Alternative Follicle Stimulating Hormone Dose Rate for Embryo Production in Dairy Cattle
David Kios1,2,3*, Victor Tsuma2 and Henry Mutembei2
1Kenya Animal Genetic Resources Centre, Ministry of Agriculture, State Department of Agriculture Research, Kenya
2Department of Clinical Studies, University of Nairobi, Kenya
3 Department of Animal Science, University of Eldoret, Kenya
Submission: January 30, 2019;;Published: February 28, 2019
*Corresponding author: Kios David, Kenya Animal Genetic Resources Centre, School of Agriculture and Biotechnology, Ministry of Agriculture, Faculty of Veterinary Medicine, University of Nairobi, Kenya
How to cite this article: David K, Victor T, Henry M. Alternative Follicle Stimulating Hormone Dose Rate for Embryo Production in Dairy Cattle. Dairy and Vet Sci J. 2019; 10(3): 555787. DOI:10.19080/JDVS.2019.10.555787
Abstract
Multiple ovulation and embryo transfer though available for the past 40 years, is seldom used in Kenya. The high cost of superovulation has been cited as a major contributory factor. The research was carried out to test the effect of four different dose rates of follicle stimulating hormone (FSH) used for superovulation. Ultrasound and manual palpation were used to determine the number of follicles and corpora lutea formed. Harvesting and grading of embryos was carried out to determine the quantity and quality of embryos produced. An average of three embryos per donor was recovered, though there was a potential of more than six embryos. One third of the donors failed to ovulate, another third produced 1 to 3 embryos while a third produced over three embryos. All donor cows had several ovulatory follicles ranging from 7.7 to 13.7, but the failure for some to ovulate needs further investigation. There was no difference in embryo quantity and quality between the different FSH dose rates. The season, technique and animal factors were shown to influence embryo production. It’s recommended that super ovulation may be carried out in Kenya using 200 mgs of FSH. This will reduce the cost of embryo production by at least 25% or more depending on the number of embryos harvested per donor.
Keywords: Multiple ovulation; Follicle stimulating hormone; Donor; Superovulation; Embryos; Dose
Abbrevations: FSH: Follicle Stimulating Hormone; GDP: Gross Domestic Product; USA: United States of America; MOET: Multiple Ovulation and Embryo Transfer; REML: Restricted Maximum Likelihood; EAApp: Eastern Africa Agricultural Productivity Project; IETS: International Embryo Technology Society
Introduction
Farmers in the rural areas, especially women, derive a larger share of their income from livestock. The livestock sub-sector plays a critical role in ensuring food and nutrition security in many countries including Kenya. With the right intervention, it will contribute to poverty reduction and wealth creation. The sub-sector is estimated to contribute 12% of the gross domestic product (GDP) in Kenya [1]. Demand for milk in Kenya is projected to rise to 12.76 billion liters per year by 2030 from 5 billion litres [2]. This demand will be met through increased milk production per cow and improved productivity. Over 80% of all milk is produced by small scale farmers in rural areas [2]. The small-scale farmers depend on own farm production of replacement heifers and purchase from established breeders.
Quality replacement heifers from established breeders are inadequate and those available are usually expensive [3]. This inadequacy is due to low adoption of reproductive technologies, improper implementation of breeding plans and poor recording [4,5]. This gap has led to a high demand for dairy breeding stock with steep rise in prices in the range of USD 2,000 to 5,000 per cow, a situation that is not only unsustainable but also out of reach to most of the small-scale farmers. Despite the high price, the replacement heifers are hardly available in the market. The small-scale dairy farmers have therefore, turned to the only breeding option available whenever their cows come on heat, namely; natural service or haphazard use of artificial insemination [6]. A conventional method of heifer production through artificial insemination has not adequately been able to satisfy farmer demands for quality dairy cattle [7]. The use of a combination of the existing assisted reproductive techniques is therefore important if supply must meet the demand. Reproductive efficiency of the top producing cows through multiple ovulation and embryo transfer (MOET) may provide the solution to this demand which has driven the prices of replacement breeding stock way above what the ordinary dairy cattle farmer can afford [7].
Friesian cattle heifers have been imported from the Republic of South Africa and Netherlands in the past three years. This was to alleviate the acute shortage of quality dairy cattle being experienced in the Country. The logistics of importation especially transportation overland is difficult and almost impossible for many farmers. Such a venture is non-tenable due to high risk of spreading diseases, effect of genotype X environment interactions, high cost of importation and shipping fever that leads to abortions and deaths from long distance travels [8].
The use of assisted reproductive technologies therefore remains the most viable option and has supported many developed countries to achieve sustainable production of milk and replacement heifers [9,10]. Multiple ovulation and embryo transfer can greatly increase the number of off spring that a genetically superior cow can produce [11,12]. The reproductive potential of a cow could be enormously enhanced considering the numerous viable ova they contain in their ovaries [3,10]. Through natural mating or artificial insemination, only a fraction of the reproductive potential of the cow is realized and the average cow will have one calf per year. Thus, a cow only produces 8 to 10 calves during her lifetime [12]. The high variability in embryo output, cost and logistics for importation of follicle stimulating hormone and other drugs, chemicals and equipment used for embryo production influence the success of a MOET program [9,11]. This high variability in embryo output has also been attributed to variations in follicular response to FSH, technical competence of the technician together with other human and animal factors [13-15].
The MOET protocol currently in use in Kenya was adopted from the United States of America (USA). Their donor cows are larger than those found in Kenya with higher weight, different physiological needs and raised in different environments. Thus, the FSH protocol for their situations may not be the most appropriate for Kenyan donor cows. This MOET protocol also recommends a blanket 30% reduction for the heifers and is silent on low weight mature cattle as kept in most farms in Kenya. This research was designed to determine the factors affecting embryo output within the dairy subsector for enhanced breeding and productivity in Kenya.
Materials and Methods
Experimental Design
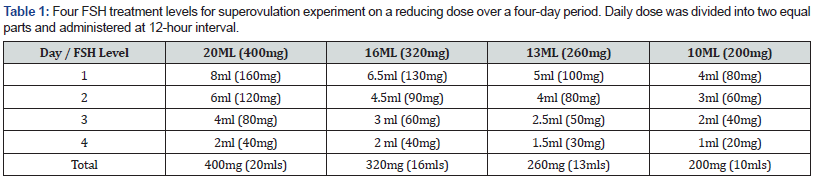
Twelve (12) donor Friesian dairy cows on their second parity, aged approximately 40 months were assigned to four different Folltropin®-V (FSH) (Bioniche animal health) dose levels; 400mgs (20mls), 320mgs (16mls), 260mgs (13mls) and 200mgs (10mls). The donors had average body condition score (BCS) of three (3) on the 1 – 5 scale, where one (1) is emaciated and five (5) is extremely fat as described by Domecq [16]. This was maintained throughout the experimental period; period 1 (P1) was in January to March, period 2 (P2) was April to June and period 3 (P3) was July to September. The controls were donors on 400mgs of FSH protocol, the standard procedure used currently in Kenya. The research was carried out at the University of Eldoret farm in Uasin Gishu County.
The experimental animals were randomly assigned to four (4) groups of three (3) donors each. A 4 x 4 cross over experimental design was used. Cross over experimental design has been shown to work well with repeated treatments comparing the response of different drugs or doses over different groups of animals [17]. The cross over design allowed the rotation of the three (3) donors in each group within the four (4) treatments randomly. The experimental donor cows crossed from one treatment to the other after a wash out period of two (2) months. The wash out period was long enough to ensure minimal cross over effects that may compound results. The effects of the four (4) FSH treatment levels on follicular development and embryo output over three different seasons were quantified and recorded.
The following dependent variables: (a) Number of follicles at the start of FSH treatment (b) Number of ovulatory follicles at the end of FSH treatment (c) Number of embryos harvested and (d) Quality of the embryos harvested were evaluated. Ovulatory follicles were defined as those with a diameter of 10 millimeters (mm) or over [5]. A17-day Synchronization and super ovulation protocol was uniformly used during the four experiments. Synchronization was carried out using a combination of progesterone and prostaglandin to bring all the animals at the same phase of the estrous cycle. It began with synchronization using a controlled internal drug release (CIDR®) (Zoetis) device impregnated with progesterone for slow release on day zero (0). The donors were also given a multivitamin injection to improve on appetite.
On day four, just prior to start of superovulation treatment, the ovaries were scanned using ultrasound. A 7.5 megahertz (MHz) dual frequency linear array probe ultrasound scanner was used. This was to visualize and count the number of follicles three millimeters (mm) or more in diameter. This was recorded and saved as digital pictures. On the fifth day, the donors were treated with (FSH) based on the four experimental levels on a reducing dose twice a day for four days (Table 1). On the evening of day seven, the donors were given prostaglandin injection which was repeated on the morning of day eight together with the withdrawal of the CIDR®. On day eight, a second ovarian ultrasound scan was carried out to determine the number of ovulatory follicles in each ovary. This was recorded and digital pictures were also stored. The donors were inseminated twice after FSH treatment with good quality semen. The first insemination being done 12 hours after observation of standing heat and 12 hours thereafter using the same sire, same batch of semen and same inseminator.
On day 16, rectal palpation combined with ultrasound was used to quantify the expected number of embryos based on the number of corpora lutea found on the ovaries. Ultrasound has led to improved understanding of follicular and corpora lutea development [18]. Corpora lutea is formed soon after ovulation from the remaining structures of ovulating follicle. The presence of a corpora lutea is a strong indicator of ovulation and can be used to estimate the number of embryos expected in such a program. This is also important for the practitioner especially when searching for the embryos. The expected output was compared with the actual number of embryos harvested.
Harvesting and grading of embryos was carried out according to the standard protocol as described by Seidel & Seidel and Bo & Mapletoft [19,20] and International Embryo Technology Society (IETS). Harvesting was carried out seven (7) days after insemination of the donors using three-way catheters introduced into the uterine horns. Flushing media was introduced slowly to the uterine horn to flush out the embryos which were then collected using an embryo filter. Searching and grading of embryos was done using a special stereoscopic microscope designed for the purpose [19].
FSH: Follicle stimulating hormone.
Statistical Analysis
All the response variables were subjected to the same analysis. Initial body weight of experimental cows was used to weight observations in the statistical analysis to take care of inadequacy of a uniform dose used on cows of varying body weight. Response was taken on the same cow at different periods resulting in a repeated measures data structure. Response values for the same cow are correlated whereas values for different cows are assumed to be independent. Consequently, a mixed linear statistical model was postulated. A parameter estimation procedure that uses restricted maximum likelihood (REML) was used to analyze the data. SAS procedure for mixed linear models’ software (SAS Institute Inc. 2004. SAS OnlineDoc® 9.1.3. Cary, NC: SAS Institute Inc.) was deployed.
Statistical Model
In the postulated linear mixed model, its stipulated that numbers of follicles, embryos and the quality constitute the dependent variable and the experimental factor hormone treatment is a fixed independent variable [21]. Period of treatment is a fixed variable whereas the variability among cows within period is random.
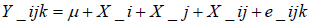
Where i=1,2,3 j=1,2,3,4 k=1,2,…..18, Y_ijk= Response of cow k that received hormone dose j in period i, μ is the overall mean effect, x_iis the fixed effect of period i, x_jis the fixed effect of hormone dose level j, x_ij is the effect of the interaction between period i and hormone dose level j, e_ijk is the random error of the measured response of cow k that received hormone dose j in period i.
Results
Follicles developed
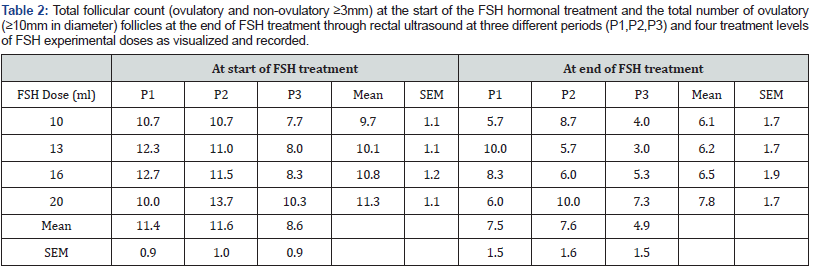
Mean number of ovulatory follicles observed at the end of FSH treatment rose marginally with the level of FSH hormone dose but did not significantly differ (Table 2). Period three mean was lower than that obtained for periods one and two but the difference was not statistically significant (P<.07) (Table 2). The highest ovulatory follicle mean count was recorded for 20ml dose in period two due to an extreme observation of 22 ovulatory follicles from one cow. Mean follicle counts slightly improved for 20ml dose from an equal response for all lower doses but not significantly. The mean ovulatory follicle counts for period three was lowest. Mean number of follicles observed at the start of the FSH treatment that progressed to ovulatory follicles ranged between 63% for the lowest FSH dose and 69% for the highest FSH dose. Period three means were lower than those observed in periods one and two particularly for lower hormone doses.
FSH: Follicle stimulating hormone, P1: January–March, P2: April–June and P3: July–September, SEM: Standard error of mean.
The highest number of follicles at the start of the FSH treatment that progressed to ovulatory follicles was at period two for 10ml FSH hormone level and period one at 13ml FSH level at 81%. The lowest number of follicles at the start of the FSH treatment that progressed to ovulatory follicles was at period three for 13ml FSH level at 38% followed by period three for 10ml level at 52%. Significance tests of fixed effects are shown in the analysis of variance (Table 3) for the two dependent variables under study; number of follicles at the start of FSH treatment and number of ovulatory follicles at the end of FSH treatment. No significant hormone dose, period or interaction effect was detected (P>.05) but intercept model fit was significant (P<.05) for the two dependent variables. Least squares mean for the fixed effects and interaction are presented below (Table 3).

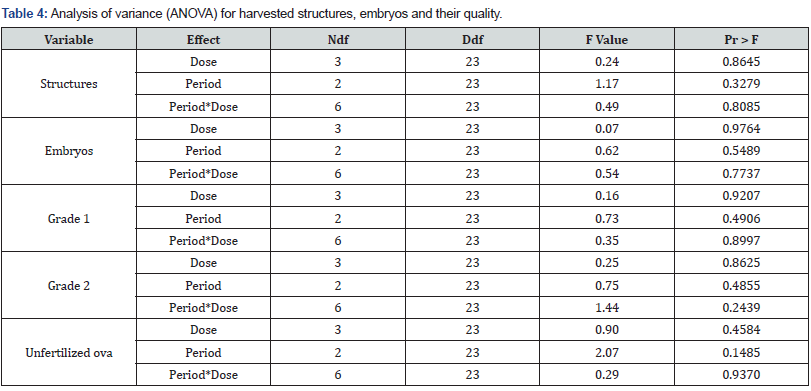
Harvested structures (embryos, unfertilized ova and degenerated embryos), embryo quantity and quality Significance tests of fixed effects are shown in the analysis of variance (Table 4) for the five dependent variables under study (structures, embryos, grade 1, grade 2, unfertilized). No significant hormone dose, period or interaction effect was detected (P>0.05) but intercept model fit was significant (P<0.05) for structures (embryos, unfertilized ova and degenerated embryos), embryos harvested and grade one embryos (Table 4).
Least squares mean for the fixed effects and interaction are presented in Table 5. Mean number of structures harvested at FSH hormone dose 400 mgs (20 ml) was marginally higher but did not significantly differ from the rest. Period three mean was lower than that obtained for periods one and two but the difference was not statistically significant (p > 0.05) (Table 5). The highest mean number of structures was harvested at hormone dose 260 mgs (13 ml) in period one and dose 400 mgs (20ml) in period two while the lowest mean yield was observed for dose 260 mgs (13ml) in period three (Table 5). Mean number of embryos harvested was not significantly different at all FSH hormone dose levels (Table 5). Period three mean was lower than that obtained for periods one and two but the difference was not statistically significant (P>.05) (Table 5). The highest mean number of embryos was harvested at hormone dose 200 mgs (10 ml) in period two and dose 260 mgs (13ml) in period one while the lowest mean yield of embryos harvested was observed for dose 260 mgs (13ml) in period three (Table 5).
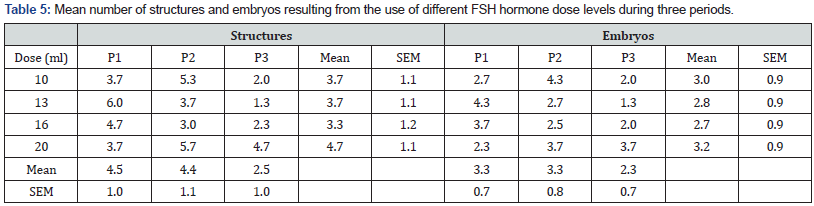
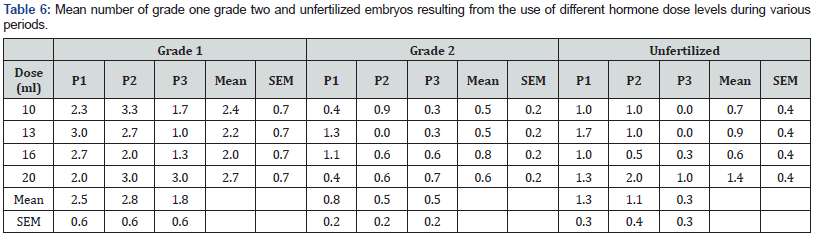
Least squares mean for the fixed effects and interaction for quality of embryos harvested are presented in Table 6. Mean number of grade one embryos harvested did not significantly differ with hormone dose or period of study (P>.05). On average, a maximum of three grade one embryos were harvested per donor cow. Fewer mean number of grade two embryos was harvested and no significant difference was observed across hormone levels or period of study (Table 6). No significant difference was observed in mean number of unfertilized ova harvested across hormone levels or period of study (P>.05) (Table 6).
Discussion
All donors responded well to super ovulation and at the end of follicle stimulating hormone (FSH) treatment, all the donors had increased number of ovulatory follicles. Ultrasound scans of the ovaries showed progressive trend in the number of ovulatory follicles with increasing levels of FSH but the differences were not significant (P>0.05). Though the higher dose of FSH is desirable, it let to over reaction in the ovarian growth as observed on one third of the donors on 20 ml FSH hormone treatment. The overreaction may delay the return to normal oestrus cycle of the donors and may be the cause of the poor embryo recovery rate and more unfertilized ova witnessed.
Other practitioners have also noticed the overreaction to 20mls level of FSH hormonal treatment in super ovulation programs in Kenya (Personal communication from Dr Maurice Cherogony of East Africa Semen and Embryo Transfer Association, 2015). Subsequent reduction of the FSH levels in such donors let to improved embryo recovery. The donor cows on the other levels of experimental FSH hormone treatment of 10 ml, 13 ml and 16 ml responded without ovarian overreaction. These levels may be suitable for average bodied donor cows such as most dairy cows found in Kenya. The lower level also reduces the cost of embryo production since FSH hormone is the most expensive item in embryo production.
The cost of super ovulation is estimated at USD 250 per donor cow [9]. FSH accounts for over 50% of this cost at USD 130 for 400 mgs (20mls) being used in the super ovulation programs in Kenya. This is the single most limiting factor that contributes to the high cost of conventional embryo production. The high embryo output variability and poor recovery rates compound the already high cost of production. The mean number of ovulatory follicles in the current study ranged from 7.7 to 13.7. This was like those observed by Peixoto [22] ranging between 7.3 and 13.8 and higher than those of Hahn (1990) who observed a range of 3 – 10 follicles [5]. Despite the large number of ovulatory follicles, not all ovulated. The proportion of follicles that ovulated ranged between 54% for 260 mgs (13ml) FSH treatment to 62% for the 400 mgs (20ml) FSH treatment. The right ovary had more ovulatory follicles compared to the left ovary and may be due to the influence of the rumen on the left ovary.
One third of the donors that failed to ovulate showed heat signs on expected dates and time. Two thirds of the donors ovulated but the number of corpora lutea where variable. One third had a few corpora lutea of between one and three, while the other one third responded well with more than three corpora lutea. Mapletoft [9] and Viana & Camago [11] had observed such a trend in several super ovulations. The reasons for such variations are not known but may be due partly to animal factors or hormone drug formulation [15]. It should be noted that, though two thirds of the donors ovulated the number of corpora lutea where highly variable. The failure by some donors to ovulate is a challenge in super ovulation because they could not be predicted using ultrasound equipment to scan the ovaries during FSH hormonal treatment. The failure to ovulate despite the growth of follicles to ovulatory stage and subsequent turning on of heat signs could be due to low levels of luteinizing hormone circulating at that time or other animal factors. Luteinizing hormone plays a critical role in ovulation process hence low levels or lack of it will result in poor ovulation hence low embryo recovery rates. Further research is needed to ascertain whether low levels of LH is the cause of the failure or other animal factors in order to improve the ratio of ovulated follicles.
Some donor animals that failed to ovulate during the first attempt responded well during the second super ovulation treatment, subsequently producing a good average number of embryos. A few donor cows failed completely to ovulate following a second super ovulation attempt despite showing growth in follicle numbers and sizes. These group of animals came on heat due to increased amount of estrogen released by the dominant follicles but failed to ovulate. Keeping records is important in embryo transfer program to allow for such donor cows to be rejected in future superovulation programs. The expected embryo yield based on ultrasound scan of ovaries and subsequent count through rectal palpation of the corpora lutea formed showed an average of 6.1 to 7.8. This was however not realized in actual mean number of structures harvested. The actual structures harvested ranged from 3.3 to 4.7 between different levels of FSH and 2.5 to 4.5 between seasons. The average number of embryos harvested was 2.9 per donor which is lower than those harvested in the United States of America with an average of six [23] and Brazil with an average of 4.1 - 7.3 [22] but higher than those of Ethiopia with an average of 2.07 [24]. This could be attributed to inexperience in embryo harvesting due to lack of regular embryo production programs.
The number of corpora lutea (6.1–7.8) were lower than those observed by Peixoto [22]. With experienced technician, this could have yielded an average of 5 to 7 embryos per donor. Lack of regular practice in embryo harvesting let to lower embryos recovered. Improvement of technical skills is needed if we are expected to meet international standards in Kenya. Most embryos (40%) were left within the uterus hence leading to perceived high production costs. Technician skills have been shown to be among the reasons for the low embryo recovery / yield [9,15,25,26]. Regular practice is needed for consistent embryo production but this has been hindered by lack of affordable FSH. FSH is imported, hence attracting freight costs on top of taxes and profit markup of the exporting and importing agents. Period three which was between July, August, September 2015 had low number of ovulatory follicles and hence low number of embryos harvested. Period three also had the lowest proportion of follicles that ovulated at 50% compared to period two (April, May, June) which had the highest proportion at 63%. Season has been shown to influence embryo yield [9,15,25]. Period three is colder than period two and one in Kenya. The animals should be kept warm through proper housing facility during such a period if optimum embryo production is to be realized.
Most of the embryos harvested were of grade one quality across the different levels of FSH and seasons. Different FSH levels had no influence on the quality of embryos produced. More unfertilized ova observed were at the higher level of FSH and this could have been contributed by the overreaction to the higher FSH dose leading to larger super ovulated ovaries. There was no documented evidence on research to ascertain the influence of FSH levels on quality of embryos. Based on observed follicular response, number of corpora lutea and actual embryo output, the experimental model using 200 mgs (10mls) of follicle stimulating hormone (FSH) is the most appropriate for use in Kenya. The ovarian response by donor cows was optimal without overreaction by the ovarian tissues. The dose was associated with low number of unfertilized ova and degenerated embryos.
The recommended protocol based on the use of 200 mgs (10mls) of FSH will reduce the cost of super ovulation by USD 65 per donor. This translates to 25% reduction in the overall cost of superovulation. Embryo production and transfer is perceived as expensive, hence the low adoption rate in Kenya [27]. This has led to infrequent, rarely used technique that is often utilized by the elite farmers and seldom by the average breeders. Though it’s a technique that is frequently used in developed countries and a few developing countries, it’s hardly a tool of choice in most Sub Sahara Africa.
Conclusion
Embryo transfer therefore, remains the most viable option provided that the correct super ovulation protocol is employed together with relevant training and experience. The use of inappropriate super ovulation protocol together with poor techniques during the cold season may lead to poor response and low recovery rates. The practitioners need to perfect the embryo harvesting techniques and sensitization of dairy cattle breeders to adopt and utilize MOET for faster dairy cattle improvement.
Acknowledgements
We acknowledge the support of DAAD the main sponsor of this research through grant number 91560720. The Eastern Africa Agricultural Productivity Project (EAAPP) for part supply of materials. The University of Eldoret (UOE), farm section, who provided the experimental animals and their management. The University of Nairobi (UON), Department of Clinical Studies, Faculty of Veterinary Medicine for research facilities. International Livestock Research Institute for the Ultrasound equipment. The teaching and support staff of UOE and UON for their support.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
“All international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Nairobi.”
References
- Kabubo-Mariara J (2009) Global warming and livestock husbandry in Kenya, impacts and adaptations. Ecological Economics 68: 1915-1924.
- KNDMP (2010) Kenya National Dairy Master Plan. Action plan and implementation strategy.
- Mutembei HM, Tsuma VT, Muasa BT, Muraya J, Erastus RM (2015) Bovine in-vitro embryo production and its contribution towards improved food security in kenya. African Journal of food agriculture and nutrition development 1: 9722-9743.
- Kios DK, VanMarle-Köster E, Visser C (2012) Application of DNA markers in parentage verification of Boran cattle in Kenya. Tropical Animal Health and Production 44(3): 471-476.
- Muraya J, Mutembei HM, Tsuma VT, Mutiga ER (2015) Characterization of Follicular Dynamics in the Kenyan Boran Cow. International Journal of Veterinary Science 4(4): 206-210.
- Lawrence FG, Mutembei HM, Lagat J, Mburu J, Amimo J, et al. (2015) Constraints to use of breeding services in Kenya. International Journal of Veterinary Science 4(4): 211-215.
- Mutembei HM, Muasa BS, Origa R, Jimbo S, Ojango JMK, et al. (2009) Delivery of appropriate cattle genotypes to Eastern African smallholder farmers through in vitro embryo production technologies, the technical procedures, prospects and challenges, Aspects of African Biodiversity. Royal Society Chemistry Journal 32: 84-90.
- Thibier M (2011) Embryo transfer, a comparative biosecurity advantage in international movements of germplasm. Revue Science Et Technique Office International des Epizooties 30(1): 177-188
- Mapletoft RJ (2012) Perspectives on Bovine Embryo Transfer. WCDS Advances in Dairy Technology 24: 83-93.
- Mapletoft RJ (2013) History and perspectives on bovine embryo transfer. Animal Reproduction 10(3): 168-173.
- Viana JHM, Carmago LSA (2007) Bovine embryo production in Brazil, a new scenario. Acta Scientiae Veterinariae 35(3): 920-924.
- Kios DK, Ongubo MN, Kitilit JK, Rachuonyo HA, Oliech GO, et al. (2013) Multiple Ovulation and embryo transfer in Kenya, a review of success rates and lessons learnt. African Journal of Education, Science and Technology 1(3): 99-105.
- Bari F, Khalid M, Hare Sign W, Murray A, Merrell B (2003) Factors affecting the survival of sheep embryos after transfer within a MOET program. Theriogenology 59: 1265-1275.
- Mollo A, Lora M, Faustini M, Romagnoli S, Cairoli F (2007) Some factors affecting embryo transfer success in dairy cattle. Journal of Animal and Veterinay Advances 6(4): 496-499.
- Lamb GC (2012) Managing embryo transfer to improve success. In: Proceedings applied reproductive strategies in Beef Cattle, Sioux Falls, SD pp. 341.
- Domecq JJ, Skidmore AL, Lloyd JW, Kaneene JB (1995) Validation of body condition scores with ultrasound measurements of subcutaneous fat of dairy cows. Journal of Dairy Science 78: 2308-2313.
- Hedayat AS, Yang M (2005) Optimal and efficient crossover designs for comparing test treatments with a control treatment. The Annals of Statistics 33(2): 915–943.
- Durocher J, Morin N, Blondin P (2005) Effect of hormonal stimulation on bovine follicular response and oocyte developmental competence in a commercial operation. Theriogenology 65: 102-115.
- Seidel EG, Seidel SM (2005) Training manual for embryo transfer in cattle. FAO Animal Production and Health Paper p. 77.
- Bo GA, Mapletoft RJ (2013) Evaluation and classification of bovine embryos. Animal Reproduction 10(3): 344–348.
- Duchateau L, Jansen P, Rowlands GJ (1998) Linear mixed models, An introduction with applications in veterinary research. International Livestock Research Institute (ILRI), Nairobi, Kenya, pp. 159.
- Peixoto MGCD, Bergmann JAG, Fonseca CG, Penna VM, Pereira CS (2006) Effects of environmental factors on multiple ovulations of zebu donors. Arquivo Brasileiro de Medicina Veterinaria e Zootecnia 58(4): 567-574.
- Hasler JF (2014) Forty years of embryo transfer in cattle, a review focusing on the journal Theriogenology, the growth of the industry in North America and personal reminisces. Theriogenology 81(1): 152– 169.
- Tadesse M, Degefa T, JeiluJemal J, Yohanis A, Seyum T (2016) Evaluation of Response to Super-Ovulation, Estrous Synchronization and Embryo Transfer in Local Zebu or Crossbred Dairy Cattle. Ethiopian Journal of Agricultural Science 26(2): 27-35.
- Arendonk JAM, Bijma P (2003) Factors affecting commercial application of embryo technologies in dairy cattle in Europe, a modelling approach. Theriogenology 59: 635-649.
- Hasler JF (2004) Factors influencing the success of embryo transfer in cattle. In: Proceedings of the WBC Congress January 2004, Québec, Canada.
- Kios D, Tsuma V, Mutembei H (2018) Factors affecting adoption of embryo transfer technology in dairy cattle in Kenya. Advances in Social Sciences Research Journal 5(8): 456–463.






























