Research 9: 129-135.
- Dinka A, Tolossa YH (2012) Coccidiosis in Fayoumi` Chickens at Debre Zeit Agricultural Research Center Poultry Farm, Ethiopia. European Journal of Applied Science 4: 191-195.
- Alamargot J (1987) Avian Pathology of Industrial Poultry Farms in Ethiopia. Paper presented at the First National Livestock Improvement Conference, Addis Ababa pp. 114-117.
- Puttalakshmamma G, Ananda K, Prathiush P, Mamatha GS, Rao S (2008) Prevalence of gastrointestinal parasites of poultry in and around Banglore. Veterinary World 1: 201-202.
- Jatau I, Sulaiman H, Musa I, Lawal A, Okubanjo O, et al. (2012) Prevalence of coccidian infection and preponderance Eimeria species in free range indigenous and intensively managed exotic chicken during hot-wet season in Zaria, Nigeria. Asian Journal of Poultry Science 6: 79-88.
- Garbi F, Tesfaye A, Woyessa M (2015) Study on prevalence of poultry Coccidiosis in Nekemte town, East Wollega, Ethiopia. African Journal of Agricultural Research 10: 328-333.
- Benisheikh AA, Tom I, Thamus ZY, Sanda KA, Biu AA (2013) Comparative studies on the prevalence of Coccidiosis in indigenous (Gallus gallus domesticus) and exotic breeds (layers) in Benisheikh town, Borno State. Journal of Medical and Applied Biosciences 5: 33-37.
- Hadipour M, Olyaie A, Naderi M, Azad F, Nekouie O (2013) Prevalence of Eimeria species in scavenging native chickens of Shiraz, Iran. African Journal of Poultry Farming 1:034-036.
- McDougald LR, Reid WM (1997) Coccidiosis. in Diseases of Poultry, (10th edn), Calnek BW (Eds.), Iowa State University Press, Ames, IA, pp. 865-883.
- Uza DV, Olorunju SA, Orkpeh JM (2001) An assessment of the disease and production status of indigenous poultry. In: Benue and Nassarawa states of Nigeria. Proceedings of the 26th Annual Conference, Nigerian Society of Animal Production, Zaria, Nigeria 26:73-75.
- Györke A, Pop L, Cozma V (2013) Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite 20: 52.
- Muazu A, Masdooq AA, Ngbede J, Salihu AE, Haruna G, et al. (2008) Prevalence and Identification of Species of Eimeria Causing Coccidiosis in Poultry with in Vom, Plateau State, Nigeria. International Journal of Poultry Sciences 7: 917-918.
- Lawal JR, Jajere SM, Ibrahim UI, Geidam YA, Gulani IA, et al. (2016) Prevalence of Coccidiosis among village and exotic breed of chickens in Maiduguri, Nigeria. Veterinary World 9: 653-659.
- Dakpogan HB, Salifou S (2013) Coccidiosis prevalence and intensity in litter based high stocking density layer rearing system of Benin. Journal of Animal and Plant Sciences 17: 2522-2526.
- Lobago F, Worku N, Wossene A (2005) Study on Coccidiosis in Kombolcha poultry farm, Ethiopia. Tropical Animal Health and Production 37: 245- 251.
- Slayer PA, Mallison AS (1995) Prevalence of Eimeria species in Broiler; Avian Diseases. American Association of Avian Pathology 41: 204-208.
- Chapman HD (2005) Perspectives for the control of coccidiosis in poultry by chemotherapy and vaccination. IX international Coccidiosis conference, Foz do Iguassu, Brazil, pp. 99-103.
- McDougald LR (2003) Coccidiosis disease of poultry (11th edn), Iowa state press. Iwoa, pp. 1001-1010.
- Urquhart G, Armour J, Duncan J, Dunn A, Jennings F (1996) Veterinary Parasitology (2nd edn), University of Glasgow, Blackwell publishing, Scotland, pp. 228-231.
- Bowman D (2009) George’s parasitology for Veterinarians. (9th edn), Saunders Elsevier, India, p. 2 -94.
- Ethiopian Livestock Sector Development Project Report No.24/93CPETH February 17: 191-199.






























