The Wheat Operation: Role of 5 mm in Postoperative Aortic Dilatation
Francesca D’Auria1*, Vincenzo Consalvo1, Antonio Nenna1, Marco Bega1, Salvatore Matteo Greco1, Mario Lusini1, Aung Myat2, Uday Trivedi2, David Hildick Smith2, Massimo Chello1 and Elvio Covino1
1 Cardiac Surgery Department, Medico University of Rome, Italy
2Cardiac Center of the Brighton and Sussex University Hospital of Brighton, UK
Submission: May 29, 2018; Published: June 21, 2018
*Corresponding author: Francesca D’Auria, Cardiac Surgery Department, Medico University of Rome, Via Alvaro del Portillo n. 200, 00128 Rome, Italy.
How to cite this article: Francesca D, Vincenzo C, Antonio N, Marco B, Salvatore M G. et al The Wheat Operation: Role of 5 mm in Postoperative Aortic Dilatation. J Anest & Inten Care Med. 2018; 7(1): 555701. DOI: 10.19080/JAICM.2018.07.555701
Abstract
Aim: The Aortic Root (AR) dilatation after Wheat Operation (WO) is an important issue to consider in the long-term Follow-Up (FU). This study aims to identify possible predictor factors involved in this late complication.
Material and Method: We retrospectively analyzed a 216 consecutive patients (150 males and 66 females) cohort who underwent WO from June 2009 to April 2018 in the Cardiac Surgery of the Policlinico Campus Bio -Medico of Rome, Italy, and in the Cardiac Center of the Brighton and Sussex University Hospital of Brighton, United Kingdom. The mean echocardiographic (TTE) FU was 44.9 ± 22.2 months. The increase of 10% in the size of the aortic root compared to the pre-operative baseline was the outcome variable. Statistical analysis used 1) the Student’s t - test or chi - square test for the comparisons between variables, 2) Kaplan – Meier’s analysis for the survival curves, and 3) the Cox’s regression models, via methodology backward stepwise after evaluation of the exploratory variables, for the assessment of the predictive value of the variables over the time. A p-value less than 0.05 was significant.
Results: No significant differences among patients underwent aortic valve repair and patients underwent aortic valve replacement (log rank = 0.917). In the group of patients underwent valve replacement, the dilation of the AR it was associated to the difference between the diameter of the prosthetic valve and the diameter of the straight vascular prosthesis (OR 0.87, P = 0.024). Based on the difference in diameter between vascular and valve prosthesis we have organized two groups. The small group (S) (n = 52), if the difference was ≤ 5 mm, and the large group (L) (n = 34) if the difference was > 5 mm. Using this grouping variable, a significant dilatation of the AR was observed in 30.8 % of the S and in the 14.7 % of the L (log rank = 0.026). A difference of more than 5 mm between the aortic valve prosthesis and the vascular prosthesis was protective for the AR enlargement in the long-term FU (OR 12:31, P = 0.033), even after adjustment for age and sex (OR 00:32, P = 0.043).
Conclusion: In our series, the difference between the size of the aortic valve prosthesis and the vascular prosthesis less than or equal to 5 mm appears to be the only factor associated with increased risk in AR dilatation after WO in the long-term FU
Keywords: Wheat operation; Aortic root; Ascending aorta aneurysm; Ascending aorta replacement; Aortic valve replacement; Aortic valve repair
Introduction
The treatment of the Ascending Aorta Aneurysms (AAA) associated with concomitant aortic valve disease includes different surgical options. In case of anatomical preservation of the Sino-Tubular Junction (STJ), a straight vascular prosthesis replaces the aneurysmal portion of the ascending aorta, leaving unaltered the Aortic Root (AR) and the Coronary Ostia (CO), while the aortic valve undergoes repair or replacement. This surgical option is the Wheat Operation (WO) [1]. Although the WO arrests the progression of the AAA and the deterioration of the valve with concomitant benefit on cardiac function. The dilation of the AR is an important issue to consider in the long-term follow-up (FU) after WO. This further enlargement can indeed lead to 1) the AR rupture, 2) the narrowing of the CO due to the traction, and/or 3) the functional anomalies of the native aortic valve or of the aortic prosthetic valve [2]. This analysis aims to identify possible predictor factors involved in the AR dilatation after the WO.
Material and Methods
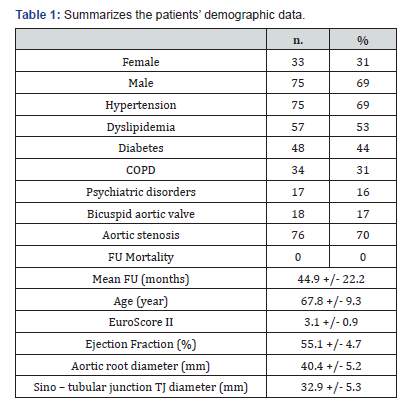
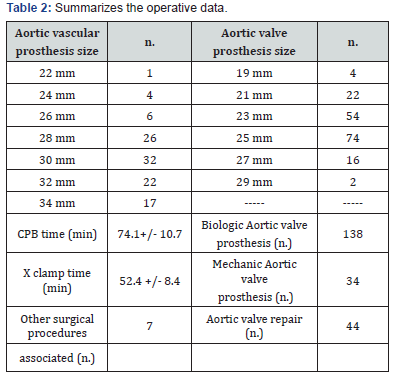
We retrospectively analyzed a 216 consecutive patients (66 females and 150 males) cohort who underwent WO from June 2009 to April 2018 in the Cardiac Surgery of the Policlinico Campus Bio -Medico of Rome. The mean echocardiographic (TTE) FU was 44.9 ± 22.2 months. Table 1 summarizes the patients’ demographic data. The increase of 10% in the size of the aortic root compared to the pre-operative baseline was the outcome variable we evaluated [3]. Statistical analysis used 1) the Student’s t - test or chi - square test for the comparisons between variables, 2) Kaplan – Meier’s analysis for the survival curves, and 3) the Cox’s regression models, via methodology backward stepwise after evaluation of the exploratory variables, for the assessment of the predictive value of the variables over the time. A p-value less than 0.05 was significant. The replacement of the aortic valve has been the treatment of choice in 172 patients, in 24 patients a commissuroplasty was performed, and in 8 patients pericardial patch valve plasty was used [4-6]. In 80% of the replacement a biological prosthetic valve have been used, in consideration of the patients age. In 6 patients it was performed coronary artery bypass grafting of one or more vessels, and one patient underwent concomitant mitral valve replacement. One patient underwent cardiac reoperation after 7 years after the first procedure for subsequent aortic valve stenosis (Table 2).
Results
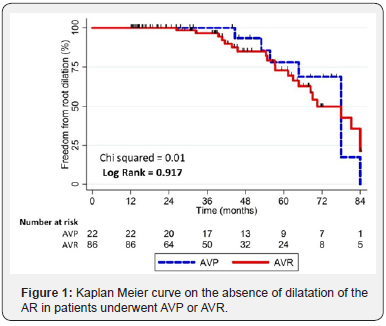
No significant differences among patients underwent aortic valve plasty and patients underwent aortic valve replacement (log rank = 0.917) were found (Figure 1). Dilation of the AR occurred in 16 of 44 patients in the AVP group (36.3%) and in 42 of 172 patients in AVR group (24.4 %). Cox’s regression analysis confirmed the no significance of this difference, since AVR showed an odds ratio 1.04 (95% CI 12:46 to 2:37) with P = 0.920.
In the group of patients underwent valve replacement, the AR dilatation was associated with the difference between the diameter of the prosthetic valve and the diameter of the straight vascular prosthesis (OR 0.87, P = 0.024) [7-10]. The mean difference between the prosthetic valve and vascular graft is 6.0 ± 2.4 mm. According to the difference in diameter between vascular and valve prosthesis we have organized two groups compared on the average value of this difference between prosthetic valve and vascular graft sizes [11]. The small group (S) (n. = 104 pts.), if the difference was ≤ 5 mm, and the large group (L) (n. = 68 pts.), if the difference was > 5 mm. At the end of the FU, a variation of the AR > 10 % compared to the preoperative size was observed in 58 patients (27 %) (Table 3).

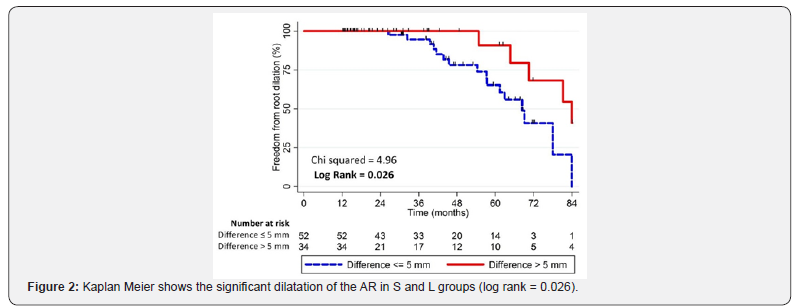
Using this grouping variable, a significant dilatation of the AR was observed in 30.8 % of the S and in the 14.7 % of the L (log rank = 0.026) as the Figure 2 shows. Cox’s regression quantified the predictive validity of this observation. This analysis showed that a difference of more than 5 mm between the aortic valve prosthesis and the vascular prosthesis is associated with a lower risk of residual AR enlargement in the long-term FU (OR 12:31, P = 0.033), even after adjustment for age and sex (OR 00:32, P = 0.043) [12-15]. The assumption of the proportional hazards was quantified using the Schoenfeld residuals test, showing a Chi square = 1.93 with 3 degrees of freedom, and it is associated with a P < 0.05. Graphics tests confirmed the validity of this analysis. These evaluations include the log-log plot as the Figure 3 shows, and the overlap between predicted and observed survival functions as the Figure 4 reports.

Discussion
The synthetic vascular grafts, including Dacron® or PTFE®, used as substitutes of the aortic wall in the WO, are widespread for many years for their ease of use, but do not reflect the biomechanical characteristics of the native vascular wall [16-20]. The compliance of the vascular prosthesis, defined as the increase in volume for increase of pressure units, is four times lower than the native aorta, and this concept of compliance mismatch between the graft and the native aorta generates blood flow anomalies with important clinical implications in the long-term period. The loss of elastic properties after replacement with vascular prostheses can cause retrograde effects, with involvement of the aortic root, the aortic valve (or prosthetic aortic valve), and the left ventricular [21].

Among the retrograde effects, the insertion of a vascular graft and the compliance mismatch determine significant changes in mechanical properties of the AR [22-25]. In the WO the aortic valve is replaced with a prosthesis which optimally responds to the fluid dynamic changes resulting from the replacement of the ascending aorta. Therefore, all retrograde shear stress loads on the AR, which is the only not prosthetic portion. In our single center experience, we observed that by using a difference of 5mm or more between the prosthetic valve and aortic vascular graft there is a smaller difference in diameter between the vascular graft and the AR [26]. This geometry determines a reduction in the dilatation of the AR in the long-term FU. On the contrary, in case between the valve and the graft there is a difference < 5 mm, the Valsalva sinus shows a more accentuated barrier effects [27]. The turbulent flow due to this barrier effects are the most plausible causes involved in the greater dilation of the AR, as the Figure 5 explains.
Conclusion
In our series, the difference between the size of the aortic valve prosthesis and the vascular prosthesis less than or equal to 5 mm appears to be the only factor associated with increased risk in AR dilatation after WO in the long-term FU. Further research, are needed to provide a more detailed explanation of this phenomena.
Disclosure
No conflict of interest declared.
References
- Anderson RH, Devine WA, Ho Sy, Smith A, McKay R (1991) The myth of the aortic anulus: the anatomy of the subaortic outflow tract. Ann Thorac Surg 52(3): 640-646.
- Sutton III John P, Ho Siew Yen, Anderson Robert H (1995) The forgotten interleaflet triangols: a review of the surgical anatomy of the aortic valve. Ann Thorac Surg 59(2): 419-427.
- Robicksek F (1991) Leonardo Da Vinci and the sinus of Valsalva. Ann Thorac Surg 52(2): 328-335.
- Brewer RJ, Deck JD, Capati B, Nolan SP (1976) The dynamic aortic root: its role in aortic valve function. J Thorac Cardiovasc Surg 72(3): 413- 417.
- Thubrikar M, Harry L, Nolan SP (1977) Normal aortic valve function in dogs. Am J Cardiol 40(4): 563-568.
- Dagum P, Green P, Nistal FJ, Daughters GT, Timek TA, et al. (1999) Deformational dynamics of aortic root: modes and phisyologic determinants. Circulation 100 (suppl II): 1154-1162.
- Bentall H, De Bono A (1968) A technique for complete replacement of the ascending aorta. Thorax 23(4): 338–339.
- Yacoub MH, Fagan A, Stassano P (1983) Results of valve conserving operations for aortic regurgitation. Circulation 68: 311-321.
- Birks EJ, Webb C, Child A, Radley Smith R, Yacoub MH (1999) Early and Long-Term Results of a Valve-Sparing Operation for Marfan Syndrome. Circulation 100(19): 29-35.
- David TE, Feindel M (1992) An aortic valve sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovas Surg 103(4): 617-622.
- Demers P, Miller DC (2004) Simple modification of “T. David-V” valvesparing aortic root replacement to create graft pseudosinuses. Ann Thorac Surg 78(4): 1479-1481.
- Miller DC (2003) Valve-sparing aortic root replacement in patients with the Marfan syndrome. J Thorac Cardiovasc Surg 125(4): 773-778.
- Wheat MW Jr, Wilson JR, Bartley TD (1964) Successful replacement of the entire ascending aorta and aortic valve. JAMA 188: 717–719.
- Gray DP, Ott DA, Cooley DA (1983) Surgical treatment of aneurysm of the ascending aorta with aortic insufficiency. J Thorac Cardiovasc Surg 86(6): 864-877.
- Watanabe G, Iwa T, Misaki T (1990) Surgical management of aneurysm of the ascending aorta with aortic insufficiency. Jpn J Cardiovasc Surg 38: 37-41.
- Tai NR, Salacinski HJ, Edwards A, Hamilton G, Seifalian AM (2000) Compliance properties of conduits used in vascular reconstruction. British Journal of Surgery 87(11): 1516-1524.
- Spadaccio C, Rainer A, Barbato R, Chello M, Meyns B (2013) The fate of large-diameter Dacron (R) vascular grafts in surgical practice: are we really satisfied? International Journal of Cardiology 168(5): 5028- 5029.
- Kelly RP, Tunin R, Kass DA (1992) Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circulation Research 71(3): 490-502.
- Morita S, Kuboyama I, Asou T, Tokunaga K, Nose Y (1991). The effect of extra anatomic bypass on aortic input impedance studied in open chest dogs. Should the vascular prosthesis be compliant to unload the left ventricle? Journal of Thoracic and Cardiovascular Surgery 102(5): 774-783.
- O’Rourke MF (2008) How stiffening of the aorta and elastic arteries leads to compromised coronary flow. Heart 94(6): 690- 691.
- Kim SY, Hinkamp TJ, Jacobs WR, Lichtenberg RC, Posniak H, et al. (1995) Effect of an inelastic aortic synthetic vascular graft on exercise hemodynamics. Annals of Thoracic Surgery 59(4): 981-989.
- De Paulis R, Matteis GM, Nardi P, Scaffa R, Bassano C, et al. (2002) Analysis of valve motion after the reimplantation type of valve-sparing procedure (David I) with a new aortic root conduit. Annals of Thoracic Surgery 74(1): 53-57.
- Robicsek F, Thubrikar MJ (1999) Role of sinus wall compli- ance in aortic leaflet function. The American Journal of Cardiology 84(8): 944- 946.
- Fokin AA, Robicsek F, Cook JW, Thubrikar MJ, Schaper J, et al. (2004) Morphological changes of the aortic valve leaflets in noncompliant aortic roots: in-vivo experiments. Journal of Heart Valve Disease 13(3): 444-451.
- Bellhouse BJ, Bellhouse FH (1968) Mechanism of closure of the aortic valve. Nature 217(5123): 86-87.
- De Paulis R, Tomai F, Bertoldo F, Ghini AS, Scaffa R, et al. (2004) Coronary flow characteristics after Bentall procedure with or without sinuses of Valsalva. European Journal of Cardio-Thoracic Surgery 26(1): 66-72.
- Bottio T, Buratto E, Dal Lin C, Lika A, Tarzia V, et al. (2012) Aortic valve hydrodynamics: considerations on the absence of sinuses of Valsalva. Journal of Heart Valve Disease 21(6): 718-723






























