Social Status Impacts Macrophage Function of Pigs
Salak Johnson JL and Shott JP*
Department of Animal Sciences, University of Illinois, USA
Submission: March 15, 2017; Published: May 5, 2017
*Corresponding author: Salak Johnson JL , Department of Animal Sciences, University of Illinois, 1207 W Gregory, 392 ASL, Urbana, IL 61801, USA, Tel: 217-333-0069; Fax: 217-333-8286; Email: johnso17@illinois.edu
How to cite this article: Salak Johnson JL , Shott J.Social Status Impacts Macrophage Function of Pigs. Dairy and Vet Sci J. 2017; 2(3): 555590. DOI: 10.19080/JDVS.2017.02.555590
Abstract
Pigs experience various stressful events including weaning, mixing, and crowding which can negatively affect well-being. These stressors may impair immune defenses and may contribute partly to disease outcome. Therefore, the aim of this study was to evaluate the effects of social status on macrophage function of pigs subjected simultaneously to cold and crowd stressors for four days. At 6-weeks-of-age, 3 unfamiliar white cross females were mixed and assigned to temperature stressor of 20°C (TNT) or 8°C (COLD) and to space stress or of 0.45m2/pig (CONT) or 0.26m2/pig (CROWD) over 6 blocks (n=72). Pigs were identified as dominant (DOM), intermediate (INT), or submissive (SUB) based on aggressive encounters over a 24-h period post-mixing. On day 4 post-treatment, pigs were sacrificed and alveolar macrophages (AMO) were isolated. Both descriptive and functional aspects were measured. Pig AMO phagocytosis was less for DOM-COLD compared to INT- or SUBCOLD pigs and less when compared to their counterparts at TNT (P<0.05; statusxCOLD). Chemotaxis was less in COLD-stressed pigs (P<0.05) and Rantes concentrations was in CROWD-pigs (P=0.06). Most effects on AMO function were due to social status of the pig. DOM-pigs had greater AMO5 sub population and Rantes than SUB-pigs. SUB-pigs had greater AMO [1,2] sub population and phagocytosis than DOM-pigs. All other AMO measures were greater among DOM-pigs and INT-pigs were similar to either DOM or SUB pigs. These results show that pig social status plays a major role in immune responsiveness in terms of macrophage function among pigs that were exposed to simultaneous cold and crowded stressors for four continuous more than the stressors alone. Pig social status influenced macrophage responsiveness regardless of stressor, thus implying that social status is an important metric to consider.
Keywords: Macrophages; Pigs; Stress; Social status
Introduction
Pigs experience various stressful events including weaning, mixing, and crowding stressors which can negatively affect well-being. These stressors may impair immune defenses that potentially increases disease susceptibility [1]. Often, stress can exacerbate opportunistic pathogenic challenges making a pig more susceptible to disease, especially respiratory disease. However, stress does not always suppress immune function and cause disease, which is partially explained by type and duration of stressor, aspect of immune system assessed, as well as social status [2]. Consequences of acute and chronic stress on immunity have been poorly documented in pigs. Moreover, data are limited on the effects of stress on alveolar macrophages especially in response to concurrent stressors such as cold and crowding stressors.
Macrophages are first line of defense again invading pathogens where they act as effectors of the immune response and belong to group of antigen presenting cells [3]. Macrophages play a crucial role in attracting and activating effector cells of the innate and adaptive immune system. Alveolar macrophages play a vital role in mitigating respiratory challenges and stress can affect the immune responsiveness of these cells. For example, macrophage function was suppressed and apoptosis increased among cold-stressed rodents [4,5]. While, tumor necrosis factor-α (TNF-α) produced by lipopolysaccharide (LPS)- stimulated macrophages was reduced among heat-stressed pigs [6]. Moreover, social status can affect the biological response initiated by a pig as it attempts to cope with some stressors [7,8]. Thus, the objective of this was to determine the effects of social status on alveolar macrophage function of pigs subjected simultaneously to cold and crowd stressors for four days in attempt to better understand the impact of stress responsiveness of pigs on disease susceptibility.
Material and Methods
Animals, housing, and experimental design
All experimental procedures were approved by the University of Illinois Institutional Animal Care and Use Committee. Six- week-old female LandracexYorkshire crossbred pigs (n=72) from the University of Illinois Swine Research Center were used in this study. For one-week, four female littermates were housed in pens with ample floor-space allowance (0.42m2/pig) within an environmentally-controlled chamber kept at an ambient temperature of 20±2°C. During the one-week acclimation period, pigs were fed ad libi tuma diet formulated to meet or exceed recommended nutrient allowances for young pigs (NRC, 2001) and water. Following the one-week adjustment period, one pig from each litter, matched for BW, was assigned to temperature treatment of either 20°C (thermoneutral; TNT) or 8°C (COLD) and to floor-space treatment of either 0.45m2/pig (adequate; CONT)or 0.26m2/pig (reduced; CROWD)for 4 days. During the 4 day treatment period pigs were offered a fixed amount of feed which was determined based on their daily feed intake during the one-week adjustment period.
Behavioral data collection
All pigs within the pen were uniquely marked with a livestock marking crayon (La-Co Industries, Elk Grove Village, IL). To determine social status, pigs were video-recorded for 24h postmixing and aggressive interactions were registered from videorecords (30frames/s). Aggressive and submissive behaviors were identified based on previously described ethogram [9]. Pigs were identified as dominant (DOM), intermediate (INT), or submissive (SUB) based on outcome of each agnostic encounter. Essentially, DOM pigs won all agnostic encounters in which they were engaged, whereas INT pigs lost one or more fights to the DOM pig. A SUB pig was identified based primarily on submissive postures and behaviors (i.e. avoidance) toward other pigs in the pen.
Pulmonary macrophage isolation and cytopins
On day 4 post-treatment, pigs were euthanized and porcine alveolar macrophages (AMO) were obtained via broncho alveolar lavage. Briefly, lungs were removed and lavaged by adding and removing sterile Hanks Balanced Salt Solution (HBSS; Gibco, Ca) three times. Lavage fluid was filtered through sterile gauze and centrifuged at 460xg for 15min, cell pellets were washed twice in HBSS, re suspended in 20mL of RPMI, counted, and cell concentrations were adjusted accordingly to assay protocol.
Cytospins were made by adding 50μl of 1:1000 dilutions of AMO, fixed and stained with Hema-3 staining system (Fisher Scientific, Houston, TX), and then 100 cells per slide were visually counted under a light microscope. The different AMO subpopulations were classified based on morphology and staining pattern as described [10,11], with minor modifications [12]. Visual morphology of cell cytoplasm and nucleus was used to qualify a particular cell into the appropriate subpopulation classification. Subpopulations 1 and 2, as well as 3 and 4, were combined as it was too difficult to differentiate between these adjacent subpopulations using light microscopy, thus final subpopulations were classified as AMO1,2; AMO3,4; and AMO5. A technician having no knowledge of animal treatments performed all cell counts.
Macrophage phagocytosis and chemotaxis
Macrophage phagocytosis was measured using a flow- cytometry-based assay [13] with minor modifications [12]. Briefly, porcine AMO were adjusted to a cell concentration of 2x106, and fluorescent beads (yellow-green, 1.0|im; Molecular Probes, Eugene, OR) were added to each sample at 10:1 (beads- to-macrophage) ratio, incubated for 40min at 37°C on a rotator plate, and then centrifuged for 5min at 1000xg. Samples were washed once in RPMI to remove non-engulfed beads, fixed in 4% paraformaldehyde, and held at 4°C until analysis. Percent fluorescence was measured using an XL flow cytometer (Beckman Coulter, Miami, FL). Data were transformed logarithmically and results expressed as total percentages of macrophages engulfing one or more beads.
The ability of cells to randomly migrate (media; control) or directly migrate (chemotaxis) toward chemokines recombinant human complement-5a (rhC5a; Sigma) and monocyte chemotactic protein-1 (rhMCP-1; R & D Systems, Minneapolis, MN) were measured using an assay adapted after [14]. Macrophages were adjusted to cell concentration of 3x106 cells/mL. The cells and the chemo attractants were separated by a polyvinylpyrrolidone-free filter with pore sizes 5μM (Neuro Probe, Cabin John, MD). Cell chambers were incubated for 1h at 37°C and 5% CO2 in a humidified incubator. A technician having no knowledge of treatments counted four fields per well via light microscopy. Homology between porcine and human chemokines and receptors, human reagents and kits were suitable for these assays per the manufacturers. At the time this study was conducted porcine reagents were not available.
Chemokine assays
Alveolar macrophage-produced chemokines were measured by enzyme linked immunosorbent assay (ELISA) and included RANTES and monocyte chemotactic protein-1 (MCP-1). RANTES is chemotactic for pro-inflammatory T-cells and monocytes and serves a central role in recruiting immune cells to infection with the help of T-cell-producedIL-2 and IFN-γ, whereasMCP-1 recruits monocytes and dendritic cells to the site of infection to broaden the innate repertoire. Concentrations of RANTES and MCP-1 from TNF-α stimulated PAM supernatants were measured using commercially available human ELISA kits (Quantikine; R&D Systems, Minneapolis, MN). Porcine AMO were diluted to 3 x107cells/mL in RPMI plus 5% FBS and seeded into 6-well plates. Plates were incubated overnight at 37°C in a humidified incubator, washed three times, then stimulated 24h with 100ng/ mL of human rTNF-α (R&D Systems). Minimal detectable concentrations were 8pg/mL for RANTES and 5pg/mL for MCP- 1, respectively.
Statistical analysis
All traits were tested for departures from normal distribution using the Shapiro-Wilk test. Data lacking normality were transformed logarithmically using log10. Minimum values for PAM subpopulations, Rantes, and MCP-1were zero, so in these cases a value smaller than the lowest non-zero number was added to all observations to allow the logarithmic transformation. A linear mixed-effects model was used to analyze all variables using the MIXED procedure of SAS (SAS Inst, Cary, NC). The main effects were temperature (two levels), space (two levels), and social rank (3 levels) and all interactions. Random effects of chamber and replicate were included. Residuals were tested for departures from assumptions.
Results
Interactive effects of stressors and social status
No significant temperaturexspaceinteractions occurred for any porcine alveolar macrophage measures assessed in this study at day 4 post-treatment. There was a significant temperaturexsocial status interaction for pAM phagocytosis, whereby SUB pigs had greater(P>0.05) phagocytosis than did either INT or DOM pigs (Figure 1).
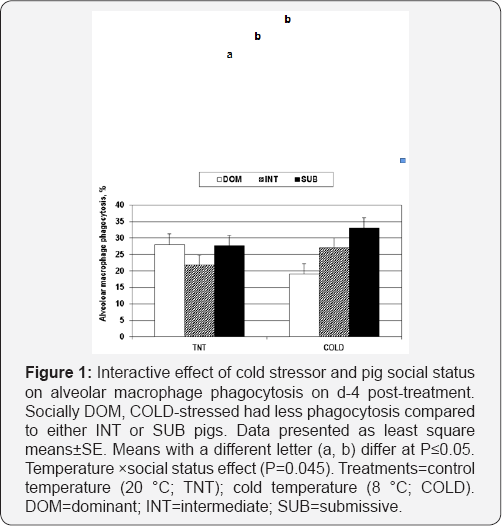
Social status

a,bMeans with uncommon superscripts within treatment are different at p<0.05.
AMO=Alveolar macrophages. AMO1,2=subpopulations 1 and 2 combined. AMO3,4=subpopulations 3 and 4 combined. AMO5=subpopulation 5. C5a=human complement-5a. MCP-1=human monocyte chemotactic protein-1
DOM = dominant INT=Intermediate SUB=submissive.
Presented in (Table 1) are the effects of pig social status on alveolar macrophages measures assessed at d 4 post-treatment. Socially SUB pigs had lower percentage of subpopulation pAM5 and reduced chemotaxis and RANTES concentrations compared to DOM pigs, with the exception of chemotaxis in response to C5a (Table 1). While, socially DOM pigs, had reduce percentages of subpopulation pAM1,2 and phagocytosis compared to SUB pigs. Socially, INT pigs had increased chemotaxis in response to C5a compared to either DOM or SUB pigs.
Temperature or space stressors
Presented in (Table 2) are the effects of cold or crowded stressors on alveolar macrophage measures assessed at d 4 post-treatment, COLD-pigs had reduced (P<0.05) chemotaxis in response to both C5a and MCP-1 (Table 2) compared to pigs at TNT. Rantes concentration tended to be reduced in CROWD-pigs compared to CTL-pigs (Table 2).

a,bMeans with uncommon superscripts within treatment are different at p<0.05
AMO=Alveolar macrophage. AMO1,2=Alveolar macrophage subpopulations 1 and 2 combined. AMO3,4= Alveolar macrophage subpopulations 3 and 4 combined. AMo5=Alveolar macrophage subpopulation 5. C5a= complement-5a. MCP-1=monocyte chemotactic protein.
Discussion
This study was designed to test the hypothesis that social status and concurrent exposure of growing pigs to cold and crowding stressors would hinder macrophage function, thereby making pigs more susceptible to respiratory disease. Macrophages are the first line of defense against invading pathogens, where they act as effectors of the immune response and belong to group of antigen presenting cells [3]. Macrophages play a crucial role in attracting and activating effector cells of the innate and adaptive immune systems. In general, there were no interactive effects of the stressors imposed within, except for reduced AMO chemotaxis in cold-stressed pigs and reduced Rantes concentration in crowded-pigs when compared to their control counterparts. The limited effects of these stressors on various macrophage measures are similar to reports by others.
Acute cold or heat stress had no effect on neutrophil phagocytosis [9] and chronic heat stress had no effect on macrophage phagocytosis, but pigs challenged with a virus had reduced macrophage phagocytosis [2]. We speculate that the limited effects of these stressors on various macrophage measures may be partly explained by types and durations of stressors and time point at which macrophage function was assessed. Moreover, it is plausible that the pigs were able to mitigate cold stress via behavioral means and that crowded stress negated the cold stress effects. Regardless, these data imply that cold and crowd stressors did not interactively or independently per se alter macrophage responsiveness of pigs, but social status greatly influenced immune responsiveness.
Stress effects on the immune system has been shown to depend on social status of pigs. Immune responses to social stressors vary based on each animal's perception and response to the stressor. Among stressed pigs, dominant pigs display greater natural killer cell cytotoxicity [7,15], lymphocyte proliferation response [16-18], and higher baseline antibody titers [16] compared with lower ranked counterparts. Among virally-infected pigs, submissive pigs had greater numbers of macrophages and subpopulation 5 and dominant pigs had lower natural killer cell cytotoxicity [19]. In this study socially submissive cold-stressed pigs had greater macrophage phagocytosis and dominant pigs had reduced, but for the most part social status had greater impact on all other macrophage measures assessed within and those data imply that dominant pigs were more immune responsive. Dominant pigs had greater RANTES production from TNF-α-stimulated macrophages. We speculate that because Rantes is a T-helper 1-associated chemokine known to recruit effector T-cells to the site of infection [20] and MCP-1 is a T-helper 2-associated chemokine known to suppress pro-inflammatory T-helper 1 cytokines was not affected especially among dominant pigs that these pigs were skewed toward a moral robust viral challenge. But, dominant pigs had greater percentages of macrophages in sub population 5 (least activated, immature cells)and less of subpopulation 1,2 (most activated, mature) and reduced phagocytosis while submissive pigs had the opposite response for these same measures which partly implies that they may be at risk if the shift in subpopulation was due to apoptosis of resident macrophages [12] found that a subpopulation 5 increased in pigs that were challenged with porcine respiratory reproductive virus due to apoptosis of mature resident macrophages. Interestingly, they also found that subpopulation 5, total macrophage numbers, and natural killer cell cytotoxicity all increased among submissive pigs that were infected with porcine respiratory reproductive virus compared to dominant pigs, yet all pigs cleared the infection without negative consequences [21-32].
It is apparent the pig social status does influence differential macrophage profile found within. For the most part, submissive had reduced macrophage measures compared to dominant pigs or the opposite response, while intermediate pigs were similar to either dominant or submissive or were the opposite of both. Based on these findings, one would speculate that dominant and intermediate pigs would have more activated immune response than submissive pigs, thus be less susceptible to disease, however these data do not imply immune suppression since neither stressor suppressed any immune measures.
Interestingly, pig social status differentially affected various macrophage measures more so than the stressors. Moreover, these findings support the theory that type and duration of stressor, aspect of the immune system measured and time points are important factors that can impact the effect of stress on the immune response. Taken together, pig social status may be the most important factor that influence the innate immune response than these afore mention stressors themselves, since social status greatly impacted the responsiveness of alveolar macrophages.
Acknowledgment
This research was supported by the Illinois Agricultural Experiment Station. The authors thank Rodriguez-Zas SL for statistical assistance. Shott J is a former University of Illinois student that is currently supported by the Division of Intramural Research, NIAID, NIH.
References
- Hermann G, Tovar CA, Beck FM, Allen C, Sheridan JF (1993) Restraint stress differentially affects the pathogenesis of an experimental influenza viral infection in three inbred strains of mice. J Neuro immunol 47(1): 83-93.
- Salak-Johnson JL, McGlone JJ (2007).
- Geissman F, Manz MG, Jung S, Sieweke MH, Merad M, et al. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327: 656-661.
- Kizaki T, Ookawara T, Izawa T, Nagasawa J, Haga S, et al. (1997) Relationship between cold tolerance and generation of suppressor macrophages during acute cold stress. J Appl Physiol 83(4): 11161122.
- Kizkai T, Suzuki K, Hitomi Y, Iwabuchi K, Onoe' K, et al. (2001) Activation and apoptosis of murine peritoneal macrophages by acute cold stress. Biochem Biophys Res Commun 283(3): 700-706.
- Klir JJ, Shahbazian LM, Matteri RL, Fritsche KL, Becker BA (1997) Effects of thermal environment on response to acute peripheral lipopolysaccharide challenge exposure in neonatal pigs. Am J Vet Res 58(4): 364-369.
- McGlone JJ, Salak JL, Lumpkin EA, Nicholson RI, Gibson M, et al. (1993) Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J Anim Sci 71(4): 888-896.
- Hicks TA, McGlone JJ, Whisnant CS, Kattesh HG, Norman RL (1998) Behavioral, endocrine, immune, and performance measures for pigs exposed to acute stress. J Anim Sci 76(2): 474-483.
- McGlone JJ (1985) A quantitative ethogram of aggressive and submissive behaviors in recently regrouped pigs. J Anim Sci 61(3): 559-565.
- Shellito J, Kaltreider HB (1984) Heterogeneity of immunologic function among subfractions in normal alveolar macrophages. Am Rev Respir Dis 129(5): 747-753.
- Choi C, Gustafson K, Chinsakchai S, Hill H, Molitor T (1994) Heterogeneity of porcinealveolar macrophage subpopulations: immune functions susceptibility to PEARS virus. Proc 13th Int Pig Vet Congress Bangkok, Thailand, p. 97.
- Sutherland MA, Niekamp SR, Johnson RW, Van Alstine WG, Salak- Johnson JL(2007) Heat and social rank impact behavior and physiology of PRRS-virus-infected pigs. Physiol Behav 90(1): 73-81.
- Jolie R, Backstrom L, Olson L, Chase C (1997) Respiratory and systemic health parameters in pigs raised in a conventional farm or in isolation. Swine Health Prod 7(6): 269-275.
- Salak JL, McGlone JJ, Lyte M (1993) Effects ofinvitro adrenocorticotrophic hormone, cortisol, and human recombinant interleukin-2 on procine neutrophil migration and luminal-dependent chemiluminescence. Veterinarty Immunology and Immunopathol 39(4): 327-337.
- Sutherland MA, Niekamp SR, Rodriquez-Za SL, Salak-Johnson JL (2006) Impacts of chronic stress and social status on various physiological and performance measures in pigs of different breeds. J Anim Sci 84(3): 588-596.
- Rudine AC, Sutherland MA, Hulber L, Morrow JL, McGlone JJ (2007) Diverse production system and social status effects on pig immunity and behavior. Livestock Science 111: 86-95.
- DeGroot J, Ruis MAW, Scholten JW, Koolhaas JM, Boersma WJA (2001) Long-term effects of social strss on antiviral immunity in pigs. Physiol Behav 73(1-2): 145-158.
- Tuchscherer M, Puppe B, Tuchscherer A, Kanitz E (1998) Effects of social status after mixing on immune, metabolic, and endocrine responses in pigs. Physiol Behav 64(3): 353-360.
- Khanna KV, Choi CS, Gekker G, Peterson PK, Molitor TW (1996) Differential infection of porcine alveolar macrophage subpopulations by nonopsonized Mycobacterium bovis involves CD14 receptors. J Leukoc Biol 60(2): 214-220.
- Janway CA, Travers P, Walport M, Shlomchik M (2005) Immunobiology: The immune system in health and disease. 6th Edition. Garland Science, New York, USA.
- Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, et al. (2009) Discovery of swine as a host for the Reston ebolavirus. Science 325(5937): 204-206.
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, et al. (2000) Nipah virus: a recently emergent deadly paramyxovirus. Science 288(5470): 1432-1435.
- Hicks TA, McGlone JJ, Whisnant CS, Kattesh HG, Norman RL (1998) Behavioral, endocrine, immune, and performance measures for pigs exposed to acute stress. J Anim Sci 76(2): 474-483.
- Holyoake PK, Kirkland PD, Davis RJ, Arzey KE, Watson J, et al. (2011) The first identified case of pandemic H1N1 influenza in pigs in Australia. Aust Vet J 89(11): 427-431.
- Kizaki TS, Oh-Ishi S, Ohno H (1996) Acute cold stress induces suppressor macrophages in mice. J Appl Physiol 81(1): 393-399.
- Kizaki T, Ookawara T, Izawa T, Nagasawa J, Haga S, et al. (1997) Relationship between cold tolerance and generation of suppressor macrophages during acute cold stress. J Appl Physiol 83(4): 11161122.
- Kizkai T, Suzuki K, Hitomi Y, Iwabuchi K , Onoe'K, et al. (2001) Activation and apoptosis of murine peritoneal macrophages by acute cold stress. Biochem Biophys Res Commun 283(3): 700-706.
- McGlone JJ (1985) A quantitative ethogram of aggressive and submissive behaviors in recently regrouped pigs. J Anim Sci 61(3): 559-565.
- McGlone JJ, SalakJ L, Lumpkin EA, Nicholson RI, Gibson M, et al. (1993) Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J Anim Sci 71(4): 888-896.
- Morrow-Tesch JL, McGlone JJ, Salak-Johnson JL (1994) Heat and social stress effects on pig immune measures. J Anim Sci 72(10): 2599-2609.
- Verbrugghe E, Boyen F, Van Parys A, Van Duen K, Croubels S, et al. (2011) Stress induced Salmonella Typhimurium recrudescence in pigs coincides with cortisol indiced intracellular proliferation in macrophages. Vet Res 42(1): 118.
- Wibawa ID, Suryadarma IG, Tsuda FM, Matsumoto Y, Ninomiya M, et al. (2007) Identification of genotype 4 hepatitis. E virus strains from a patient with acute hepatitis E and farm pigs in Bali, Indonesia. J Med Virol 79(8): 1138-1146.






























