Prevalence of Major Gastrointestinal Nematode and Degree of Parasite Infestation in Sheep of Bako Agricultural Research Center Community Based Breeding Program Project Small Holder Farms at Horro District
Abebe Tibebu, Yobsan Tamiru* and Debela Abdeta
School of Veterinary Medicine, College of Medical and Health Science, Wollega University, Nekemte, Ethiopia
Submission: November 01, 2018;Published: December 04, 2018
*Corresponding author: Yobsan Tamiru, school of Veterinary Medicine, College of Medical And Health Science, Wollega University, P.O.Box, 395, Nekemte, Ethiopia
How to cite this article: Abebe T, Yobsan T, Debela A. Prevalence of Major Gastrointestinal Nematode and Degree of Parasite Infestation in Sheep of Bako Agricultural Research Center Community Based Breeding Program Project Small Holder Farms at Horro District. Dairy and Vet Sci J. 2018; 8(3): 555740. DOI: 10.19080/JDVS.2018.08.555740
Abstract
A cross-sectional study was conducted from November 2017 to April 2018 on 384 randomly selected sheep in two purposively selected peasant associations of Horro district with the objective of determining the prevalence and degree of gastrointestinal nematode infestation in sheep. Fecal samples collected from all study animals were subjected to parasitological investigation including simple test tube floatation and McMaster egg counting techniques for screening and counting the eggs of observed gastrointestinal (GIT) nematodes. The study found that the overall prevalence of gastrointestinal nematodes in sheep was 36.7% (141/384). Among the identified nematode parasite egg, strongyle eggs 64(16.66%) were the most prevalent followed by Trichuris 30(7.8%) and 29(7.55%) Strongyloidiasis. There was a significant difference (P < 0.05) in prevalence of GIT nematodes between different body conditions and age of the study animals. Animals with poor body conditions 75 (46.4%) were highly infected than animals with medium 51 (38.7%) and good body condition 15 (20%).
The study revealed that the prevalence of nematodes significantly higher (p<0.05) in younger animals than in adults. However, there was no significant difference (P>0.05) in the prevalence of GIT nematodes in case of peasant associations and sex of animals. Based on the EPG result, the study animals were classified as light81 (57.4%), moderate 34 (24.1%) and severe 26 (18.4%) infection. The majority of examined animal had the EPG count in average of less than 800 which is lightly infested. Hence, this finding indicated that helminths parasites are more prevalent in the study area. So that, proper screening and monitoring of the sheep should be carried out regularly, regular and strategic deworming programmes should be carried out, and. further studies on should be done to know the seasonal prevalence of GIT parasites of sheep.
Abbrevations:°C: Degree Centigrade; BARC: Bako Agricultural Research Center; BCS: Body Condition Score; CI: Confidence Interval; CSA: Central Statistics Authority; DF: Degree of Freedom; E: East; ELISA: Enzyme Linked Immunosorbent Assay; EPG: Egg per Gram of faeces; GINs: Gastrointestinal Nematodes; GIT: Gastro Intestinal Tract; ILRI: International Livestock Research Institute; KG: kilogram; KM: Kilo Meter; L1: First stage larvae; L2: Second stage larvae; L3: Third stage of larvae; L4: Fourth stage of larvae; L5: Fifth stage of larvae; M.a.s.l: Meter above sea level; MG: Milligram; Mm: millimeter; N: North; P: Precision value; SPSS: Statistical Package for Social Science; X2: Chi Square
Introduction
Ethiopia lies within the tropical latitudes of Africa, and has an extremely diverse topography, a wide range of climatic features and a multitude of agro-ecological zones, which makes the country suitable for different agricultural production systems. This in turn has contributed to the existence of a large diversity of farm animal genetic resources in the country [1]. The country has the largest population of small ruminants in Africa. The latest estimate of small ruminant population gives 23.6 million sheep and 23.3 million goats [2]. Small ruminants play a significant role in maintaining household stability by providing meat, milk, skin and wool, generate cash income and play traditional social and religious roles [3,4].
Sheep and goats are the most numerous of man’s domesticated livestock and are especially important in more extreme climates of the world. Over two-thirds of the total population of sheep and goats occur in the less developed countries where they often provide major contribution to farming enterprises [5]. Among the small ruminants in Ethiopia, sheep are the dominant livestock, providing up to 63% of cash income and 23% of the food subsistence value obtained from livestock production [6]. Regardless of the large size of the sheep population in the country and the huge potential therein; productivity per animal and the contribution of this sub-sector to the national economy is relatively low due to multitude of constraining factors including malnutrition, diseases, improper health care and management problems [7].
The epidemiology of gastro-intestinal (GIT) parasites in livestock varied depending on the local climatic condition, such as humidity, temperature, rainfall, vegetation and management practices. These factors largely determine the incidence and severity of various parasitic diseases in a region [8]. Clinical diagnosis of GI nematodes is difficult, since the signs are not pathognomonic. However, diagnosis of gastrointestinal nematode infections plays a major role in investigating parasite epidemiology. The ante mortem diagnosis of nematode infections in livestock has been based on the detection of nematode eggs or larvae in the faeces by microscopic examination using the methods of flotation and/or larval culture. Quantifying of the egg per gram of feces is the best way of estimating parasite loads [9].
Despite the large livestock population of Ethiopia, the economic benefits remain marginal due to prevailing diseases, poor nutrition, poor animal production systems, reproductive inefficiency, management constraints and general lack of veterinary care [10- 13]. Parasitic diseases are global problem and considered as a major constraint in weight gain, health and product performance of livestock. They cause lowered productivity and high economic losses affecting the income of small holder farming communities. These effects are varied and more pronounced in sheep and goats compared to those seen in other species of livestock [14].
Nematodes (round worms) are free-living unsegmented worms, which have cylindrical form, tapering at either ends. Their body is covered with a colorless, somewhat translucent layer called the cuticle, are elongated in shape and an alimentary canal is present [15]. They have separate sexes and exhibit both direct and indirect life cycle, are found in fresh water, the sea and the soil and are among the most successful parasites of plants and animals [16]. Gastro intestinal nematodes of greatest importance in small ruminants are Haemonchus, Osophagustomum, Chabertia, Bunostomum, Ostertagia, Trichostrongylus, Gaigeria which lays eggs of similar feature like strongyle and identified by fecal culture at larval level. Whereas Trichuris, Nematodirus and Strongyloides produce distnict eggs [17]. The major weapons used for gastrointestinal problem are “Anthelmintic” used since long to combat the problem of gastrointestinal parasitism but due to the excessive use of that drugs developed the resistance. The frequent use of these anthelmintics over many years has inevitably led to the development of drug resistance to each class in parasitic nematodes [18].
Although considerable work has been done on endo parasites of sheep in many parts of Ethiopia, there was no previous study carried out on prevalence and intensity of the ovine gastrointestinal nematodes in the study area even though, there is large population of small ruminants especially sheep. Therefore, the objectives of this study were: To determine the prevalence of gastrointestinal nematodes and associated risk factors in sheep at Horro district, Western Oromia and to determine the degree of gastrointestinal infestation in Horro district, Horro guduru wollega zone, Western oromia.
Literature Review
Ovine Gastrointestinal Nematodes
Gastrointestinal nematodes are numerous parasites which develop within the digestive tract (abomasum, intestines) of domestic ruminants. They include a range of nematode species, which belong to the order Strongylida [19]. Gastro-intestinal nematode parasitic infection is one of the major health problems in the world [20] reported that nematode infections affect the health of millions of people and animals, causing huge economic loss in livestock farming. The consequences of nematode infection include: reduced feed intake and weight gain, reduced immunity, lower fertility, a reduction in milk production and work capacity, treatment expenses and death in critical infections [21,22]. In general, the nematodes are the most numerous animals on earth. Nematodes make up a large assemblage of worms of relatively simple structure with a widespread distribution, their cylindrical, non-segmented bodies distinguishing them easily from other helminthes [23]. The degree of infestation by gastrointestinal nematode parasites can be evaluated by counting the eggs of those parasites under microscope. Degree of nematode infection depends mainly upon the age of the host, the breed, the parasite species involved, and the epidemiological patterns which include husbandry practices and physiological status of the animals. More importantly, environmental conditions such as temperature, rainfall and humidity are major factors to the development of nematode eggs and free-living stages.
Morphology
Concerning the morphology of nematodes, the body is elongated, cylindrical and tapered at the extremities. The body is also unsegmented and covered with cuticle which is thick and continuous with the cuticular lining of the buccal cavity, the oesophagus, the rectum and the distal portions of the genital ducts [24].
General Life Cycle of Gastrointestinal Nematode
Most GIT nematodes have the same life cycle. Majorities are oviparous, and the eggs are similar and very characteristic type, and immediate transfer of infection from one host to another does not occur. The life cycle of the nematode may be direct or include an intermediate host. The sexes are usually separated (male and female). However, all the economically important gastrointestinal parasites of small ruminants have direct life cycles, requiring no intermediate hosts [25]. The mature parasites (worms) breed inside the host and lay eggs which pass through the host and are shed in the faeces. After the eggs pass out of the host, they hatch into first-stage larvae (L1) and moult into second-stage larvae (L2) under appropriate conditions of temperature and humidity. The larvae need moisture to develop and move. During this time the larvae feed on bacteria and L2 moult into infective larvae (L3), which migrate out of the faeces and up blades of grass. When an animal (sheep) grazes, they may ingest parasite larvae along with the grass. Normally L3 can moult into fourth-stage larvae (L4) within 2-3 days, remaining for further 10-14 days to moult into young adult parasites.
Factors Affecting Nematode Infestation
The number of infective larvae L3 ingested by the host, which in turn is influenced by the climate, the amount of protection of larvae provided by vegetation, the livestock density and the grazing pattern of the ruminant’s present. The rate at which acquired resistance develops in the host is influenced by the species of the parasite and host, genetic factors, nutrition and physiological stress (e.g., parturition). The intrinsic multiplication rates of the species of parasites present which are controlled by the fecundity, pre-patent period, environmental development and survival rates of these species. Management particularly, grazing patterns, use of anthelmintic, including the timing and frequency of administration [26].
Effect of Nematode Parasites on Animals
Effect of larval stages on the host: Considerable damage is caused by fourth-stage larvae (L4) of abomasal parasites (Haemonchus, Ostertagia and T. axei). The L3 enter the mucous membrane in the wall of the abomasum within six hours of entering the host and will usually stay in the mucous membrane for about two to three weeks. If large numbers of Haemonchus, Ostertagia and T. axei larvae enter the abomasum, the host will be affected. The larvae of Trichostrongylus in the small intestine may cause severe damage to the intestinal mucous membrane with similar effects. This development may be accompanied by destruction of the mucous membrane, the extent of which depends on the numbers of inhibited larvae emerging. The (L4) of Haemonchus is a blood sucker in the abomasum. Animals infected with large numbers of larvae therefore may suffer from anemia before the parasite eggs can be detected in the animal’s faeces [27].
Effect of Adult Worms on the Host: Infections with gastrointestinal nematodes usually involve several different species of parasites, which may have an additive pathogenic effect on the host. The pathogenic effect of gastro-intestinal parasites may be sub-clinical or clinical. Young animals are most susceptible. Severe blood and protein loss into the abomasum and intestine due to damage caused by the parasites often results in oedema. Some nematode species, especially those that suck blood, such as Haemonchus, Bunostomum and Oesophagostomum, are responsible for specific clinical signs. Haemonchus is the most pathogenic of the blood suckers and infections with large numbers of this parasite often result in severe anaemia in the host (Figure 1).

Epidemiology
Helminths are most frequently a problem in young animals reared in permanent animals’ pasture, although cases of severe diseases may occur in adult animals kept in sub urban paddocks and subjected to overcrowding and poor management [28]. Many low to moderate infection are sub clinical although, they may cause reduced weight gain and performance. Young, non-immune animals are most susceptible and manifest clinical disease which may include diarrhea, colic and hypoproteinaemia [29]. Pasture larval levels increase markedly during the summer months when conditions are optimal for rapid development of eggs to L3. There is also increasing evidence that may infective L3 ingested during autumn show a degree hypobioxis under ileum in the large intestinal mucosa until the following spring [28]. Resistance affected adversely by stress and nutritional deficiencies. Moderate infection can be tolerated by a good plane of nutrition and management with in similarly infected but poorly [30].
Factors affecting Epidemiology Nematode Parasites
The development, survival and transmission of the free-living stages of nematode parasites are influenced by micro-climatic factors within the faecal pellets and herbage. These include sunlight, temperature, rainfall, humidity and soil moisture. Under optimal conditions (high humidity and warm temperature), the development process takes 7 to 10 days, but for H. contortus a more rapid translation of eggs to larvae can occur in warm wet conditions. In most African countries, the temperatures are permanently favorable for larval development in the environment. Development of trichostrongylid larvae occurs in a temperature range of approximately 10-36 °C. The optimal humidity requirement for free- living stage development of most species is 85%. Although desiccation is lethal for the free-living stages of parasite worms, the important nematode parasites can survive such conditions either as embroynated eggs or as infective larvae [31].
The seasonal fluctuations in numbers and availability of the infective larval stages are also influenced by the level of contamination of the pasture (Figure 2). The latter is controlled by the biotic potential (fecundity) of the adult parasites in the host, the density of stocking, and the immune status of the host. Although different species of GI nematodes of small ruminants have varying egg-producing capacities, H. contortus is one of the most prolific nematodes. A female H. contortus may produce thousands of eggs each day, and larval numbers on pasture can rapidly increase during the wet seasons [28].
Diagnosis of Nematode Infestation
Clinical diagnosis of GIT nematodes of sheep and goats needs history of the area, history of anti-helminthes treatment, grazing history, age of animal and clinical signs manifested by the disease. But as GIT nematodosis share common clinical manifestations with other diseases laboratory diagnosis is important. The diagnosis of nematode parasites of small ruminants is based on demonstrating the presence of their eggs, or larvae, in faecal samples, or the presence of parasites recovered from the digestive tracts of the animals [32].
The following diagnostic procedures for helminth infections of small ruminants are relevant to African conditions. Faecal examination by means of the modified McMaster technique for the enumeration of worm eggs and larval differentiation by faecal culture methods are the most common routine means for the diagnose helminthosis in small ruminants. The strong lid nematode genera produce eggs that are similar in appearance and cannot be easily discriminated, which means that genus identification cannot accurately be made by faecal examination alone. To identify nematodes in faecal samples, faecal cultures are required to yield L3 larvae, which generally can be differentiated to genus level. Nematodirus, Strongyloides and Trichuris species have eggs that can be differentiated by their distinct morphological features [33].
Laboratory Diagnosis
Although there is much current interest in the use of serology as an aid to the diagnosis of helmenthosis, particularly with introduction of ELISA test, diagnosis GIT parasitic infections still depend mostly on parasitological findings of eggs and/ or parasite in fecal samples [28].
Faecal Examination
Fecal examination for the detection of worm eggs is most common and routine work in GIT nematode diagnosis. Examination of faces for nematode eggs may vary from a simple direct smear to more complex methods involving centrifugation and the use of flotation fluids [34].
Direct Fecal Smear Examination
The presence or absence of worm eggs in fecal sample using direct smear of fresh faces on microscope slide and examination under low power objective microscope is routine procedure. However, this technique is only useful to detect nematode eggs when it exists high concentration in faces. Other disadvantages of direct techniques include difficulty to identify them since the eggs are partially covered by debris materials and quantitative results could not be obtained although it is fast and easy technique [34].
Concentration Techniques
Light infections are not easily detected using direct smear; therefore, concentration technique was developed to overcome the short coming of direct smear. The concentration techniques that are widely used include the use of salt or sugar solution and centrifugal concentration techniques. In both cases the logic behind is to concentrate the nematode eggs in each portion of sample or processed fecal material. In flotation the type of egg recovered is related to specific gravity of solutions; half saturated sodium chloride with specific gravity of 1.125 is capable of floating Trichostrongloids and strong lid eggs while fully saturated sodium chloride with specific gravity of 1.204 is preferred as generalpurpose solution [35].
Egg Counting Technique
The demonstration of a parasitic element in excreta includes: the presence of parasite. However, this information is not always enough. In the case of gastrointestinal strongylosis, the number rather than the presence of parasites is important. A technique called Mac Master. This technique is said to be easily applicable low technology parameter to indicate the level of infestation and degree of worm burden in some instances. The method enables to determine the number of eggs per gram of faces, although it is difficult to relate directly with the burden of parasites in large ruminants, still it is widely used, and best correlation was observed in small ruminants and the method is also used to detect anti helmhentic resistance.
Fecal Culturing
Grazing sheep and goats usually have mixed nematode infections. Only few nematode parasites have characteristic eggs that enables as to differentiate to genus level (Nematodirus spp, Trichuris spp, strongyloides spp,) but those trichostrongyle and strongyles are not easily differentiated, for this reason fecal culturing and larval identification based on the keys available is useful technique.
Treatment
Small holder farmers and pastoralists of Ethiopia practice varying degrees of parasite control in their livestock. These practices range from the use of anthelmintic drugs of varying quality, to the use of traditional medicines [36]. The prophylactic treatment of nematode infection depends basically on the use of anthelmintics [37]. Notable, the availability of safe, broad spectrum anthelmintics has helped to reduce the incidence of a great number of worm diseases (Figure 3). In general, anthelmintic groups are greatly effective against the immature and mature stages of virtually all the important gastrointestinal nematodes as well as many extra intestinal helminth species [38]. The drugs of choice for small ruminants’ nematode infection areIvermectin 0.2mg/kg, oxfendazole 5mg/kg, fenbendazole 5mg/kg, levamisole 2.5mg/kg, albendazole 4-8mg/kg and febantel 5-10mg/kg. These anthelminthic have high activity against mature and immature stage nematode. Antibiotics are also given to prevent secondary bacterial infection [39].

Control and Prevention
Control of endoparasite is the most desirable although internal parasite problem is usually related to management practices that increases exposure. Whereas ongoing preventive management practices minimizes losses caused by parasitic infection. Control of nematode infection in small ruminants may be achieved by pasture management. Animal must be removed from infected ground, placed on dry pasture and supplied with clean drinking water. Draining and resting pasture during dry summer kill many larvae that readily survive cold winter. Their feces should not be used for fertilizing lands on which crops for green feeding are grown, moist grasses should not be given to animals, and adult should not graze together with young stock [39].
Proper Nutrition
The strongest link between nutrition and parasitism has been illustrated between protein intake and resistance to GINs infection. The most dramatic has been the abolishment of the peri parturient egg rises in lambing ewes by providing protein accordingly. Supplementation with phosphorus has been shown to prevent worm establishment [40].
Pasture Management
A safe pasture is one that had no sheep grazed on it for 6 months during cold weather or 3 months during hot/dry weather. Weaning sheep at 2 months of age and rotating them through pastures ahead of the adults will minimize the exposure to large numbers of infective larvae. Pastures should be rotated following any administration anthelmintic to the animals [41]. Nematophagous Fungi: Act as a biological control agent. Nematophagous fungi are micro-fungi which utilize nematode larvae as their main source of nutrients. The fungi are ingested by ruminants pass through the digestive tract and colonize fecal material. Three predaceous fungi have been identified but only one is suitable for including in ruminant diets. Duddingtoniaflagrans has thick-walled spores that can be fed to ruminants and passes safely through to the feces. The spores must be fed daily to maintain the reduction in L3 numbers [42].
Anthelmintic Usage
If possible, anthelmintic use should be restricted to 2 or 3 times per year by combining anthelmintic use with the epidemiology of nematode infection. Regular monthly dosing, as practiced on some farms, cannot be recommended. Use the full dose of an anthelmintic as well as alternate the type of anthelmintic used. The generally accepted view is that anthelmintics should be alternated on an annual basis [43].
Materials and Methods
Description of the study area
The study was conducted in selected peasant associations of Horro district by the help of Bako agricultural research center because it has community-based breeding program project supported by international livestock research institute with objective of improving breeding in horro sheep at two selected sites of the district namely Laku Ingu and Githilo Dole. Which are considered as the origin of horro sheep, presence of large sheep populations, and presence of ear tag for suitable sampling and known history of anthelmentics treatments. There are 135 small holder farmers in the community based breeding program project those have 12-15 sheep on average. Horro district is in Horo Guduru wollega Zone of Oromia regional state at the distance of 328km to west of Addis Ababa. Horro Guduru Wollega zone is located between 09º29´N and 37º26´E, at an altitude of 2,296` m.a.s.l, with a uni-modal rainfall ranging between 1200mm- 1800mm. The rainy season occurs from April to mid-October. Maximum temperature of 23-27 are reached from January to March, and temperature range of 7 °C -15 °C is normal from October to November.
Study Population
The study animals comprised indigenous Horro sheep of randomly selected small holder farms from 135 small extensive farms kept together on communal grazing pasture of the peasant association day time and separately housed at night by individual house holders. Those small farms owned by small holder farmers under community-based breeding program of Bako Agricultural Research Center supported by ILRI at both peasant associations. Single small farm contains an average flock size of 12-15 animals. The study animals will be taken from those selected peasant association’s small holder farmers depending on their total number of animals. A total of 384 sheep were sampled from both site regardless of sex, age, body condition score etc.Animal in each selected districts were grouped into age groups as young (<1year) and adult (>1 year) based on birth register and body conditions (poor, medium and good) as per Kempster.
Study Design
Cross sectional study was conducted to determine prevalence of major gastrointestinal nematode and degree of parasite infestation in sheep of Bako Agricultural Research Center community-based breeding program project small holder farms at Horro district. The study sites were selected purposively whereas farmers and the individual sample animals were selected by simple random sampling method. Depending on numbers of eggs counted, animals were then categorized as lightly, moderately and severely (massively) infected according to their egg per gram of faeces (EPG) counts. Egg counts from 50-799, 800-1200 and over 1200 eggs per gram of faeces were considered as light, moderate and massive infection respectively [28].
Sample Size and Sampling Method
Simple random sampling strategy was followed to collect feces from the individual animals. Those all study sheep were from two purposively selected peasant association of Horro district managed together for the most part and grazed on permanent communal pastures. Due to study animals were under community-based breeding program the proportion of female to male is high. Sample size for fecal collection was determined using the formula given by Thrusfield. Accordingly, using expected prevalence of 50% at 95% confidence intervals and 5% desired absolute precision a sample size of 384 animals were collected because no previous work in district even in those farms. Sample size determined by the following formula.
Where N= Sample size
P= expected value
d= desired absolute precision
Then by taking p=50% and d=5%
N =1.962 x 0.5(1-0.50)/0.05
N=384
Study Methodology
Sample Collection and Laboratory Analysis
An average of 5gm of fecal sample was collected from rectum of each sampled sheep those were not dewormed for at least three months in to a plastic container and clearly labeled corresponding to detailed information recorded and transported for examination. After collection the fecal samples were preserved with 10% of formalin to prevent the egg from hatching during transportation because the study site is far from the laboratory where the samples processed then taken to Bako Agricultural Research Center (BARC) animal health laboratory for coprological investigation. When samples were reached in the laboratory they were immediately stored in the refrigerator (4 °C) until they were processed for further preservation. Floatation techniques were employed to diagnose eggs of nematodes using saturated sodium chloride (40%) as flotation fluid and the slides prepared were examined under microscope (x10). Eggs of the different nematodes were identified on the base of morphological appearance and size of eggs and Macc master egg counting technique was also done for positive samples to categorize the severity of the infestation level. The degree of faecal egg output per gram was determined as described by Hansen & Perry [44] in mixed infection with different GI nematode species.
Data Analysis
Data generated from laboratory investigations will be recorded and coded using Microsoft Excel spreadsheet (Microsoft Corporation) and analyzed using SPSS version 20 statistical software. Descriptive statistics will be used to determine the proportion of diseases based on sex, peasant associations, body condition and age. Chi-square test and the p-value will be used to determine the presence of association among the different variables and the major animal health problems caused by parasites. A calculated two value at the specified degree of freedom(df) greater than the tabulated value of two at that df will be reported as having a significant association and vice versa. A p-value less than 0.05 will be considered as having statistically significant. 95% confidence interval will be used for interpreting the result.
Results
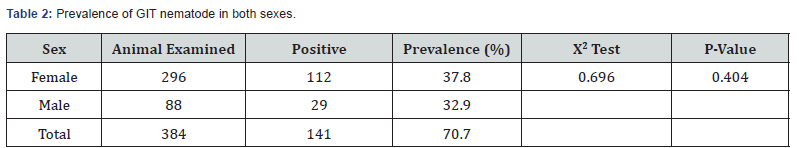
The overall prevalence of gastrointestinal nematodes in sheep during the study period was 36.7% from the positive samples, 64 (16.66%) were positive for strongyle type egg, 30 (7.81%) were positive for Trichuris egg, 29 (7.55%) were positive for strongyloides egg for single infection and gastrointestinal nematodes were appeared as multiple infection in 18 positive animals as Strongyle and Trichuris 10 (2.60%), Strongyle and Strongyloides 6 (1.56%), Trichuris and Strongyloides 2(0.52%) (Tables 1& 2). Most of the samples were found with single infection 123 (87.23%) than multiple infections (12.76%). The prevalence of gastrointestinal nematode parasite was higher in female sheep (37.8%) than male (32.9%) in the study area. Among age groups, higher prevalence (59.29%) was observed in young animals as compared to adult (27.3%) (Table 3).

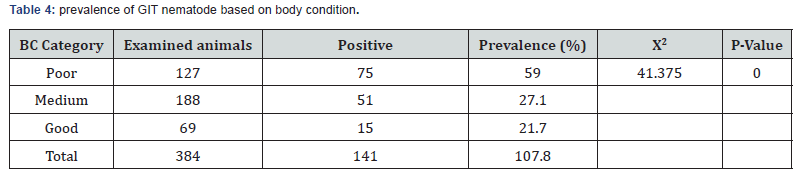
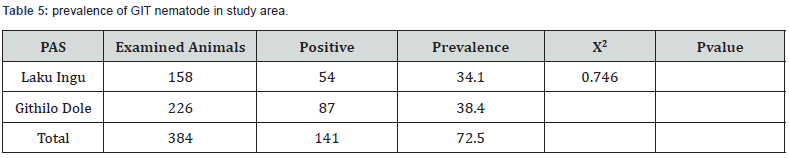
The prevalence of gastrointestinal nematode was higher in female sheep (37.8%) than male (32.9%). Of the total 384 sheep examined, 127, 188 and 69were categorized as having poor, medium and good body condition scores. Infection prevalence was significantly higher in animal with poor body condition when compared to that of medium and good body condition scores. The overall infection prevalence according to body condition grades, 59.1%, 27.1% and 21.7% with poor, medium and good, respectively (Table 4). The prevalence of gastrointestinal nematode was higher in Githilo Dole (38.459%) than Laku Ingu (34.2%) with almost minimal difference.
Results of Quantitative Fecal Egg Counts
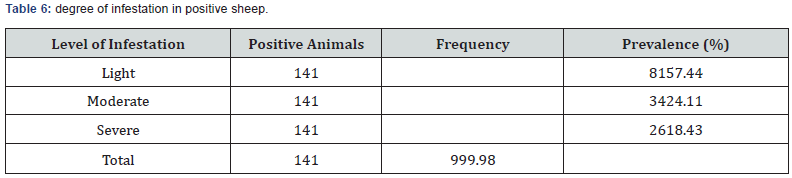
Fecal samples positive for GIT nematodes in this study were subjected to Mc Master egg counting chamber for EPG count to determine the degree of severity of parasitic infestations. The majority of positive study animal had the EPG count of less than 800.The animals positive for nematodes by floatation technique was subjected to McMaster technique and are classified as lightly (57.4%), moderately 34 (24.1%) and severely 26(18.4%) infected with GIT nematodes (Tables 5 & 6).
Discussion
Many studies showed that gastrointestinal nematodes are the leading causes of productivity losses in small ruminant production in Ethiopia [45]. The coprological examination done for this study using simple test tube faecal floatation techniques revealed that an overall gastro-intestinal nematode infection prevalence of 36.7% of sheep originating from this area which were being parasitized at least by one type of gastrointestinal nematodes.
In this study, infections with strongyles were the dominant one among the examined animals. Infections with Strongyloidiasis spp and Tricuris were also identified with limited proportions accordingly. This agrees with several studies conducted so far [46-48]. who reported high proportion of strongyle infection? This might be since ruminants have different level of resistance for different species of parasitic infections and several nematode parasites produce strongyle like egg which only identified on larval stage by fecal culture.
The present study shows no statistically significant differences (P > 0.05) between two sex groups, male (32.9%) and female (37.8%). This finding agrees with report by Assefa & Sissay [49], with gastrointestinal helminths affecting both sex groups equally. This is due to equal exposure of both sexes, and they are from similar agro-ecology. The variation may occur in the intensity of infection due to post-parturient parasite rise in lambed sheep. The absence of association between sexes is inconsistent with previous reports [50,51]. Nevertheless, itis agree with a higher prevalence of helminth infection in female animals. It is assumed that females are more prone to parasitism during pregnancy and per-parturient period due to stress and decreased immune status [28].
In this study, a significant difference was observed in prevalence of nematode infection in relation to body condition score where a higher prevalence of gastrointestinal nematodes parasites was recorded in poor (59%) and moderate (27.1%) body conditioned animals as compared to animals having good (21.7%) body condition. This finding agrees with [52-54]. In addition, [55] indicated that animals with poor condition are highly susceptible to infection and may be clinically affected by worm burdens as compared to well-fed healthy animal. Moreover, Knox et al. [56] observed that a well-fed animal was not in trouble with worms, and usually a poor diet resulted in more helminth infections. The study further revealed that small ruminants with medium and poor body condition score have higher prevalence rate of nematodiasis infection which is consistent with previous reports. This might be due to either well-fed animals have good immunity or parasitic infection leads to poor immunological response to the fecundity of the parasites.
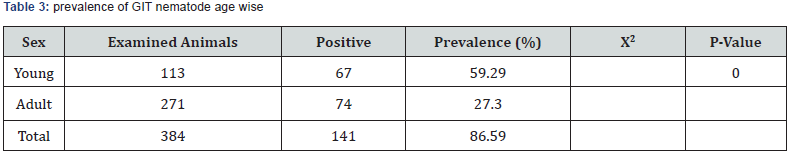
Age wise observation revealed statistically significant difference in infestation of parasites between ages with prevalence of 59.2% in young and 27.3% in adult sheep. This finding disagrees with reports from Gambia and Semi-arid part of Kenya that indicated that GIT helminthes affect both ages equally [57,58]. The present finding agrees with most literatures that young animals are more susceptible to parasite infection than sheep older than 1 year of age. The researchers justified the result that it could be because adult animals may acquire immunity to the parasite through frequent challenge and expel the ingested parasite before they establish infection [59]. The finding also agrees with reports of higher prevalence in young animals in Ethiopia. Age was considered as an important risk factor in GIT helminthiasis [60]. Several authors have documented that adult and old animals develop acquired immunity against helminth infections as they get mature due to repeated exposure [28].
The current study has shown the presence of mixed infection characterized by the presence of two or more nematode parasites in sheep which agrees with the findings of other researchers in the country and elsewhere. These Mixed infections have been suggested to be an important cause of morbidity and loss of production in sheep. Moreover, the presence of interaction and compromization of the immune system of the host by mixed infections described increase in their susceptibility to other diseases or parasites.
In relation to peasant associations the prevalence of GIT nematodes is relatively high in Githilo Dole (38.4%) as compared with Laku Ingu (34.1%). However, the difference in prevalence between the two sites was not statically significant (P>0.05). This also agrees with report by [61-63]. Significant difference was not reported in animals reared in similar geographical areas. In this study, absence of association between location and prevalence in sheep could be due to relative similarity in agro-ecology between study locations, similar deworming strategy by Bako Agricultural Research Center and a relatively similar management systems practiced by farming communities.
Conclusion and Recommendation
The gastrointestinal nematodes of sheep are one of the important parasitic diseases that obviously result in reduced productivity of sheep. Therefore, the high prevalence of nematode infection observed in the study area indicates potential contribution to limiting the productivity and compromised wellbeing of the animals. The infection was found higher in animals with poor body conditions than medium and good body conditioned sheep. Therefore, attention should be given to animals with poor body condition in control of the nematode infections. According to the results of this study, the prevalence of gastrointestinal nematode was found 36.7%.
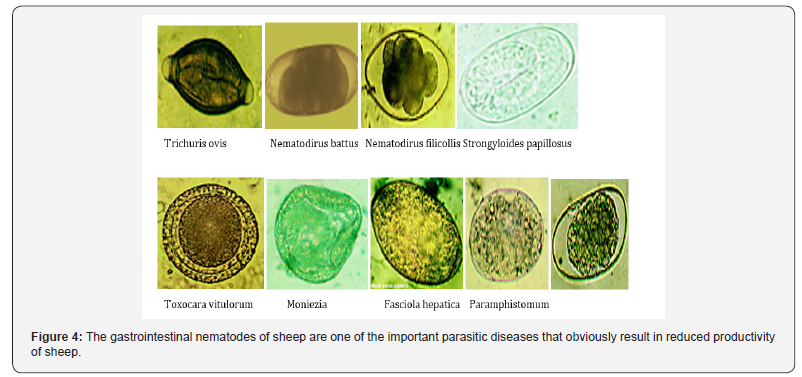
Generally, gastrointestinal nematodes were prevalent in Horro district and sheep of the study area were infected with diversified gastrointestinal nematodes that can seriously affect the health and productivity of the animals. These parasites affected all age and sex groups prevailing agro-climatic conditions like overstocking of the animals, grazing of young and adult animals together with poorly drained land provide an ideal condition for the transmission of the endoparasites to build up clinical infestation of the host [64]. Furthermore, weak status of animal health services and lack of proper management, crop-livestock mixed farming is highly practiced, and most land is cultivated so that many species of animals including sheep are kept together on communal grazing land which is the source of parasitic infection. They give the first line to draught animals and forced sheep to graze behind on overstocked areas which lead them to graze close to the ground and on fecal materials, causing in the uptake of higher numbers of infective larvae. All in all, the finding suggests that the study area is favorable for the continual maintenance and successive transmission of helminthes parasites to vulnerable hosts [65- 67]. Many animals were sub-clinically infected without attracting understanding of farmers to undertake control measures (Figure 4).
Based on the above conclusions, the following points are forwarded as recommendations:
a. Regular de-worming program using broad spectrum anthelmintic and good management practices should be implemented to minimize pasture contamination with larvae.
b. Further epidemiological study should be conducted in the area including environmental factors like management conditions that helps to design an appropriate control measures.
c. Quantitative method of study should be conducted to determine the parasite load and it is effects on the different body parameters.
d. Detailed study should be conducted to clearly identify nematode parasites using fecal culture and postmortem examination in the study area.
e. Separating the most susceptible young animals from adults, this is a possible source of contamination.
f. Proper screening and monitoring of the sheep should be carried out regularly in the sheep
g. Furthermore, parasitic control and prevention should be implemented in the area.
References
- Anon (2004) State of Ethiopian’s Animal Genetic Resources- Country Report. A Contribution to the First Report on the State of the World’s Animal Genetic Resources. Institute of Biodiversity Conservation (IBC). Addis Ababa, Ethiopia. p. 74.
- CSA (Central Statistics Authority) (2004) The 2001/2002 Ethiopian Agricultural sample enumeration (EASE) Executive Summary, Addis Ababa, Ethiopia.
- Devendera C, Mcleroy GB (1982) Goat and sheep production in tropics. Singapore, ELBI with Longman p. 1-7.
- El-Azazy O (1995) Seasonal changes and inhibited development of the abomasal nematodes of sheep and goats in Saudi Arabia. Vet Parasitol 58: 91-98.
- Tony W (2007) The veterinary epidemiology and economics research unit (VEERU), School agriculture, policy and development. The University of reading, United Kingdom.
- Zelalem A, Fletcher I (1991) Small ruminant productivity in the central Ethiopian mixed farming systems. In: IAR, Addis Ababa, Ethiopia: (4th edn), Tumwasorn, Livestock Improvement Conference, pp: 141-147.
- Haileleul N (2002) Study on prevalence of GIT helminths of small ruminants in and around WolaytaSoddo, southern Ethiopia. DVM Thesis Faculty of veterinary medicine, Addis Ababa Thesis, Debre-Zeit, Ethiopia.
- Takelye B (1991) Epidemiology of endoparasites of small ruminants in sub-Saharan Africa. In: Proceedings of the 4th National livestock Improvement Conference, Addis Ababa, Ethiopia, p: 7-15.
- Roeber F, Jex R, Gasser R (2013) Impact of gastrointestinal parasitic nematodes of sheepand the role of advanced molecular tools for exploring epidemiology and drug resistance-an Australian perspective. The University of Melbourne, Australia, Journal of pharmaceutical policy and practice 6: 153.
- Mukasa-Mugerwa E, Lahlou-Kassi A, Anindo D, Rege JEO, Tembely S, et al. (2000) Between and within breed variation in lamb survival and the risk factors associated with major causes of mortality in indigenous Horro and Menz sheep in Ethiopia. Small Ruminant Research 37: 1- 12.
- Tibbo M, Woldemeskel M, Gopilo A (2001) An outbreak of respiratory disease complex in sheep in central Ethiopia. Tropical Animal Health and Production 33: 355-365.
- Kassa HB (2003) Livestock and Livelihood Security in the Harar Highlands of Ethiopia: Implications for research and development. Doctoral thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, Europe.
- Tibbo M, Mukasa-Mugerwa E, Woldemeskel M, Rege JEO (2003) Risk factors for mortality associated with respiratory disease among menz and Horro sheep in Ethiopia. The Veterinary Journal 165: 276-287.
- IqbalZ, Akhtar M, Khan MN, Riaz M (1993) Prevalence and economic significance of haemonchosis in sheep and goats slaughtered at Faisalabad abattoir. Significance of haemonchosis in sheep and goats slaughtered at Faisalabad abattoir. Pakistan J Agric Sci 30: 51-53.
- Urquhart GM, Armour J, Dunca JL, Dunn AM, Jennings FW (1997) Veterinary Parasitology, (2nd edn), Blackwell Science Ltd. London, UK
- Soulsby EJ (1982) Helminthes Arthropds and Protozoa of Domesticated Animals. (7th edn), Bailliere, Tindall and Cassell, London, UK.
- Hassan DI, Musa-Azara IS Mohammed J, Zanwa IA (2013) Influence of Age, Sex and Season on Haematology and Serum Chemistry of Red Sokoto Goats in Lafia, Nasarawa State Nigeria. Int J Agric Sc and Vet Med 1: 4.
- Singh H, Rai HS, Singh NK, Kaur A (2002) Prevalence of helminthic infection in sheep in Ludhiana. J Vet Parasitol 19(2): 97- 101.
- Troncy PM (1989) Helminthes of livestock and poultry in tropical veterinary parasitology .UK: CAB International, TCTA, 11(15): 24-54.
- Gillian S, Behnke JM, Buttle DJ, Duce LR (2004) Natural plant cysteine proteinases as anthelmintics? TRENDS in Parasitology. 20: 322-327.
- Hale M (2006) Managing internal parasites in sheep and goats.
- Fikru R, Teshale S, Dhuguma R, Kiros Y (2006) Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. International Journal Applied Research for Veterinary Medicine. 4: 51- 57.
- Jasmer DP, Goverse A, Smant G (2003) Parasitic nematode interactions with mammals and plans. Annual Review of Phytopathology. 41: 245- 270.
- Jacobs RD, Hogsette JA, Butcher GD (2003) Nematode parasites of poultry (and where to find them). Institute of Food and Agricultural Sciences, University of Florida, USA.
- Sissay MM, Uggla A, Waller PJ (2007) Epidemiology and seasonal dynamics of gastrointestinal nematode infections of sheep in a semiarid region of eastern Ethiopia. Veterinary Parasitology 143: 311-321.
- Coffey L, Hale M, Terrill TH, Mosjidis JA, Miller JE, et al. (2007) Tools for managing internal parasites in small ruminants: Sericea lespedeza
- Radostitis OMS, Gay KW, constable RD (2007) Helmenths of digestive tract. In: A textbook of the disease of cattle, sheep, horse, pig and goat’s veterinary medication (10thedn), sounders Elseveir, London, UK, pp. 1378-1383.
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW (1996) Veterinary Parasitology, (2nd edn), Blackwell Science, United Kingdom, pp. 307.
- Zajac AM, Conoboy GA (2006) Veterinary clinical parasitology (7th edn), Blackwell, USA.
- Radostits M, Gay C, Hinchcliff W, Constable D (2006) Nematode diseases of the alimentary tract. In: Veterinary Medicine, A textbook of the diseases of cattle, horses, sheep, pigs and goats, (10th edn), pp. 1541-1553.
- O’Connor L, Walkden-Brown S, Kahn L (2006) Ecology of the freeliving stages of major trichostrongylid parasites of sheep. Veterinary Parasitology 142: 1-15.
- Kassai T (1999) Veterinary Helminthology. Butterworth-Heinemann. Reed Educational and Professional Publishing Ltd, Oxford, USA pp. 260.
- Van Wyk J, Cabaret J, Michael L (2004) Morphological identification of nematode larvae of small ruminants and cattle simplified. Veterinary Parasitology 119: 277-306.
- Van Wyk J, Cabaret J, Michael L (2004) Morphological identification of nematode larvae of small ruminants and cattle simplified. Veterinary Parasitology 119: 277-306.
- Bowman DD (1999) Georgis parasitology for Veterinarian’s (7th edn), USA: W.B. Saunders company, pp. 144-220.
- Adugna G (1990) Black Head Ogaden sheep under traditional management practices in south-eastern Ethiopia. In: Rey B, Lebbie SHB, Reynolds L (Eds.), Small Ruminant Research and Development in Africa. Proceedings of the first Biennial Conference of the African Small Ruminant Research Network, p. 10-14.
- Mickael R, Fabrice G, Le Vern Y, Kerboeuf D (2003) Modulation of multidrug resistance (MDR) system the nematode Haemonchus contortus by changing cholesterol content: effects on resistace to anthelmitics. Journal of Antimicrobial Chemotherapy. 52: 180-187.
- Kohler P (2001) The biochemical basis of anthelmintic action and resistance. International Journal for Parasitology. 31: 336-345.
- Upadhayay AK (2005) Text book of preventive veterinary medicine as per VCL syllables International book distribution Co, India, Pp: 387- 390.
- Bentounsi B, Trad R, Gauz N, Kohil K, Cabaret J (2006) Gastrointestinal nematode J Cabaret. Gastrointestinal nematode resistance to benzimidazols in a sheep. Veterinary Record, 158: 634-635.
- Jackson F, Waller P (2008) Managing refugia. Tropical Biomedicine, 25: 34-40.
- Afak M (2003) Parasite control practices and anthelmintic resistance against gastrointestinal nematodes of sheep. Ph.D. Thesis, University of Agriculture, Department of Veterinary Parasitology, Faisalabad, Pakistan.
- Rahmann G, Seip H (2006) Alternative strategies to prevent and control endoparasite diseases in organic sheep and goat farming p. 49-90.
- Hansen JW, Perry BD (1994) The epidemiology, diagnosis and control of helminth parasites of ruminants. A hand book, (2nd edn), ILRAD, Nairobi, Kenya p: 1–79.
- Demelash B, Yilma J, Hassen C (2006) Ovine Helminthosis is Major Health Constraints to Productivity of Sheep in Ethiopia. Faculty of Veterinary Medicine, Awassa University, Awassa, Ethiopia.
- Diriba L, Birhanu A (2013) Prevalence of ovine gastrointestinal nematodes in and around Asella, South Eastern Ethiopia. J Vet Med Anim Health 5: 222-228.
- Tesfaye H (1998) Ovine and bovine helminthiasis in Kelela, South Wollo. In: Proceedings of EVA conference, Addis Ababa, Ethiopia, p. 30.
- Abebe W, Eseyas G (2001) Survey of ovine and Caprine gastrointestinal Helminthosis in eastern part of Ethiopia during the dry season of the year. Revue Med Vet 152: 379-384.
- Assefa D, Sissay L (1998) Preliminary investigation on the seasonal occurrence of parasites around Sheno. In: 5th national conference of society of animal production ESAP, Addis Ababa Ethiopia, pp: 123-137.
- Regassa F, Teshale S, Reta D, Yosef K (2006) Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. Int J Appl Res Vet Med 4(1): 51-57.
- Nigatu K (2008) Gastrointestinal Helminthosis of Sheep in Awi Zone, northwestern Ethiopia. Global Veterinaria, 12: 121-129.
- Keyyu D, Kyvsaard N, Monrad J, Kassuku A (2005) Epidemiology of gastrointestinal nematodes in cattle on traditional, small-scale dairy and large-scale dairy farms in Iringa district, Tanzania. Vet Parasitol 127: 285-294.
- Negasi W, Bogale B, Chanie M (2012) Helminth parasites in small ruminants: prevalence, species composition and associated risk factors in and Around Mekelle Town, Northern Ethiopia. Europ. J Biol Sci 4 (3): 91-95.
- Gonfa S, Basaznew B, Achenef M (2013) An Abattoir Survey on Gastrointestinal Nematodes in Sheep and Goats in Hemex-Export Abattoir, Bishoftu (Debre Zeit), Central Ethiopia. J Adv Vet Res 3: 60-63.
- Odoi A, Gathuma M, Gachuiri K, Omore A (2007) Risk factors of gastrointestinal nematode parasite infections in small ruminants kept in smallholder mixed farms in Kenya. BMC Vet Res 3(6): 1186-1746.
- Knox R, Torres-Acosta F, Aguilar-Caballero J (2006) Exploiting the effect of dietary supplementation of small ruminants on resilience and resistance against gastrointestinal nematodes. Vet Parasitol 139(4): 385-393.
- Waruiru RM, Mutune MN, Otieno RO (2005) Gastrointestinal parasite infections of sheep and goats in a semi-arid area of Machakos District, Kenya. Bull Anim Health Prod Afr 53(1): 25-34.
- Fritsch T, Kaufmann J, Ptister K (1993) Parasite spectrum and seasonal epidemiology of gastrointestinal nematodes of small ruminants in Gambia. Vet Parasitol 49(2-4): 271-283
- Shah-Fischer M and Say R (1989) Manual of Tropical Veterinary Parasitology. CAB International. The Technical Center for Agricultural and Rural Cooperation (CTA).
- Raza MA, Iqbal Z, Jabbar A, Yaseen M (2007) Point prevalence of gastrointestinal helminthiasis in ruminants in southern Punjab. Pakistan. J Helminthol 81: 323-328.
- Lemma, Abera (2013) Prevalence of ovine gastrointestinal nematodes in and around Asella, South Eastern Ethiopia. J Vet Med Anim Health 5(8): 222-228.
- Coles G (2005) Anthelmintic resistance - looking to the future: a uk perspective. Research in Veterinary Science 78: 99-108.
- Dagnachew S, Amamute A, Temegen W (2011) Epidemiology of gastrointestinal helminthiasis ofsmall ruminants in selected sites of North Gondarzone, Northwest Ethiopia. Ethiop Vet J 15(2): 57-68.
- European Medicines Agency, Veterinary Medicines and inspections (2006) Guidline on the SPC for anthelimintics. Doc.Ref.Emea/Cvmp/ Ewp/170208/2005- Consultation. London, UK
- Kaplan RM (2004) Drug resistance in nematodes of veterinary importance: a status report. TRENDS in Parasitology 20: 477- 481.
- Prichard RK (2005) Is anthelmintic resistance a concern for heartworm control? What can we learn from the human filariasis control programs? Veterinary Parasitology. 133: 243-253.
- Wolstenholme A, Fairweather L, Prichard RK, Samson-Himmelstjerna GV, Sangster N (2004) Drug resistance in veterinary helminths. Trends in Parasitology 20: 469-476.






























