Prevalence Major Metacestodes of Ruminant Slaughtered at Elfora Export Abattoir and Public Health Importance
Muluneh Hailu1*, Ermias Worku2, Getahun Endale2 and Abaysew Ayele2
1 College of Veterinary Medicine and Agriculture, Addis Ababa University, Ethiopia
2Department of Biotechnology, School of Engineering and Technology, Sharda University, India
Submission: February 11, 2019; Published: March 21, 2019
*Corresponding author: Muluneh Hailu, College of Veterinary Medicine and Agriculture, Addis Ababa University, P.O. Box 34, Bishoftu, Oromia, Ethiopia
How to cite this article: Muluneh H, Ermias W, Getahun E, Abaysew A. Prevalence Major Metacestodes of Ruminant Slaughtered at Elfora Export Abattoir and Public Health Importance. Dairy and Vet Sci J. 2019; 10(5): 555797. DOI: 10.19080/JDVS.2019.10.555797
Abstract
A cross-sectional study was carried out from November 2015 to March 2016 in the Elfora export Abattoir, in the Bishoftu city of Ethiopia with the objective of estimates the prevalence, organ distribution, viability of metacestodes, to identify major risk factors and to assess the level of risk perception of community about zoonotic cestodes. Out of the total 785 small ruminants examined for the presence of hydatid cysts and coenurus cerebralis an overall prevalence of 7.39% and 3.8% was recorded, respectively. Of the total 400 goats examined for hydatid cysts, 6.8 % and coenurus cerebralis,5% were found positive. There was no significant difference in the prevalence of coenurus cerebralis in sheep and at different age groups and in both species. However, young goats found significantly affected by Coenurosis. More Hydatid infected sheep were found in Negelle where as 11.7 % of goats with Hydatid cyst was found in Yabello zone. Organ distribution hydatid cyst revealed that lung and liver were found frequently infected. Out of 49 sheep with hydatid cysts, 57.1 % harbored hydatid cysts in lung, 36.7 % in liver, 2.04% in kidney and 4.08 % in muscle. In sheep, a total of 49 cysts were examined to identify cyst fertility or viability out of these 24%, 20%, 28% and 28% were identified as fertile, non-viable, sterile and calcified, respectively. From a total of 27 lung cysts there was no fertile cyst detected in goats.
The total 119 Hydatid cysts being collected from the infected animals were distributed as in the lungs 63.03 %, liver 33.6 % and kidneys 3.36 %. The retrospective survey revealed that T. saginata/taeniosis is a wide spread problem in study area. The prevalence of sex was statistically significant. Of a total of 74684 patients admitted for stool examination in the two private clinics and one referral hospitals in Bishoftu, 495 (0.61%) Taeniosis cases were registered between September 2005–August 2007 E.C. Education status created significant role in risk of exposure in which tertiary educated individuals has low risk than illiterates and other levels of education. From a total of 100 people 69% knew that tapeworm could spread from animals to humans. There was significant difference in high risk group where large number of Christians experienced consumption of raw meat (p = 0.001). In general, the low risk group was more likely to agree that tape worm infection and its consequences were serious. Interestingly, high risk groups were also more likely to agree that the absence of access to cooking facilities, inspected meat or abattoir slaughtered meat increase their chances for the consumption of raw meat there by tends to attain the parasite. These results suggest that the high prevalence of metacestode infestations in this area is a great concern for both medical and veterinary authorities to design therapeutic and preventive programmes to overcome this problem.
Keywords: Cestodes; coenurus cerebralis; Hydatid Cysts; Taeniosis; Viability
Introduction
Ethiopia’s estimated livestock population is often said to be the largest in Africa. In the country, there were approximately 57.8 million cattle, 28 million sheep, 28.6 million goats, 1.23 million camels and 60.5 million poultry [1]. Ethiopia’s great livestock potential is not properly exploited due to different factors such as traditional management system, limited genetic potential, lack of appropriate disease control policy and lack of appropriate veterinary services. Apart from this [2]. Foods of animal origin are often the preferred source of protein. However, if not properly prepared or handled, they can lead to food-borne infections [3]. In a country confronted with challenges of an ever-rising human population and food shortage, such enormous losses caused by helminthes parasites, ‘the silent predators’, are intolerable [4]. Moreover, these diseases are also known to cause public health problems as humans can be infected from accidental ingestion of parasite eggs/larvae passed into the environment with faeces from definitive hosts [5,6].
Cestodes of the family Taeniidae infect dogs and humans as the definitive host and are transmitted to a wide range of intermediate host species where they cause Coenurosis, Hydatidosis, and Cysticercosis, respectively. Infestations with the larval stage of some species of Taenia are not only of public health importance, but also of veterinary significance because they cause economic losses due to condemnation of infected offal or meat [7]. The infestation may lead to lower production and even death of the animals in cases of heavy infestations [8]. Hydatidosis and Taeniosis are parasitic zoonoses that present major public health problems in lower income countries and some industrialized countries [7,9-11]. The prevalence is considered to be higher in developing countries because of poor sanitation, traditional cattle husbandry systems and inadequate meat inspection facilities [11,12]. As a result, the quality of human life, the aesthetic value of meat and the trading of meat and offal are compromised [11,13].
Hydatid cyst is the metacestodes of the tapeworm Echinococcus granulosus. Adult worms have been reported to be found in small intestines of dogs and wild carnivores like the wolf and fox. Infested carnivores eliminate eggs with their faeces. Herbivores (intermediate host) become infested with the eggs on account of having fed on contaminated pastures [14]. Man is infected incidentally up on ingestion of infective eggs in contaminated water, vegetables and other food or through direct contact with dog. Possible intermediate hosts for Cysticercus cerebralis are sheep and goats, for Cysticercus bovis are cattle and buffalo, and for hydatid cysts, domestic ungulates and man act as an intermediate host [15]. Consumption of offal containing viable cyst results in infection of definitive host carnivores including dogs. The adult tapeworm in definitive host is harmless unlike the metacestodes in the intermediate host animals that is responsible for immense economic and medical importance in infected host [16-18]. The remarkable biotic potential of E. granulosus is known by the fact that a heavily infested dog may carry as many as 40,000 tapeworms, shedding approximately 1,000 eggs per 2 weeks [19]. Clinical hydatidosis is uncommon in animals, but hydatid cysts in the liver and other tissues at slaughter are widespread and cause condemnation and economic loss [20].
Coenurosis, the bladder worm stage of Taenia multiceps predominantly develops in the brain and spinal cord of many mammal species, including human [21-24]. Coenuruses due to larval stage of Taenia multiceps can occur in both an acute and a chronic disease form. Acute coenuruses occurs during the migratory phase of the disease, usually 10 days after ingestion of the large number of tape worm eggs. Young lambs aged 6-8 weeks are most likely to show signs of acute disease. The signs are associated with an inflammatory and allergic reaction. There is transient pyrexia and relatively mild neurological signs such as listlessness and a slight head aversion. Occasionally the signs are more severe and the animal may develop encephalitis, convulse and die within 4-5 days [24]. The infection is acquired in cattle by grazing on pasture contaminated with faeces of humans. Cysticercus bovis infection in cattle may not show any clinical disease and therefore goes un-noticed except during abattoir meat inspection. These larvae remain embedded in the tissues of cattle posing serious public health threats. The observations were reinforced by a probabilistic model developed by Kyvsgaard [25] which showed that over 85% of infected animals may be missed during routine meat inspection.
Hydatidosis and taeniosis is of public health and economic importance not only in areas of endemicity but also in non-endemic countries due to the migration of infected people and livestock exchange, their products, and potentially contaminated produce or other fomites which promotes emergence in previously free-disease areas [26]. They are frequently reported from different corners of the country [27-29], and the disease is much more common in rural areas of Ethiopia where dogs and domestic animals live in a very close association [30]. Additionally, where home slaughtering of cattle, sheep, goats and camels is still predominant and uncooked offal and carcass wastes are normally given for dogs and cats, peoples to eat the ingestion of raw or undercooked beef dishes such as ‘‘kurt’’ and ‘’kitfo’’ [31-33]. However, there is lack of recent information on some major metacestodes in East Shoa Zone of Oromia Regional State particularly in and around Bishoftu. This area is known for its commercial, domestic and export abattoirs growing in number currently. To establish appropriate strategy for prevention and controls, it is very important to know public perception about the risk of the diseases and up to date epidemiological information is needed on zoonotic parasites and their public health importance’s.
Therefore, the objectives of current study are:
a. To determine the prevalence, organ distribution, viability of metacestodes
b. To identify major risk factors associated with metacestodes in cattle, sheep and goats slaughtered at Elfora export abattoir.
c. To assess the level of risk perception by community about zoonotic cestodes.
Materials and Methods
Study Animals
All local breeds’ sheep and goats that originated from neighboring localities and/or regions for slaughter in Elfora export abattoir were included in the study population. Consequently, all male sheep & goats were subjected for the study and age of the animals were grouped based on dentition, for those which have not erupted permanent incisor teeth, are classified as young, while those with pair or more permanent incisor teeth erupted were classified as adult [34]. All cattle inspected were adult males and from similar agro-ecological sites and husbandry systems
Study Design and Sample Size
A cross-sectional study type was carried out from November 2015 to April 2016. The total number of animals required for the study was calculating based on the formula given by Thrusfield [35]. A stratified random sampling procedure was employed to carry out this study. By rule of thumb where there is no information for an area it is possible to take 50% of expected prevalence. Using 5% degree of absolute precision, 384 animals need to be sampled. For this study, the required sample size was 384, but in order to increase precision, it was maximized to a sample size of 384 for cattle, 385 for sheep and 400 for goats. Whereas, a questionnaire and retrospective type of study were Based on the formula recommended by Arsham [36] N= 0.25/SE2, S E=5%), N=100, Where N=sample size, SE=standard error assuming the standard error of 5% at a precision level of 0.05 and the confidence interval of 95%. the sample size for the questionnaire survey was expected to be100 for each site.
Sampling Method
Active Abattoir Survey: Active abattoir survey was conducted during routine meat inspection on randomly selected cattle, sheep and goats. During ante-mortem examination of each study animal was given an identification number and its age and origin was recorded. After slaughtering the cattle, sheep and goats, post-mortem examination was carried out using standard procedures recommended by FAO/UNEP/WHO [37,38]. During post-mortem examination, carcasses and their respective organs was carefully examined for the presence of metacestodes. Visual inspection and palpations followed by multiple incisions in livers, kidneys, lungs, hearts, spleens and other organs were made to detect metacestodes.
Cyst fertility and viability test: All the positive samples were transported to Addis Ababa University, College of Veterinary Medicine and Agriculture, Veterinary Parasitology Laboratory for confirmation of cyst viability. The viability of the cysts (C. bovis) was examined by using 30% ox bile solution diluted in normal saline and incubated at 37 °C for 1 to 2h. A cyst was regarded as viable if the scolex evaginated according to Gracey [39].
For the case of hydatid cysts, individual cysts were grossly examined for degeneration, then according to the size (not too small) hydatid cysts in cattle, sheep and goats were selected for fertility study. To reduce intracystic pressure, the cyst wall was penetrated, using needle and it was cut with scalpel and scissors. The contents were then without any protoscoices, calcified, non-viable (cysts with dead protoscoices) and viable or fertile (cysts with live protoscoices). To determine viability of the protoscolices, a drop of cyst fluid was placed on a microscopic glass slide and cover slip was applied and observed for the motility of flame cells activity like peristaltic movement. When it becomes doubtful for motility, a drop of 0.1% aqueous eosin solution was added and examined under a light Microscope for taking the dye. Live protoscolices did not take the dye whereas, the dead ones did according to Daryani [40].
Questionnaire survey: To determine the infection rate and associated risk factors of human taeniosis, 100 volunteer respondents from different sex, age, level of education, occupation and religion were selected using random sampling based on willingness to participate in the questionnaire survey.
Data Management and Analysis
The data collected from abattoir and questionnaire was stored into Microsoft excel. Logistic regression was employed to analyze the association of metacestodes cyst occurrence with the potential risk factors using SPSS.ver.20 (USA) statistical software. The degree of risk factors association with the disease occurrence was further analyzed using odd ratios. Chi-square (X2) test was used to determine the variation in infection, prevalence between species, ages and origins. Questionnaire survey data was summarized using descriptive analysis and important factors were tested with logistic regression for their contribution for the occurrence of taeniosis in human. For the questioner survey we were used Health belief model (HBM) to assess the risk perception level.
Results
Prevalence of Metacestode
In the current study, a total of 1169 animals comprising 384 cattle, 385 sheep and 400 goats slaughtered in Elfora abattoir were examined for the presence of metacestodes. Out of the total 785 small ruminants examined for the presence of hydatid cysts and C.cerebralis an overall prevalence of 7.39% and 3.8% was recorded, respectively. Of the total 400 goats examined for hydatid cysts, 27(6.8 %) and C.cerebralis, 20(5%) were found positive. Likewise, of the 385 sheep examined, 31(8.1%) and 10(2.6%) were found positive for hydatid cysts and coenurus cerebralis, respectively. Statistical analysis showed that there was no significant difference among the species (P>0.005) (Table 1).
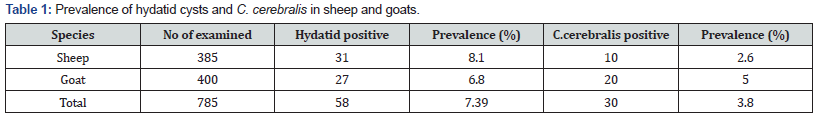
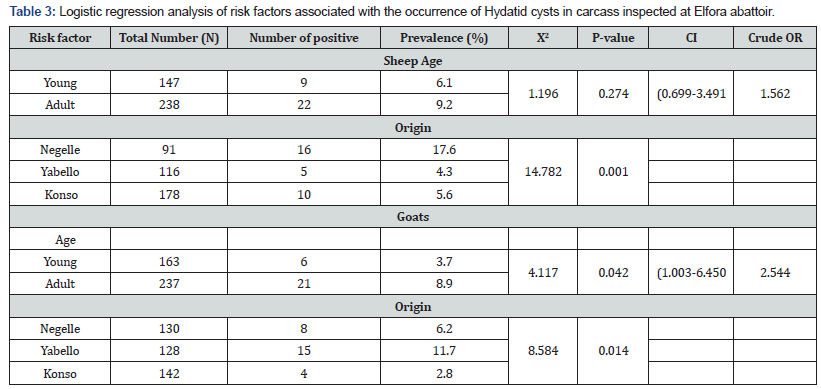
The risk of exposure to C.cerebralis based on different origin and age groups of both species were examined. Higher prevalence C.cerebralis was found in young of both sheep and goats compared adult groups. There was no significant difference in the prevalence of coenurus cerebralis in sheep at different age groups and in both species due to geographical origin. However, young goats found significantly affected by Coenurosis. Prevalence of Hydatid cysts revealed significant variation in the origin of both species of small ruminants (Tables 2-4). There was statistically significant difference in origin and age of the goats (p<0.05). where old goats by far had higher opportunities of obtaining hydatid cyst than young ones. More Hydatid infected sheep were found in Negelle (17.6%) where as 11.7 % of goats with Hydatid cyst was found in Yabello zone. In General, the prevalence of HC in sheep were higher compared with goats but not significance difference(P>0.05) Table 5.
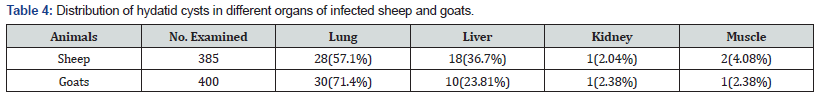
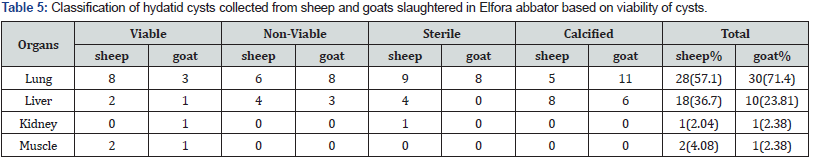
Organ distribution hydatid cyst revealed that lung and liver were found frequently infected. Out of 49 sheep with hydatid cysts, 28 (57.1 %) harbored hydatid cysts in lung, 18 (36.7 %) in liver, 1(2.04%) in kidney and 2(4.08 %) in muscle. Similarly, in goats, out of 42 with hydatid cysts, lungs accounted for 30 (71.4 %), liver 10(23.81 %), kidney 1(2.38 %) and 1 (2.38 %) harbored hydatid cysts in muscle (Table 6). In sheep, a total of 49 cysts were examined to identify cyst fertility or viability. From those 12 (24%), 10(20%), 14 (28%) and 14 (28%) were identified as fertile, non-viable, sterile and calcified, respectively. Fertile cysts were mostly detected in lung which were 8 (66.7 %) of the whole fertile cysts. However, most of cysts in the liver 8 (66.7%)) were found calcified (Table 7). Viable cysts were higher in lungs compared to liver although not statistically significant (p > 0.05). In goats, a total of 42 cysts were examined to identify cyst fertility. From those 6 (14.29 %), 11 (26.19 %), 8 (19.05 %) and 17 (40.48 %) were identified as fertile, non-viable, sterile and calcified, respectively. From a total of 27 lung cysts there was no fertile cyst detected in goats. Rather non-viable cysts in goats were found mostly in lungs which were 11(40.74 %) of the whole cysts (Table 6).

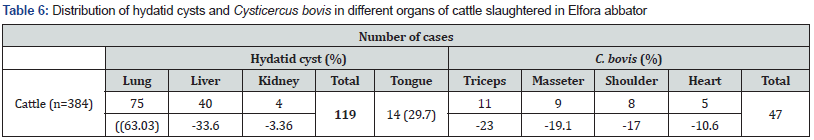
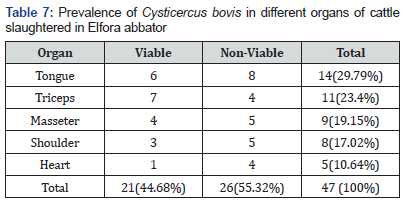
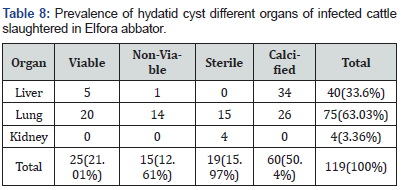
All 384 cattle inspected were adult males and from similar agro-ecological sites and husbandry systems. Of those, 17 (4.4 %) were infected with cysticerci. A total of 47 cysticerci were collected and occurred in decreasing order, in the tongue 29.79% (14), triceps muscle 23.4% (11), masseter muscle 19.15% (9), shoulder muscle 17.02% (8) and heart 10.64% (5) (Table 7). Out of 47 cysticerci, 21 (44.68 %) were viable and 26 (55.32 %) were non-viable. Viable cysticerci were observed in the triceps (7), tongue (6), masseter (4) shoulder (3) and heart (1) muscles, in descending order. Non-viable cysts were recovered from tongue (8), masseter (5), shoulder (5), triceps (4) and heart (4) muscles, in decreasing order (Table 8). Out of a total of 384 cattle carcasses, 47 (12.2 %) were infected with hydatid cysts, a total of 1 19 hydatid cysts being collected from the infected animals. Of these cysts, 25 (21.01 %) were viable,15(12.61%) non-viable, 19 (15.97 %) sterile and 60 (50.4 %) calcified. Cysts were found in the lungs75 (63.03 %), liver 40 (33.6 %) and kidneys 4 (3.36 %).
Discussion
The present study revealed that the overall prevalence of cysticercosis in cattle slaughtered at Bishoftu Elfora abattoir was 4.4 %, which is comparable to the prevalence of 4.4% reported from Jimma [41] 7.5% from Addis Abeba [42,43], 8.2% from Tigray region [44], 6.7% from Kombolcha Abattoir [45]. However, it was higher than the reported prevalence of 3% in Zeway [46], 3.11% in central Ethiopia, 3.6% in Addis Abeba [47], 2.98% in Nekemte and 2.93% in Jimma abattoir [48]. The prevalence of cysticercosis bovis in this study was relatively lower as compared with previous reports from different abattoirs of Ethiopia, such as 11.3% from Wolaita Soddo [49], 22.9% from Hawassa municipal abattoir [50], 26.25% from Southern Ethiopia Abunna and from other countries 16% from Upper Egypt [51], 72.2% from Nigeria [52] and other endemic areas in Africa and Asia by other authors [53-55].
The variation of prevalence in different study sites may be due to variations in personal and environmental hygiene, religion, culture and feeding habits of the population and their production systems [56]. The difference in prevalence rate could also attribute due to the limitation of conventional method of meat inspection which is less sensitive to pick all animals that are infected with T. saginata metacestodes. Experimental studies showed a 5-50 times higher prevalence rates by complete slicing of the predilection sites [57]. Observations indicated that except for dead and degenerated cysts which form white and fibrotic lesions viable cysts pass to human consumption without being detected due to carless meat inspection as described by Dorny & Garedaghi [11,55]. The majority of the findings in Ethiopia were based on surveys carried out on carcasses subjected to routine meat inspection procedures. Hence, the same limitations with which meat inspection shares globally were reflected in the results of the present study. For instance, Onyango-Abuje [58] in low and high-risk areas of Kenya reported 0 and 31.47% of prevalence by meat inspection and 13.33% and 80.42% prevalence by serology respectively.
During inspection, C. bovis was found in different organs with higher number of cysts encountered in the tongue (14; 29.79%), followed by triceps muscle (11; 23.4%), masseter muscle (9; 19.15%), shoulder (8; 17.02%) and heart (5; 10.64%). Other studies carried out elsewhere showed that tongue; heart and masseter appear were the most frequent locations for cysticerci [55]. Further, Abunna & Getachew [59] reported triceps as being frequently affected by the cyst. However, the current study showed that the most frequently affected organ with the highest number of cysts was the tongue which agrees with the report of Bedu & Garedaghi [55]. It is evident from the result that other organs such as triceps, masseter muscle and heart were also frequently affected predilection sites for C. bovis which is similar to earlier reports in various endemic areas [60,61]. The results of viability test showing highest proportion of viable cysts in triceps muscles was comparable to the works of Tembo, Shimeles & Emiru [62,63]. Hydatidosis is known to be livestock and public health important disease and for establishment of a control strategy, detailed information on local epidemiology and significance of the disease must be known. The present study showed that the prevalence of hydatid cyst in sheep, goats and cattle were 8.1%, 6.8% and 12.2%, respectively.
The prevalence of hydatidosis in cattle recorded in this study 12.2% agreed with the findings of Belina 9.4% in Harar, Regassa [49] 15.5% in Woliata and Kebede [28] 16% in Wolita sodo. In contrast, the present study disagrees with the reports of Alemayehu [64] 54.8% in Assela, Kebede [29] 48.9% in Debre Markos, Tigist, (2009) 36.58 % in Jimma & Wubet [65] 62.96% in Bale Robe. The prevalence of cystic echinococcosis in cattle in other countries has been reported to be lower than that in the cattle at Elfora Abattoir; for example, studies have quoted prevalence of 8.56% in Tunisia, Lahmar [66] 8.28% in Saudi Arabia, Ibrahim 7.4% in Turkey Sariozkan & Yalcin [67] 6% in Sudan Omer [68] and 4.2% in Arusha Tanzania, Nonga & Karimuribo [69]. This variation in prevalence of cystic echinococcosis among cattle of different areas in Ethiopia and in the countries could be attributed mainly to the strain difference of Echinococcosis granulosus that exist in different geographical location [70] and other factors like differences in agroecology, the times at which studies took place, stocking rates and movements of animals, animal husbandry systems, awareness, culture and religion of the society, and attitude to dog sin different regions of the country [71].
Regarding organ distribution, the current study showed that lungs (63.03%) were the most preferred predilection site for hydatid cysts followed by liver (33.6%) which agrees with other study in cattle in Ethiopia such as Tolossa and Getaw. This might be due to the fact that cattle are slaughtered at older age, during which period the liver capillaries are dilated and most oncospheres pass directly to the lung. It is also possible for the hexacanth embryo to enter the lymphatic circulation and be carried via the thoracic duct to the heart and then trapped in the lungs [72]. Furthermore, the lungs and liver possess the first. great capillaries encountered by the migrating echonocouccss oncosphere (hexacanth embryo) which adopt the portal vein route and primarily negotiate hepatic and pulmonary filtering system sequentially before any other peripheral organ is involved [73]. Our overall finding that 50.4 % of hydatid cysts were calcified, 21.01% fertile (viable), 12.61% sterile and 15.97% non-viable implies that most hydatid cysts from cattle are not infective to the final hosts. This finding supports previous arguments by several investigators in Ethiopia that assume sheep to have a greater role than cattle as an intermediate host of cystic echinococcosis.
The fertility rate among different organs also showed varied proportion. Accordingly, cysts in the lungs took the higher proportion of fertility rate agrees with earlier reports [74]. It has been stated that the relatively softer consistency of the lung tissue allows the easier development of the cyst and this may be aggravated due to reduced immunological compatibility of animals at their older age of infection. However, our finding is in contrast with a report from Tunisia of the higher fertility of hepatic compared with pulmonary cysts in cattle [75] this is most probably because different strains of hydatid cyst occur in the two countries. The high prevalence and fertility of pulmonary cysts in the present study suggests that the lung is the most important organ as a source of infection to dogs of the area. Unlike the case in lungs, relatively higher number of calcified cysts encountered in the liver (24%). The liver is firm in consistency and lack suitable matrix for long term cyst survival and hence the cyst degenerates earlier than the once found in lungs. The higher number of calcified cysts in the liver could also be attributed to relatively higher reticuloendothelial cells and abundant connective tissue reaction of the organ. The variation between tissue resistances of the infected organs may also influence the fertility rate of the hydatid cysts [29].
An overall prevalence of 58(7.4%) hydatid cysts was recorded in examined small ruminants in the prevalence of CE in sheep (8.1%) and goats (6.8%). In sheep 8.52% prevalence was recorded and this value agrees with Assefa & Tesfay, Getachew, Abunna, Azlaf, Dakkak & Elmahdi [76-78], who reported (8.02), (8.52), (8.05), (10.58%) and (10.3%) prevalence in sheep. However, Haridy & Njoroge [79,80] observed lower hydatid cyst infection in sheep; (0.33%) and (3.6%) respectively. On the other hand, the prevalence in goats in the present study was 6.8% which is agreement with the study of Saeed [81] 6.2%, Dalimi [82] 6.3%, Yeshiwork [83] 6.8% and Getaw 6.7% was is not in agreement with the study of Haridy & Njoroge [79,80], who reported 4.5% and 3.4% respectively. This variation probably due to the reason explained in the discussion part of bovine hydatidosis.
The higher prevalence of cystic echinococcosis in sheep than goats in the present study was most probably due to the fact that goats feed mainly by browsing than grazing unlike in sheep and due to the close grazing to the rot of grasses behavior of sheep on pastures contaminated with oncospheres of E. granulosus on the pasture [28,29,61]. This finding suggests the importance of sheep as the main reservoir of infection in maintaining and perpetuation of the domestic life cycle of E. granulosus in the region [28]. On the other hand, categorical analysis of age in this study demonstrated higher infection rate of cystic echinococcosis in adult sheep and adult goats as compared to their young ones. This can be attributed to two factors: firstly, higher age reflects a much longer period of exposure to infective egg stage in the pasture, and secondly, the chances detecting cysts at meat examination are higher in aged animals due to their bigger size. In younger animals, either hydatid cysts are not developed into detectable size, which can be easily missed during post-mortem examination. Indeed, the present study as well many other studies elsewhere [84] have shown higher infestation rates of hydatid cysts in older animals.
In this study, livers and lungs were the most frequently infected visceral organs to hydatid cysts in both host species examined. This is explained by the fact that livers and lungs possess the first great capillary sites encountered by the migrating Echinococcus oncosphere (hexacanth embryo) which adopt the portal vein route and primarily negotiate the hepatic and pulmonary filtering system sequentially before any other peripheral organs are involved. The location of cysts and cyst morphology are influenced not only by host factors but also by parasite factors such as the strain of E. granulosus involved [85]. The observation in this study that the lungs in both sheep and goats were found to be more commonly infested with hydatid cysts than the liver agrees with previous findings of Marshet & Oryan [86,87]. The lungs are considered of having the first large capillary fields encountered by the blood-borne oncospheres. In addition to this, the presence of greater capillary beds in the lungs than in other organs and soft consistency of the lung might also allow easy growth of cysts. The development of hydatid cyst occurs occasionally in other organs and tissues when oncosphere escapes into the general systemic circulation in both heart and spleen, no cysts were observed from both sheep and goats [88].
The higher viability rates of pulmonary cysts than hepatic cysts in both sheep and goats in the current study agree with those of Kebede & Getachew [29,77]. This might be due to softer consistency of tissues that allows the easier development of cyst and the viability. Among other zoonotic cestodes, coenuruses is endemic in Ethiopia, especially in the highland sheep where 75% of the population is found [89]. The presence of freely roaming dogs on grazing land greatly contributes to the existence of the disease. Dogs are routinely fed on offal, including sheep and goats head are not dewormed. Thus, maintaining the C. cerebralis -Taenia multiceps cycle [23].
The findings of the present study revealed that up to 3.78% of sheep and goats slaughtered at Hashim Export Abattoir in Bishoftu were found to be infected with Coenuruses cerebralis with prevalence’s of 3.9% in goats and 2.5% in sheep. The result of the current study in sheep is consistent with the report of Abo-Shehada in Jordan (3% in sheep), Varma & Malviya [90] in India (2.88% in sheep). But is slightly lower than Sharma &C hauhan [23] in Ethiopia (5% in sheep) and Oryan [91] in Iran (9.8% in sheep). The most probable reason for the variation of the results in different countries is supposed due to variations in climatic, geographical management of the study animals and the final dog hosts and social conditions.
Higher prevalence of coenuruses was recorded in young 6.8% animals than adult 1.9% small ruminants. Comparative results were reported by Morris [92], 5% in sheep, Adem & Hayelome [93] 4% in goats and 5.3% in sheep in different parts of Ethiopia. Previous studies show that clinical coenurosis in sheep is common in young animal [94]. This higher prevalence in young sheep and goats is most probably attributed to under developed immunity in young animals thus higher infection rate in these animals whereas the adults have acquired immunity [95].
The prevalence of human taeniosis was recorded based on the and questionnaire survey and indicated an overall infection rate of 41% which demonstrates the importance of taeniosis in Bishoftu town, surrounding kebeles and in the areas of animal origins. The high prevalence of taeniosis recorded in Hawassa 64.2%, Ziway 56.7% Jimma 56.7%. The prevalence of T. saginata varies from country to country and even differs within the same country from area to area depending on factors, such as variation in the habit of raw meat consumption, meat inspection procedures practiced, awareness of patients about the clinical pictures and transmission of the disease, variation in personal and environmental hygiene, and other factors related to the variation in the prevalence of taeniosis among countries. Moreover, some individuals in a society may become shy to tell openly about taeniosis infection and that may undermine the true infection rate of the disease.
The researchers recognize the consumption of raw or undercooked beef in Ethiopia. Previous reports from Ethiopia indicated that consumption of raw or inadequately cooked beef was strongly associated with T. saginata infection. Reports have indicated that the prevalence of T. saginata taeniosis may also vary in relation to age, sex, religion, educational status and income of individual. The present study showed that there was strong association between age of the respondents and the prevalence of T. saginta infection, which agrees with previous reports by Hailu & Abunna. Taeniosis was more reported from respondents above 20 years of age /adults than from younger respondents. This may imply that the habit of raw meat consumption increases with age. Younger people, mostly students cannot afford to buy beef for raw consumption as most raw meats are consumed at the butcher’s house and are more expensive (of high quality) than the one that is taken away for preparation at home. The current result was also supported by Cabaret [12] where he reported a high prevalence of T. saginata/cysticercosis in sub-Saharan Africa especially East Africa.
This study has also clearly demonstrated the impact of religion on raw meat consumption. The proportion of taeniosis infection was higher in the Christian respondents than in Muslim respondents. Tibo & Abunna have also reported similar observations in different parts of Ethiopia suggesting that the tradition of raw beef consumption is more important in the Christian community. High prevalence of metacestodes infestation in the present study could be due to high population of carnivores particularly stray dogs in the grazing area of domestic ruminants and lack of proper efforts in segregating domestic and wild carnivores from livestock or their grazing areas. Feeding offal of ruminants to dogs also enhance completion of the life cycle. The results of the present study indicate the importance of metacestodes infestation in this area. Their significance is not only because they have great economic importance resulting in losses due to condemnation of the infected organs and downgraded carcasses but it is also because of the zoonotic aspects of some of these infestations such as cysticercosis, hydatidosis and coenurosis. In addition, substantial economic loss due to treatment of human taeniosis remains to be evaluated.
In other countries, the prevalences of 0.5-3% human infestations with T. saginata have recently been reported from different parts of Iran [96,97]. In another study Daryani [98] reported that 14% of vegetables imported to and 16% of those cultivated in Ardabil, north-western Iran were contaminated with T. saginata eggs. Human cases of hydatidosis are regularly reported from different regions of Iran and it is one of the most important zoonotic diseases prevalent in different parts of this country [99,100]. Population studies on human hydatidosis, using serological and ultrasonographical methodologies, have shown 3.5-5.9% infestation in different areas of Iran [100,101]. Although there are reports of human coenurosis in the world [102-186].
Conclusion and Recommendations
Zoonotic metacestodes are a public health risk and causes considerable economic loss via decreasing livestock production and condemnation of offals in slaughter houses. The current abattoir survey proved that zoonotic metacestodes like hydatidosis, Cysticercus bovis and coenurus cerebralis are the most and highly prevalent in slaughtered ruminants [187-203]. The retrospective and the question- naire survey showed that taeniosis is a widespread problem with higher prevalence among the resident of Bishoftu town. Religion, open air defecation, presence of backyard slaughtering practices, habit of raw meat consumption, Age, and Sex were identified to be the most important risk factors for the disease occurrence in the study area [204-208].
Based on the above conclusive remarks, the following recommendations are forwarded:
a. Public education is required to avoid the consumption of raw and undercooked meat, keep their self-hygiene, prohibition of backyard slaughter, proper disposal of condemned offal’s.
b. Fencing of abattoirs and denying access of stray dogs in to abattoirs must be strictly applied.
c. Sustainable community-based control strategies against zoonotic metacestodes should be designed and implemented.
d. Impact of zoonotic cestodes on meat export market should be further studied in export abattoirs including different species of slaughtered animals
e. Further studies on the prevalence and economic importance of metacestodes in different zones of region involving different hosts (including wildlife) as well as on existing status in human would be mandatory to establish a clear information system for launching a control programme.
References
- Central Statistical Authority (2016) Federal Democratic Republic of Ethiopia Agriculture sample survey, Addis Ababa. Staticall bulletin 2: 583.
- Ayele S, Assegid W, Belachew H, Jabbar MA, Ahmed MM (2003) Livestock marketing in Ethiopia: A review of structure, performance and development initiatives. Socio-economics and Policy Research Working Paper 52. ILRI (International Livestock Research Institute), Addis Ababa, Ethiopia.
- Karshima NS (2012) A multidisciplinary approach in the control of zoonoses in Nigeria. Journal Veterinary Advance 2(12): 557-567.
- Biffa D, Jobre Y, Chakka H (2006) Ovine helminthosis, a major health constraint to productivity of sheep in Ethiopia. Animal Health Research Rev 7: 107-118.
- Jenkinsa DJ, Romig T, Thompson RCA (2005) Emergence/re-emergence of Echinococcus spp. a global update. International Journal of Parasitology 35: 1205-1219.
- Ashrafi K, Valero MA, Panova M, Periago MV, Massoud J, et al. (2006) Phenotypic analysis of adults of Fasciola hepatica, Fasciola gigantica and intermediate forms from the endemic region of Gilan, Iran. Parasitology International 55: 249-260.
- Thompson RCA (1995) Biology and systematics of Echinococcus. In: Echinococcus and hydatid disease. Thompson RCA, Limbery AJ (Eds.), Wallingford UK: CAB International p. 1-50.
- Radfar MH, Tajalli S, Jalalzadeh M (2005) Prevalence and morphological characterization of Cysticercus tenuicollis (Taenia hydatigena cysticerci) from sheep and goats in Iran. 75: 6.
- Mehrabani D, Oryan A, Sadjjadi SM (1999) Prevalence of Echinococcus granulosus infection in stray dogs and herbivores in Shiraz, Iran. Veterinary Parasitology 86: 217-220.
- Utulas M, Esatgil D, Tuzer S (2007) Prevalence of hydatidosis in slaughtered animals in Thrace, Turkey. Parasitology Dergisit 31: 41-45.
- Dorny P, Vercammen F, Brandt J, Vansteenkiste W, Berkvens D, et al. (2000) Sero- Epidemiological Study of Taenia saginata Cysticercosis in Belgian Cattle. Veterinary Parasitology 88: 43- 49.
- Caparet J, Geerts S, Madeline M, Ballandonne C, Barbier D (2002) The use of urban sewage sludge on pasture: the cysticercosis threat. Veterinary Research 33: 575-597.
- Conteh L, Engels T, Molyneux DH (2010) Socioeconomic aspects of neglected tropical diseases. Lancet 375: 239-247.
- Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, et al. (2007) Prevention and control of cystic echinococcosis. Lancet Infectious Disease 7(6): 385-394.
- Kumsa B (1994) Hydatidosis in Nekemet: prevalence in slaughtered cattle and sheep estimated economic loss and incidence in stray dog. DVM Thesis, Addis Ababa University, Faculty of Veterinary Medicine, Debre zeit, Ethiopia.
- Azlaf R, Dakkak A (2006) Epidemiological study of the cystic echinococcosis in Morocco. Veterinary Parasitology 137:83-89.
- Bettelli G (2009) Echinococcosis: costs, losses and social consequences of neglected zoonoses. Veterinary Research Community 33(1): 47-52.
- Ibrahim MM (2010) Study of cystic Echinococcosis in slaughtered animals in Al Baha region, Saudi Arabia: interaction between some biotic and abiotic factors. Acta Tropical 113: 26-33.
- Schantz PM, Kern P, Brunetti E (2006) Echinococcosis. In: Guerrant R, Walker DH, Weller PF (Eds.), Tropical infectious diseases: principles, pathogens and practice, (2nd edn), Saunders, Philadelphia pp. 1104- 1326.
- Eichenberger RM, Stephan R, Deplazes P (2011) Increased sensitivity for the diagnosis of Taenia saginata cysticercus infection by additional heart examination compared to the EU-approved routine meat inspection. Food Control 22: 989-992.
- Tafti AK, Oryan A, Maleki M (1997) Pathologic changes due to Coenurosis in a wild ewe in Iran. Journal of Veterinary Parasitology 11: 65-68.
- Ing MB, Schantz PM, Turner JA (1998) Human coenurosis in North America: case reports and review. Clinical Infectious Disease 27: 519- 523.
- Sharma DK, Chauhan PPS (2006) Coenurosis status in Afro-Asian Region - a review. Small Ruminant Research 64: 197-202.
- Oryan A, Goorgipour S, Moazeni M, Shirian S (2012) Abattoir prevalence, organ distribution, public health and economic importance of major metacestodes in sheep, goats and cattle in Fars, southern Iran. Tropical Biomedecine 29: 349-359.
- Kyvsgaard NC, Ilsøe B, Henriksen SA, Nansen P (1990) Distribution of T. saginata cysts in carcasses of experimentally infected calves and its significance for routine meat inspection. Research Veterinary Science 49: 29-33.
- Mamuti W, Yamasaki H, Sako Y (2002) Usefulness of hydatid cyst fluid of Echinococcus granulosus developed in mice with secondary infection for serodiagnosis of cystic Echinococcosis in humans. Clinical and Diagnostic Laboratory Immunology 9(3): 573-576.
- Biluts H, Minas M, Bekele A (2006) Hydatid disease of the liver: a 12 year of surgical management. East Central Africa Journal Surgery 11: 54-59.
- Kebede N, Abuhay A, Tilahun G, Wossene A (2009) Financial loss estimation, prevalence and characterization of hydatidosis of cattle slaughtered at Debre Markos municipality abattoir. Ethiopia. Tropical Animal Health and Production 41: 1787-1789.
- Kebede N, Mitiku A, Tilahun G (2009) Hydatidosis of slaughtered animals in Bahir Dar Abattoir, Northwestern Ethiopia. Tropical Animal Health Production 41: 43-50.
- Fromsa A, Jobre Y (2011) Infection prevalence of hydatidosis (Echinococcus granulosus, Batsch, 1786) in domestic animals in Ethiopia: a synthesis report of previous surveys. Ethiopia Veterinary Journal 15(2): 11-33.
- Teka G (1997) Food Hygiene Principles and Food Born Disease Control with special Reference to Ethiopia. Addis Ababa University, Faculty of Medicine, Department of Community Health, Addis Ababa, Ethiopia.
- Tembo A (2001) Epidemiology of Taenia saginata taeniosis and cysticercosis in three selected agro climatic zones in central Ethiopia, MSc thesis, Faculty of Veterinary Medicine, Addis Ababa University and Free University of Berlin.
- Kumar A, Tadesse G (2011) Bovine cysticercosis in Ethiopia: A review. Ethiopian Veterinary Journal 15: 15-35.
- Alemu Y, Merkel RC (2008) Sheep and goats’ production and Handbook for Ethiopia. p. 2.
- Thrusfield M (2005) Veterinary epidemiology, (2nd edn), Blackwell Science, USA, pp. 182-198.
- Thrusfield M (2005) Veterinary epidemiology, (2nd edn), Blackwell Science, USA, pp. 182-198.
- FAO/UNEP/WHO (1994) Guidelines for echinococcosis/hydatidosis surveillance, prevention and control. Rome: Food and Agriculture Organization of the United Nations pp. 147.
- Swai I, Schoonmann K (2012) A survey of zoonotic diseases in trade cattle slaughtered at Tanga city abattoir: a cause of public health concern. Asian Pacific Journal of Tropical Biomedical 2: 55-60.
- Gracey JF, Collins DS, Huey RJ (1999) Meat Hygiene, (3rd edn), W.B. Saunders Company Ltd, USA, pp. 669-678
- Daryani A, Aiaei R, Arab R, Sharif M (2006) Prevalence of hydatid cyst in slaughtered animals in Northwest Iran. Journal of Animal Veterinary Advance 5: 330-334.
- Getaw A, Beyene D, Ayana D, Megersa B, Abunna F (2010) Hydatidosis: prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, Central Oromia, Ethiopia. Acta Tropical 113: 221-225.
- Kebede N, Tilahun G, Hailu D (2009) Current status of bovine cysticercosis of slaughtered cattle in Addis Ababa Abattoir, Ethiopia. Tropical Animal Health and Production 41: 291-294.
- Kebede W, Hagos A, Girma Z, Lobago F (2009) Echinococcosis/ hydatidosis: its prevalence, economic and public health significance in Tigray region, North Ethiopia. Tropical Animal Health Production 41: 43-50.
- Kumar A, Gebretsadik B (2008) Occurrence of cysticercosis in cattle of parts of Tigray region of Ethiopia. Journal Haryana Veterinarian 47: 88-90.
- Endris J, Negussie H (2011) Bovine Cysticercosis: Prevalence, Cyst Viability and Distribution in Cattle Slaughtered at Kombolcha Elfora Meat Factory, Ethiopia. American- Eurasian Journal of Agriculture and Environmental Science11: 173- 176.
- Bedu H, Tafess K, Shelima B, Woldeyohannes D, Amare B (2011) Bovine Cysticercosis in Cattle Slaughtered at Zeway Municipal Abattoir: Prevalence and its Public Health Importance. Journal of Veterinary Science and Technology 2(108): 2157- 7579.
- Ibrahim N, Zerihun F (2012) Prevalence of Tania Saginata Cysticercosis in Cattle Slaughtered in Addis Ababa Municipal Abattoir, Ethiopia. Global Veterinarian 8: 467-471.
- Tolosa T, Tigre W, Teka G, Dorny P (2009) Prevalence of bovine cysticercosis and hydatidosis in Jimma municipal abattoir, Southwest Ethiopia. Onderstepoort Journal of Veterinary Research 76: 323-326.
- Regassa A, Abunna F, Mulugeta A, Megersa B (2009) Major Metacestodes in cattle slaughtered at Wolaita Soddo Municipal abattoir, Southern Ethiopia: Prevalence, cyst viability, organ distribution and socioeconomic implications. Tropical Animal Health Production 41: 1495-1502.
- Belachew M, Ibrahim N (2012) Prevalence of Cysticercosis Bovis in Hawassa Municipal Abattoir and its Public Health Implication. American- Eurasian Journal of Scientific Research 6: 238-245.
- Basem R, Amal S, Asmaa A, Hussein I, Arafa M (2009) Occurrence of Cysticercosis in cattle and buffaloes and T. saginata in man in Assiut Governance of Egypt. Veterinary World 2(5): 173-176.
- Ikpeze O, Eneanya O, Christine I, Ekechukwu W (2008) Significance of meat inspection in the estimation of economic loss due to bovine cysticercosis. Animal Research International 5(3): 896-899.
- Opara MN, Ukpong UM, Okoli IC, Anosike JC (2006) Cysticercosis of Slaughtered Cattle in Southeastern Nigeria. Annual New York Academics Science 1081: 339-346.
- Muneyeme M, Munangandu HM, Muma JB, Nambota AM, Biffa D (2010) Investigating effects of parasite infection on body condition of the Kafue basin. Biomedical Research 3: 346-346.
- Garedaghi Y, Rezaii Saber A, Saberie Khosroshahi M (2012) Prevalence of Bovine Cysticercosis of Slaughtered Cattle in Meshkinshahr Abattoir. Iran Journal of Animal and Veterinary Advances 11(6): 785-788.
- Megersa B, Tesfaye E, Regassa A, Abebe R, Abunna F (2009) Bovine cysticercosis in Cattle Slaughtered at Jimma Municipal Abattoir, South western Ethiopia: Prevalence, Cyst viability and Its Socio-economic importance. Veterinary World, 3(6): 257-262.
- Minnozo J, Gusso R, De Castro E, Lago O, Soccoi V (2002) Experimental Bovine Infection with Taenia Saginata eggs: Recovery rates and Cysticerci Location. Brazilian Archives of Biology and Technology 45: 451- 455.
- Onyango-Abuje JA, Joseph MN, Moses K, Steven HW, Patrick L, et al. (1996) Seroepidemiological survey of Taenia saginata cysticercosis in Kenya. Veterinary Parasitology 64: 177-185.
- Getachew B (1990) Prevalence and significance of Cysticercus bovis among cattle slaughtered at Debre zeit abattoir. Unpublished DVM thesis, Addis Ababa University, Faculty of Veterinary Medicine, Debre Zeit, Ethiopia.
- Hailu D (2005) Prevalence and risk factors for Taenia saginata cysticercosis in three selected areas of Eastern Shoa. Faculty of Veterinary Medicine, Addis Ababa University. MSc. Thesis, Debre zeit, Ethiopia.
- Dawit T, Tewodros S, Tilaye D (2012) Public health and economic significance of Bovine cysticercosis in Wolaita Soddo, Southern Ethiopia. Hawassa University, School of Veterinary Medicine, Ethiopia. Global Veterinary 9(5): 557-563.
- Shimeles D (2004) Epidemiology of T. saginata, Taeniasis and cysticercosis in North Gonder zone. DVM Thesis, Faculty of Veterinary Medicine, Addis Ababa University, Debre-Zeit, Ethiopia.
- Emiru L, Tadesse D, Kifleyohannes T, Sori T, Hagos Y (2015) Prevalence and public health significance of bovine cysticercosisat Elfora Abattoir, Bishoftu, Ethiopia. Journal Public Health Epidemiology 7(2): 34-40.
- Alemayehu L (1990) The prevalence of Hydatidosis in cattle, sheep and goat and Echinococcus granulosus in dog’s in Arsi administration region. DVM thesis, Faculity of Veterinary Medicine, Addis Ababa University, Debrezeit, Ethiopia.
- Wubet S (1988) Prevalence of cattle hydatidosis and its economic significance in Bale Robe municipal abattoir. DVM thesis, Faculty of Veterinary Medicine, Addis Abeba University, Debrezeit, Ethiopia.
- Lahmar S, Trifi M, Naceur SB (2012) Cystic echinococcosis in slaughtered domestic ruminants from Tunisia. Journal of Helminthology 12: 1-8.
- Sariozkan S, Yalcin C (2009) Estimating the production losses due to cystic echinococcosis in rumiants in Turkey. Veterinary Parasitology 163: 330-334.
- Omer RA, Dinkel A, Romig T (2010) A molecular survey of cystic echinococcosis in Sudan. Veterinary Parasitology 169: 340-346.
- Nonga HE, Karimuribo ED (2009) A retrospective survey of hydatidosis in livestock in Arusha, Tanzania, based on abattoir data during 2005- 2007.Tropical Animal Health Production 41: 1253-1257.
- McManus DP (2006) Molecular Discrimination of Taeniid Cestodes. Parasitology International 55: 531-537.
- Kebede N, Mitiku A, Tilahun G (2010) Retrospective survey of human hydatidosis in Bahir Dar, north-western Ethiopia. Eastern Mediterranean Health Journal 16: 937-941.
- Arene FOI (1985) Prevalence of hydatidosis in domestic livestock in the Niger Delta. Tropical Animal Health and Production 17: 3-5.
- Eckert J, Deplazes P (2004) Biological, epidemiological and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clinical Microbiology Reviews 17: 107-135.
- Gebremeskel B, Kalayo K (2009) Prevalence, viability and fertility study of bovine cystic echinococcosis in Mekelle city, Northern Ethiopia. Revue Med Vet 160: 92-97.
- Budke CM, Deplazes P, Torgerson PR (2006) Global socioeconomic impact of cystic echinococcosis. Emerging Infectious Disease 12: 296-303.
- Assefa A, Tesfay H (2013) Major causes of organ condemnation and economic loss in cattle slaughtered at Adigrat municipal abattoir, northern Ethiopia. Veterinary World 6(10): 734-738.
- Getachew D, Almaw G, Terefe G (2012) Occurrence and fertility rates of hydatid cysts in sheep and goats slaughtered at Modjo Luna Export Slaughter House, Ethiopia. Ethiopia Veterinary Journal 16: 83-91.
- Elmahdi IE, Ali QM, Magzoub MM, Saad MB, Roming T (2004) Cystic echinococcsis of livestock and humans in Central Sudan. Academic Journal of Animal Diseases 98: 473-479.
- Haridy FM, Ibrahim BB, Morsy TA (2000) Sheep-dog-man. The risk zoonotic cycle in Hydatidosis. Journal Egyptian Society of Parasitology 30: 423-429.
- Njoroge EM, Mbithi PM, Gathuma JM, Wachira TM, Gathura PB, et al. (2002) A study of Cystic echinococcosis in slaughter animals in three selected areas of northern Turkana, Kenya. Veterinary Parasitology 104: 85-91.
- Saeed I, Kapel C, Saida LA, Willingham L, Nansen PP (2000) Epidemiology of Echinococcus granulosus in Arbil province, northern Iraq. Journal of Helminthology 74: 83-88.
- Dalimi AG, Motamedi M, Hosseini B, Mohammadian H, Malaki Z, et al. (2002) Echinococcosis /Hydatidosis in western Iran. Journal of Animal Production Sciences 105: 161-71.
- Yeshiwork A (2009) Prevalence survey on hydatidosis in small ruminants slaughtered at Haramaya Municipal Abattoir, DVM Thesis, Faculty of Veterinary Medicine, Haramaya University, Ethiopia.
- Baswaid SH (2007) Prevalence of hydatid cysts in slaughtered sheep and goats in Hadhramout, Yemen. Assiut University Bulletin of Environmental Research 10: 67-71.
- Eckert J, Conraths FJ, Tackmann K (2000) Echinococcosis: an emerging or reemerging zoonosis? International Journal Parasitology 30: 1283- 1294.
- Marshet E, Asamre K, Bekele J, Anteneh T, Abera M, et al. (2011) The status of cystic echinococcosis (hydatidosis) in small ruminants slaughtered at Addis Ababa municipal abattoir. Journal Veterinary Animal Advance 10: 1445-1449.
- Oryan A, Moazeni M, Amrabadi O, Akbari M, Sharifiyazdi H (2015) Comparison of distribution pattern, pathogenesis and molecular characteristics of larval stages of Taenia multiceps in sheep and goats. Small Ruminant Research 132: 44-49.
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jenning FW (1996) Veterinary parasitology, (2nd edn), Blackwell Science, UK. pp 307.
- Bekele T, Woldeab T, Lah11our-Kssi A, Sherington J (1992) Factors affecting morbidity and mortality on farm and on station in the Ethiopian highland sheep. Acta Tropical 52: 99-109.
- Varma TK, Malviya HC (1989) Prevalence of coenurosis in sheep, goat and pigs in Bareilly, Utar Pradesh. Journal of Veterinary Parasitology 3: 69-71.
- Oryan A, Moghaddar N, Gaur SNS (1994) Metacestodes of sheep with special reference to their epidemiological status, pathogenesis and economic implications in Fars Province, Iran. Veterinary Parasitology 51: 231-240.
- Morris RS (1988) The effect of disease on productivity and profitability of livestock: how showses between 1999 and 2009 at two university medical centres. Tropical Biomedeca 28: 450-456.
- Hayelome M (2008) A thesis submitted to the faculty of veterinary medicine, Haramaya University, in the partial fulfillment for the requirements for the attainment of the degree, Doctor of Veterinary Medicine.
- Scala A, Cancedda GM, Varcasia A, Ligios C, Garippa G, et al. (2007) A survey of Taenia multiceps coenurosis in Sardinian sheep. Veterinary Parasitology 143: 294-298.
- Gemmell MA, Lawson JR, Roberts MG (1987) Population dynamics in echinococcosis and cysticercosis: evaluation of the biological parameters of Taenia hydatigena and Taenia ovis and comparision with those of Echinococcus granulosus. Parasitology 94: 161-180.
- Kia EB, Masoud J, Yalda A, Mahmoudi M, Farahani H (2005) Study on Human taeniasis by administering anti- Taenia drugs. Iranian Journal of Public Health 34: 47-50.
- Solaymani AR, Jamali SR, Hoseini K (2011) Intestinal obstruction due to Taenia saginata. Tehran University Medical Journal 69: 136-140.
- Daryani A, Ettehad GH, Sharif M, Ghorbani L, Ziaei H (2008) Prevalence of intestinal parasites in vegetables consumed in Ardabil, Iran. Food Control 19: 790-794.
- Mamishi S, Sagheb S, Pourakhbari B (2007) Hydatid disease in Iranian children. Journal of Microbiology, Immunology, and Infection 40: 428- 431.
- Sarkari B, Sadjjadi SM, Beheshtian MM, Aghaee M, Sedaghat F (2010) Human cystic echinococcosis in Yasuj district in southwest of Iran: an epidemiological study of seroprevalence and surgical cases over a ten-year period. Zoonoses and Public Health 57: 146-150.
- Sadjjadi SM (2006) Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitology International 55: S197-S202.
- Antonios SN, Mina SN (2000) A case report of human coenurus cerebralis in Tanta, Egypt. Journal of the Egyptian Society of Parasitology 30: 959-960.
- Acha PN, Szyfres B (2003) Helminthiases: Cestodiases, Text book of zoonoses and communicable diseases common to man and animals, (3rd edn), pan America Health Organization 3: 162-163.
- Allan JC, Craig PS, Garcia noval J, Mencos F, Liu D, et al. (1992) Coproantigen detection for immune diagnosis of echinococcosis and taeniosis in dogs and humans. Parasitology 104: 347-355.
- Alum A, Rubino JR, Ijaz MK (2010) The global war against intestinal parasites - Should we use a holistic approach? International Journal of Infectious Disease 14: 732-738.
- Assefa A (2015) Public Health Impact of Bovine Cysticercosis in Ethiopia: A review. Journal of Veterinary Science Research 1(1): 0-10.
- Attindehou S, Salifou S (2012) Epidemiology of cestodes infections in sheep and goats in Benin. Veterinary Research 5(3): 59-62.
- Avcioglu H, Terim KA, Yildirim A (2012) Clinical, morphological and histopathological features of bovine coenurosis: case reports. Revue de Med Vet 163(3): 295-298.
- Bekele T, Mukasa-Mugerwa E, Kasali OB (1988) The prevalence of cysticercosis and hydatosis in Ethiopian sheep. Veterinary Parasitology 28: 267-270.
- Belino EO (1975) Some observations T saginata Cysticercosis in slaughtered cattle in Nigeria. Interernational Jounal of Zoonosis 3: 92-99.
- Benifla M, Barrelly R, Shelef I, El-On J, Cohen A, et al. (2007) Huge hemispheric intraparenchymal cyst caused by Taenia multiceps in a child. Case report. Journal Neurosurgical 107(6): 511-514.
- Bilger B, Dinkel A, Merli M, Mackenstedt U (1999) Echinococcus multilocularis in animal hosts: new data from Western Europe. Helminthologia 36: 185-191.
- Biswas D (2013) Ultrasound diagnosis and surgical treatment of coenurosis (GID) in bengal goat (Capra hircus) atchittagong metropolitan area, Chittagong, Bangladesh. Science Journal of Veterinary Advance 2(5): 68-75.
- Budke CM, Campos-Ponce M, Qian W, Torgerson PR (2005) A canine purgation study and risk factor analysis for echinococcosis in a high endemic region of the Tibetan plateau. Veterinary Parasitology 127: 43-49.
- Central Statistical Agency (2008) Summary and statistical report of the 2007 population and housing census, Federal Democratic Republic of Ethiopia Population Census Commission, Addis Ababa, Ethiopia.
- Central Statistical Authority (2009) Federal Democratic Republic of Ethiopia Agriculture sample enumeration statistical abstract pp. 446-539.
- Cheesbrough M (2005) District Laboratory Practice in Tropical Countries. (2nd edn), Cambridge University Press, Cambridge, UK, pp. 454.
- Cheesbrough M (2006) District Laboratory Practice in Tropical Countries. (2nd edn), Cambridge University Press, Cambridge, UK, pp. 434.
- Christodoulopoulos G (2007) Two rare clinical manifestations of coenurosis in sheep. Veterinary Parasitology 143: 368-370.
- Christodoulopoulos G, Theodoropoulos G, Petrakos G (2008) Epidemiological survey of cestode - larva disease in Greek sheep flocks. Veterinary Parasitology 153(3): 368-373.
- Dakkak A (2010) Echinococcosis/hydatidosis: a severe threat in Mediterranean countries. Veterinary Parasitology 174(1-2):2-11.
- Dawit S (2004) Epidemiology of T. saginata Taeniasis and Cysticercosis in North Gonder Zone, North Western Ethiopia. DVM Thesis, FVM, AAU, Debre Zeit, Ethiopia.
- Deressa A, Tilahun T, Tadesse A, Beyene M, Gebrewold G, et al. (2012) Assessment of Coenuruscerebralis and its economic impact in sheep brain harvested at Ethiopian Health and Nutrition Research Institute, Ethiopia. International Journal of Livestock Research, 2(2): 217-226.
- Desta B (1995) Ethiopian traditional herbal drugs, part 1: Studies on the toxicity and therapeutic activity of local taenicidal medication. Journal of Ethno pharmaceutical 45: 7-33.
- Dohoo I, Martin W, Stryhn H (2009) Veterinary Epidemiological Research, (2nd edn), AVCInc. Prince Edward Island, Canada, pp. 100-105.
- Doyle MP, Beuchat LR, Montaville TJ (1997) Food Microbiology. Fundamentals and Frontiers. Center for Food Safety and Quality Enhancement. Department of Food Science and Technology, University of Georgia. Washington, USA.
- Eichenberger RM, Lewis F, Gabriël S, Dorny P, Torgerson PR, et al. (2013) Multi-test analysis and model-based estimation of the prevalence of Taenia saginata cysticercus infection in naturally infected dairy cows in the absence of a ‘gold standard’ reference test. International Journal Parasitology 43(10): 853-859.
- Eom KS, Rim HJ (1999) Morphologic descriptions of Taenia asiatica. Korea Journal Parasitology 31: 1-6.
- Fakhar F, Sadjjadi SM (2007) Prevalence of hydatidosis in slaughtered herbivores in Qom Province, Central Part of Iran. Veterinary Research Community 31: 993-997
- FAO (1982) Echinococcus/hydatidosis: surveillance, prevention and control. FAO/UNEP/WHO guidelines. FAO Animal Production and Health Paper No. 29. Rome, Italy.
- Fertig DL, Dorn CR (1985) Taenia saginata cysticercosis in an Ohio cattle feeding operation. Journal of America Veterinary Medical Association pp. 1281-1286.
- Flisser A, Craig PS, Ito A (2011) Cysticercosis and taeniosis Taenia saginata, Taenia solium and Taenia saginata. In: Zoonoses. Biology, Clinical Practice, and Public Health Control,
- Frolova AA (1982) Epidemiology of Taeniosis. Zoonoses Control Collection of Teaching Aids for International Training Course. V.II, Moscow, Russia.
- Garedaghi Y, Rezaii Saber AP, Saberie Khoroshahi M (2011) Prevalence of bovine Cysticercosis of Slaughtered Cattle in Meshkinshahr Abattoir. American Journal of Animal Veterinary Science 6(3): 121- 124.
- Garippa G, Varcasia A, Scala A (2004) Cystic echinococcosis in Italy from the 1950s to present. Parasitology 46: 387-391.
- Gasser R, Chilton NB (1995) Characterization of taeniid cestode species by PCR-RFLP of its ribosomal DNA. Acta Tropical 59: 31-40.
- Giadinis ND, Psychas V, Polizopoulou Z, Papadopoulos E, Papaioannou N, et al. (2012) Acute coenurosis of dairy sheep from 11 flocks in Greece. New Zealand Veterinary Journal 60(4): 247-253.
- Gicik Y, Kara M, Arsalan MO (2007) Prevalence of Coenuruscerebralis in sheep in Kars Province, Turkey. Bull Veterinary Institute Pulawy 51(3): 379-382.
- Godara R, Katoch R, Yadav A, Khajuria JK, Borkataki S (2011) Coenurosis in small ruminants: an overview. Veterinary Practice 12(1): 102-105.
- Gonzalez LM, Montero E, Morakote N, Puente S, Diaz DE, et al. (2004) Differential diagnosis of Taenia saginata and Taenia saginata asiatica taeniosis through PCR. Diagnostic Microbialogy Infectious Diseases 49: 183-188.
- Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A (2012) Worldwide epidemiology of liver hydatidosis including the Mediterranean area.
- Gül Y, İssi M, Özer S (2007) Clinical and pathological observations of flock of sheep showing epileptoid spasm related to Oestrosis and Coenurosis. FÜ Sağlık Bil Derg 21: 173-177.
- Harrison LJS, Sewell MMH (1991) The Zoonotic Taeniae of Africa. In: Parasitic Helminths and Zoonoses in Africa. Unwin Hyman, London, UK, pp. 54-56.
- Hashim MA, Rashid MH, Nooruddin M (2000) Extraneural coenuriasis in Bengal goats. 4. Treatment. Bangladesh Veterinary, 17(1): 46- 49.
- Inechukwu BI, Onwukeme KE (1991) Intraocular coenurosis: a case report. Br Journal of Opthalmology 75(7): 430-431.
- Jabbar A, Swiderski Z, Mlocicki D, Beveridge I, Lightowlers MW (2010) The ultrastructure of taeniid cestode oncospheres and localization of host-protective antigens. Parasitology 137: 521-535.
- Jia WZ, Yan HB, Guo AJ, Zhu XQ, Wang YC (2010) Complete mitochondrial genomes of Taenia multiceps, T. hydatigena and T. pisiformis: additional molecular markers for a tapeworm genus of human and animal health significance. BMC Genomics 11: 447.
- Jibat T, Ejeta G, Asfaw Y, Wudie A (2008) Causes of abattoir condemnation in apparently healthy slaughtered sheep and goats at HELMEX abattoir, Debre Zeit, Ethiopia. Revue de Médical Véterinary 159(5): 305-311.
- Khalil LF, Jones A, Bray RA (1994) Keys to the Cestode Parasites of Vertebrates. Wallingford, Oxon, CAB International, UK.
- Kheirandish R, Sami M, Azizi S, Mirzaei M (2012) Prevalence, predilection sites and pathological findings of Taenia multicepscoenuri in slaughtered goats from south-east Iran. Underreport, Journal of Veterinary Research 79(1): 1-5.
- Lescano AG, Zunt J (2013) Other cestodes: sparganosis, coenurosis and Taenia crassiceps cysticercosis. Handbook Clinical Neurology 114: 335-345.
- Lightowlers MW, Rolfe R, Gauci CG (1996) Taenia saginata: Vaccination against Cysticercosis in cattle with recombinant oncosphere antigens. Experimental Parasitolology 84: 330-338.
- Lloyd S (2011) Other cestode infections. Hymenoleposis, diphyllobothriosis, coenurosis, and another adult and larval cestodes. In: Zoonoses. Biology, Clinical Practice, and Public Health Control. MACMILLAN education Ltd, ACCT. p. 6-10
- Loos-frank B (2000) An up-date of Verster’s (1969) Taxonomic revision of the genus Taenia (Cestoda) in table format. Systmatic Parasitology 45: 155-183.
- Maeda GE, Kyvsgaard NP, Nansen C, Bogh HO (1996) Distribution of Taenia saginata Cysts by Muscle group in Naturally Infected Cattle in Tanzania. Preventive Veterinary Medecine 28(2): 81-89.
- Mann I (1984) Environmental Hygienic & Sanitary Based on Concept of Primary Health as a tool for Surveillance, Prevention and Control of Taensiasis /Cystcercosis. Current Publication in Health Research Tropics 36: 127 - 140.
- McManus DP, Thompson RC (2003) Molecular epidemiology of cystic echinococcosis. Parasitology 127: 37-51.
- McManus DP, Zhang W, Li J, Bartley PB (2003) Echinococcosis. Lancet 362: 1295-1304.
- McManus DP, Smyth JD (1986) Hydatidosis: changing concepts in epidemiology and speciation. Parasitology Today. 2(6): 163-168.
- Miran BM (2013) coenurosis in slab-slaughtered sheep and goats in ngorongoro district: prevalence and predisposing factors of the disease, Mscthesis, adissertation submitted in partial fulfilment of the requirements for the degree of Master of Science in parasitology of sokoine university of agriculture. morogoro, tanzania. p. 58.
- Miran MB, Nzalawahe J, Kassuku AA, Swai ES (2015) Prevalence of coenurosis in sheep and goats at three slaughter slabs in Ngorongoro District, Tanzania. Tropical Animal Health Production 47(8): 1591- 1597.
- Moghaddar N, Oryan A, Gaur SNS (1992) Coenurosis in cattle in Iran. Journal of Applied Animal Research 2: 119-121.
- Molyneux DH (2004) Neglected” diseases but unrecognized successes-- challenges and opportunities for infectious disease control. Lancet 364: 380-383.
- Moro P, Schantz PM (2006) Cystic echinococcosis in the Americas. Parasitology International 55: 181-186.
- Morris DL, Richards KS, Clarkson MJ, Taylor DH (1990) Comparison of albendazole and praziquantel therapy of Echinococcus granulosus in naturally infected sheep. Veterinary Parasitology 36: 83-90.
- Muqbil NA, Al-Salami OM, Arabh HA (2012) Prevalence of unilocularhydatidosis in slaughtered animals in Aden Governorate-Yemen. Jordan Journal of Biology Science 5(2): 121-124.
- Navarrete N, Jersic MI, Denis R (1995) Comparación de tres técnicas en el diagnostic serológico de la hidatidosis humana. Bol Chil Parasitol 50: 97-100.
- Njau BC, Scholteus RG, Kasili O (1990) Parasites of sheep at the International Livestock Centre for Africa, Debre Behan Station, Ethiopia. Preventive Veterinary Medicine 9: 267-277.
- Nourani H, Pirali Kheirabadi KH, Rajabi H, Banitalebi A (2010) An unusual migration of Taenia hydatigena larvae in a lamb. Tropical Biomedicine 27: 651- 656
- Nunes Dda S, Gonzaga HT, Ribeiro Vda S, da Cunha JP, Costa-Cruz JM (2013) Taenia saginata metacestode antigenic fractions obtained by ion exchange chromatography: potential source of immunodominant markers applicable in the immunodiagnostic of human neurocysticercosis. Diagnostic Microbiology Infectious Diseases 762: 36-41.
- OIE (2000) Manual of Standards for Diagnostic Tests and Vaccines. Cysticercosis pp. 423-428.
- OIE (2004) Cystecercosis: In manual of diagnostic tests and vaccines for terrestrial animals.
- Oryan A, Gaur SN, Moghaddar N, Delavar H (1998) Clinico-pathological studies in cattle experimentally infected with Taenia saginata eggs. JS Afr Veterins Assocation 69: 156-162.
- Oryan A, Moghaddar N, Gaur SN (1995) Taenia saginata cysticercosis in cattle with special reference to its prevalence, pathogenesis and economic implications in Fars Province of Iran. Veterinary Parasitology 57: 319-327.
- Palmer SR (2011) Oxford textbook of zoonoses: biology, clinical practice, and public health control, Oxford University Press, New York, USA.
- Palmer SR, Lord Soulsby EJL, Torgerson PR, Simpson DH (2012) Oxford University Press, Oxford, UK, pp. 644-649.
- Pandey GS, Sharma RN (1987) A survey of Pulmonary diseases at Lusaka Abattoir in Zambia. Bulletin of Animal Health and Production in Africa 35: 336-338.
- Parihar NS (1988) Pathology of coenurus cerebralis in ovine subclinical infections. Indian Journal of Animal Science 58: 539- 543.
- Parija SC (1996) Text Book of Medical Parasitology. Protozoology and Helminthology. Text and Color Atlas. All Indian Publishers and Distributors Registered.
- Pierre C, Civatte M, Chevalier A, Terrier JP, Gros P, et al. (1998) diagnostic des helminthes en anatomie pathologique. Medical Tropical (Mars) 58: 85-97.
- Reniecke RK (1983) Veterinary Helimntology. Butterworths. Food Safety and Handling, Epidemiological Findings from an Out Break of Cysticecosis in Feed lot Cattle. JAVMA 205(1):75-86.
- Romig T, Omer RA, Zeyhle E (2011) Echinococcosis in sub-Saharan Africa: emerging complexity. Veterinary Parasitology 181: 43-47.
- Scala A, Varcasia A (2006) Updates on morphobiology, epidemiology and molecular characterization of coenurosis in sheep. Parassitologia 48(1-2): 61-63.
- Schantz PM (1982) Echinococcosis. In: Jacobs L, Arambuloz P (Eds.), CRC handbook series in zoonoses 38: 129 -131
- Scott PR (2012) Diagnosis and treatment of coenurosis in sheep, University of Edinburgh, Easter Bush Veterinary Centre, Roslin, Midlothian, Scotland, United Kingdom. Veterinary Parasitology 189(1): 75-78.
- Skerritt GC, Stallbaumer MF (1984) Diagnosis and treatment of coenuriasis (gid) in sheep. Veterinary Research 115: 399- 403.
- Soulsby EJL (1982) Helminths, arthropods and protozoa of domesticated animals, (7th edn), Bailliere Tindall, London. Lead and Febiger. Philadelphia, USA, pp. 119-127.
- Suwan Z (1995) Sonographic findings in hydatid disease of the liver: Comparison with other imaging methods. Annual Tropical Medical Parasitology 89: 261-269.
- Symth JD (1994) Introduction to Animal Parasitology. (3rd edn), Cambridge University press, London, UK, pp. 334-340.
- Taresa G, Melaku A, Bogale B, Chanie M (2011) Cyst viability, body site distribution and public health significance of bovine cysticercosis at Jimma, south west Ethiopia. Global Veterinaria 7: 164-168.
- Tavassoli M, Malekifard F, Soleimanzadeh A, Tajik H (2011) Prevalence of Coenuruscerebralis in sheep in Northwest of Iran. Veterinary Research Forum 2(4): 274-276.
- Tesfaye D, Sadado T, Demissie T (2012) Public Health and Economic Significance of Bovine Cysticercosis in Wolaita Soddo, Southern Ethiopia. Global Veterinaria 9: 557-563.
- Thompson RC, McManus DP (2002) Towards a taxonomic revision of thegenus Echinococcus. Trends Parasitology 18: 452-457.
- Tigist N (2009) Prevalence and economic importance of bovine hydatidosis in Bahir Dar municipal abattoir. DVM thesis, School of Veterinary Medicine, Jimma University, Jimma, Ethiopia.
- Torgerson PR, Karaeva RR, Corkeri N, Abdyjaparov TA, Kuttubaev OT (2003) Human cystic echinococcosis in Kyrgystan: an epidemiological study. Acta Tropical 85: 51-61.
- Torgerson PR, Budke CM (2003) Echinococcosis - an international public health challenge. Research in Veterinary Science 74: 191-202.
- Ugbomoiko US, Ariza L, Heukelbach J (2008) Parasites of importance for human health in Nigerian dogs: high prevalence and limited knowledge of pet it shows it is assessed? In: proceeding of New Zealand Society of Animal Production 48: 117-123.
- Upadhayay AK (2005) Text book of preventive medicine, first edition, Int. book distributing company (Publ. Div.), pp. 452-454.
- Uslu U, Guclu F (2007) Prevalence of coenurus cerebralis in sheep in Turkey. Medical Weterynarina 63(6): 678-680.
- Van De N, Le TH, Lien PT, Eom KS (2014) Current status of taeniosis and cysticercosis in Vietnam. Korean Journal Parasitology 52: 125- 129.
- Varcasia A, Jia WZ, Yan HD, Manunta ML, Pipia AP, et al. (2012) Molecular characterization of subcutaneous and muscular coenurosis of goats in United Arab Emirates. Veterinary Parasitology 190(3): 604- 607.
- Varcasia A, Tosciri G, Coccone GNS, Pipia AP, Garippa G, et al. (2009) Preliminary field trial of a vaccine against coenurosis caused by Taeniamulticeps. Veterinary Pathology 162(3): 285-289
- Vink WD, Lopes Pereira A, Nota and De Balogh KKIM (1997) Prevalence of Coenurosis in goats in tete province, Mozambique. France, pp: 72-99.
- Wanzal W, OnyangoAbuje JA, Kang’ethe EK, Zessin KH, Kyule NM, et al. (2003) Analysis of post mortem diagnosis of Bovine cysticercosis in Kenyan cattle. Online Journal of Veterinary Research 7: 1-9.
- WHO (1983) Guidelines for Surveillance, Prevention and Control of Taeniosis/ Cysticercosis. In: Gemmell M, Matyas Z Pawlowski, Souls EJL (Eds.), WHO, Geneva. VPH 83: 49-207.
- WHO (1996) Investing in health research and development Report of the committee on health research relating to future intervention options? Geneva, WHO (World Health Organization), Switzerland, pp. 278.
- Yoder DR, Eblell ED, Hancock DD, Combs BA (1994) Public Veterinary Medicine: World Journal of Gastroenterology 18: 1425-1437
- Yoshino T, Momotani E (1988) A case of bovine coenurosis (Coenurus cerebralis). Veterinary Science 50: 433-438.






























