Investigating the Expression of NMDA Receptors and Cognitive Function in Children with ADHD: A Comparative Study
Sajad Haghshenas1,2*, Mohammad Reza Zarrindast3, Mohammad Nasehi4, Soolmaz Khalifeh4 and Peyman Hasani-abharian1
1 Department of Cognitive Neuroscience, Institute for Cognitive Science Studies, Tehran, Iran
2 Shahid Beheshti University, Tehran, Iran
3 Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
4 Cognitive and Neuroscience Research Center (CNRC), Amir-Almomenin Hospital, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
Submission: July 20, 2023; Published: September 13, 2023
*Corresponding author: Sajad Haghshenas, Department of Cognitive Science Studies, Shahid Beheshti University, Iran, Email: Haghshenassjd@gmail.com
How to cite this article: Sajad H, Mohammad Reza Zarin d, Mohammad N, Peyman Hasani A, Soolmaz K. Investigating the Expression of NMDA Receptors and Cognitive Function in Children with ADHD: A Comparative Study. Glob J Intellect Dev Disabil. 2023; 12(4): 555841. DOI:10.19080/GJIDD.2023.12.555841
Abstract
Emerging evidence from clinical, genetic, and animal model studies suggests that N-methyl-D-aspartate (NMDA) glutamate receptors (NMDAR) may contribute to the pathophysiology and etiology of neurological and psychiatric disorders. Patients with impaired NMDA receptors often experience psychological symptoms. Therefore, we hypothesized that NMDAR receptors play a key role in the development of attention deficit hyperactivity disorder (ADHD). In this comparative analytical study, we utilized the Western blotting method to assay the expression levels of NMDA subunits NR1 and NR2 in the blood plasma of 50 male individuals diagnosed with ADHD, comparing them to 20 healthy controls. The findings from the Western blotting analysis provide support for the hypothesis that individuals with ADHD exhibit significantly lower levels of NR1/2 receptors compared to those without the disorder. Further research is needed to explore the potential causal relationship between reduced NR1/NR2 receptor levels and the development of ADHD.
Keywords: NMDA receptors; NR1; NR2; ADHD; Expression
Introduction
Structures
NMDA receptor is a heterotetramer consisting of two NR1 subunits, which bind to glycine, and two NR2 subunits, which bind to glutamate. The binding of both glycine and glutamate is required for the activation of the receptor and the subsequent opening of its ion channel. These receptors are further divided into four subtypes (GluN2A-D) [1]. Each subunit contains an amino-terminal domain, an agonist-binding domain, a transmembrane domain, and a carboxy-terminal domain [2,3]. The NR1 subunit contains the channel pore-forming domain, while the NR2 subunit contains the binding site for regulatory molecules such as Mg2+, Zn2+, and polyamines [1]. In addition, the carboxy-terminal domain of the NR2 subunit contains several protein-protein interaction domains that allow the receptor to interact with other intracellular proteins and signalling pathways [3]. The complex structure of the NMDA receptor allows for its regulation by a variety of factors, including post-translational modifications, and protein-protein interactions [4,5].
Function
NMDA receptors play an essential role in synaptic plasticity, learning and memory, as well as in the pathophysiology of various neurological and psychiatric disorders. Hansen et al. [6], discussed the role of these receptors in the induction of LTP and proposed a model of synaptic plasticity where the activation of NMDA receptors results in calcium ion influx and the subsequent activation of intracellular signalling pathways that strengthen synaptic connections. The diversity of NMDA receptor subunits allows for fine-tuning of synaptic transmission and dysfunction of NMDA receptors is implicated in various neurological and psychiatric disorders [1]. The association between NMDA receptor’s disfunction and cognitive state is well stablished. Liu and colleagues’ study [7] showed that the dysfunction of NMDA receptors may contribute to the cognitive decline in Alzheimer’s disease. Also, in another study Coyle et al. [8], confirmed the association between the NMDA receptor hypofunction, particularly a reduction in the activity of the NR2A subunit and the development of cognitive deficits observed in schizophrenia.
Furthermore, the impairment of these receptors may play a role in the development of depression [2]. Sanacora et al. [9] reported that the administration of ketamine, a noncompetitive NMDA receptor antagonist, produced rapid and significant antidepressant effects in patients with treatment resistant depression. As a result, several therapeutic drugs that interact with NMDA receptor have been developed to treat major depressive disorders. In 2014 Dang and colleagues provided a comprehensive review of these novel therapeutic drugs and their potential in targeting the glutamatergic system for treating MDD [10].
NMDA receptors have been linked to the underlying causes of ADD and ADHD. Although the precise mechanisms are still not completely understood, studies indicate that dysfunction of NMDA receptors may play a role in the observed symptoms of these disorders [11]. This could be due to the impaired synaptic plasticity, which relies on proper functioning of NMDA receptors, or the modulation of dopamine neurotransmission. The involvement of NMDA receptors in ADD/ADHD is an active area of research, and further investigations are necessary to fully comprehend their specific role and develop more targeted treatments.
Pharmacology
NMDA receptor antagonists such as ketamine and phencyclidine (PCP) have been used as anaesthetics and recreational drugs, and as noted earlier have potential therapeutic effects for the treatment of MDD and other psychiatric disorders. Namely, Tariq et al. [12] studied the effect of NR2B-selective antagonists such as MK-0657 and CP-101,606 and confirmed for their therapeutic potential in the treatment of depression. Additionally, preclinical studies such as the one conducted by Large (2007) have shown that antagonists selective for the NR2B subunit of the NMDA receptor, such as ifenprodil and Ro25-6981, have antipsychotic-like effects in animal models of schizophrenia. However, there is a lack of consistent evidence indicating that there are differences in the overall levels of NR1 and NR2 receptors between individuals with schizophrenia and those without the disorder [13]. Furthermore, NR2A selective antagonists have been proposed as a potential treatment for Alzheimer’s disease [14]. For instance, D-cycloserine, an NMDA receptor agonist has been shown to enhance learning and memory and may have potential therapeutic effects for the treatment of cognitive deficits in disorders such as Alzheimer’s disease [7,15].
There is growing evidence that both the dopamine and glutamate systems are involved in attention-deficit hyperactivity disorder (ADHD). In ADHD, there may be excessive activity in the glutamate system, which is linked to a less active state in the dopamine system which will result in ADHD symptoms such as mood swing, altered focus and attention problems (Kristiansen et al., 2007). Many authors have reported the presence of serum NMDA antibodies in varying proportions of patients with ADHD; however, many others have not been able to confirm this. Because of the contradictory findings reported in various studies, more definitive research on this issue is required. Hence, this study investigated the NR1/NR2 subunit of NMDA receptors in patients with ADHD (n=50) and healthy controls (n=20) to evaluate if ADHD behaviours is associated with the expression level of NMDA receptor subunits of NR1/NR2.
ADHD
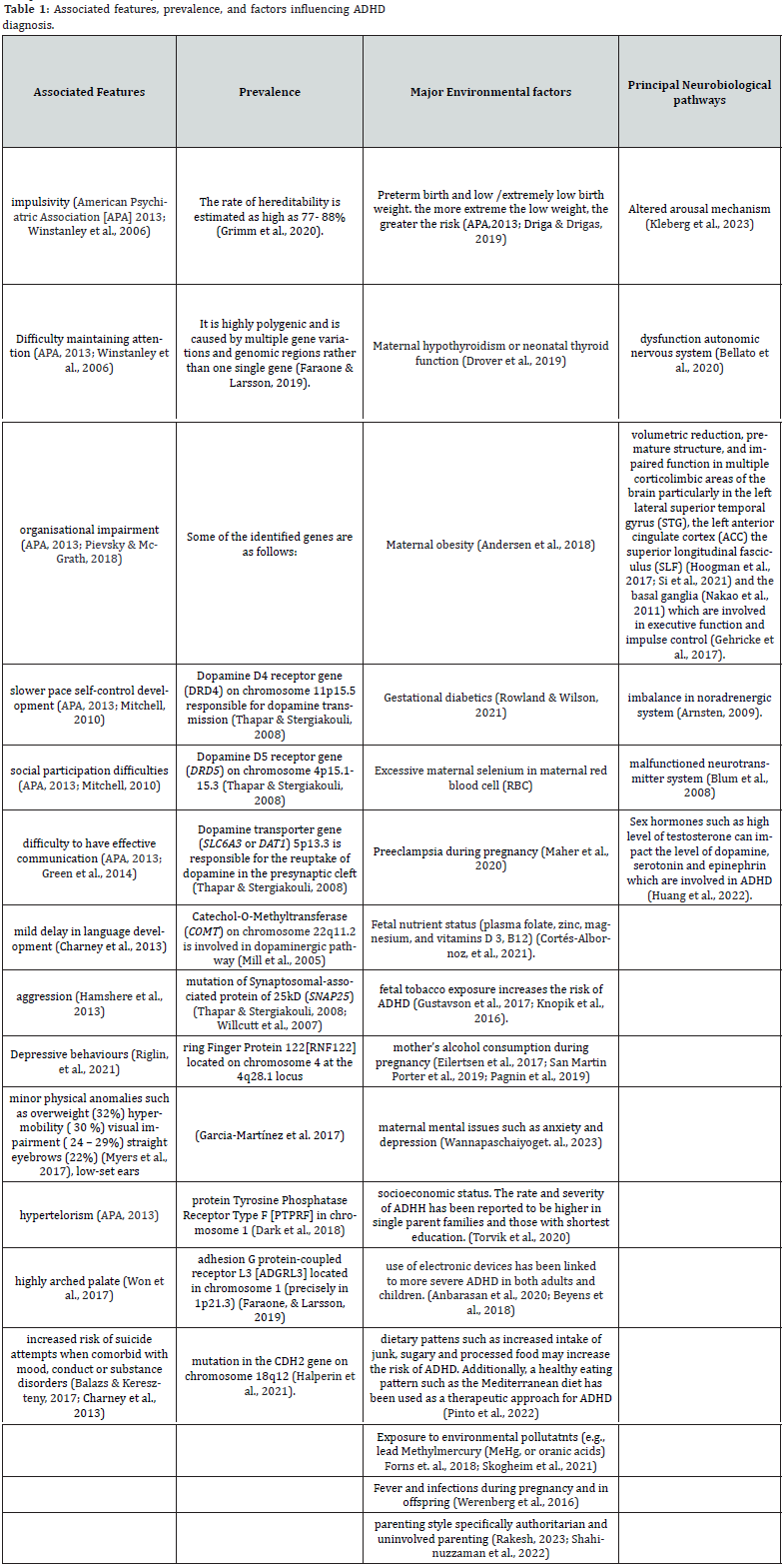
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental syndrome that affects both children and adults, characterized by symptoms of hyperactivity, impulsivity, and inattention. According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [15], Diagnostic, ADHD can be divided into three subgroups, combined-type ADHD (ADHD-C), predominantly inattentive-type ADHD (ADHD-I), predominantly hyperactive/impulsive ADHD. Clinically, the most common subtypes are ADHD-I and ADHD-C (Willcutt, 2012). The dopamine hypothesis of ADHD proposes that abnormalities in dopamine neurotransmission contribute to the condition, as stimulant drugs that enhance dopamine levels are effective in treating symptoms [13]. Genetic studies have revealed connections between the onset and severity of ADHD symptoms and both the quantity and functionality of glutamate receptors. Likewise, other studies have indicated that impaired glutamate receptors can lead to dysfunction in the dopaminergic systems, resulting in symptoms such as depression, anxiety, difficulty in focusing, and memory disturbances. Table 1 summarizes the associated features of ADHD, explores its prevalence, and discusses the genetic and environmental factors linked to the condition.
Materials and Methods
Participants
After obtaining approval for the research and acquiring the ethical code from the Ethics Committee of the university, the diagntostic tests were prepared, and a referral letter was obtained for the Roshd rehabilitation clinic. The inclusion criteria for participants were as follows: (a) meeting the diagnostic criteria for mixed ADHD as assessed by the Structured Clinical Interview of DSM-IV [15], (b) being between 6 and 15 years of age, and (c) being male. Limiting the selection to a single gender was done to mitigate potential confounding factors associated with gender differences. The exclusion criteria involved individuals with a history of severe neurological and neuropsychological problems other than ADHD, as well as those with severe physical illness.
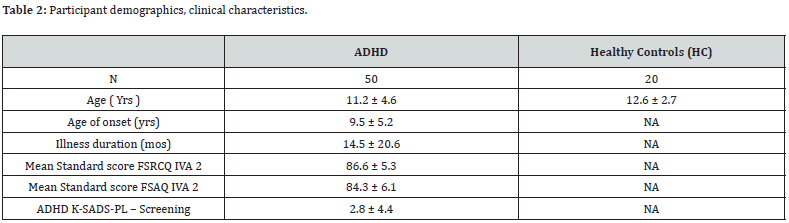
To recruit healthy subjects, advertisements were utilized, targeting siblings of the patients at Roshd clinic. These potential participants were screened and matched in terms of age and gender to the patient group. Demographic data for both patients and normal controls are summarized in table 2. The selection of study participants was based on convenience sampling [16]. The final sample consisted of 70 male individuals aged 6 to 15 years, including 50 subjects diagnosed with mixed ADHD and 20 agematched healthy controls. Prior to the assessments and cognitive testing, all participants refrained from taking any medication for a minimum of 24 hours. The demographic characteristics and sample size are presented in table 2.
Ethical considerations
Prior to data collection, written informed consent was obtained from parents and children who participated in this work after providing detailed information about the study to ensure their informed participation. Additionally, the anonymity of the participants and the confidentiality of the collected information was guaranteed. All personal and sensitive information collected from participants was treated with the utmost confidentiality. Identifying information, such as names and contact details, was removed and replaced with unique codes to ensure the anonymity of participants. Only authorized researchers had access to the data, and all digital files were password protected. Every effort was made to minimize any potential harm to the participants. The research protocol was designed to avoid any physical, psychological, or emotional harm. Participants were not exposed to any undue stress or discomfort during the study, and they were debriefed after the study to ensure their well-being.
Diagnostic assessment of ADHD
All ADHD participants underwent a thorough diagnostic process conducted by a consultant psychiatrist with expertise in ADHD. This process involved the utilization of pre-assessment questionnaires and semi-structured interviews aligned with the diagnostic criteria outlined in the DSM-5 [15]. To enhance the validity of the diagnosis, a trained assessor, working as part of the research team, administered the K-SADS-PL (Kiddie Schedule for Affective Disorders and Schizophrenia) [17], and the IVA2 (Integrated Visual and Auditory Continuous Performance) [18], tests. The assessor received specialized training from a consultant child and adolescent psychiatrist to ensure accurate administration and interpretation of these assessment tools. Trained administrators introduced the purpose of the K-SADSPL [17,19], to the parents and the child, explaining the scoring requirements to transition from the screening interview to the diagnostic supplement. Questions were posed to both the parent and the child, with the assessor integrating their responses. While the ADHD module of the K-SADS-PL involves parent-child participation, we primarily relied on the parent’s or caretaker’s accounts of the child’s behavior. In the screening interview, responses were rated as absent (coded as 1), subthreshold levels (coded as 2), or threshold levels (coded as 3). This interview focused on four items related to ADHD symptoms, namely: (a) “Difficulty sustaining attention,” (b) “Easily distracted during task or play activities,” (c) “Difficulty remaining seated,” and (d) “Impulsivity.” The IVA-2 CPT [18] assesses attention and impulsivity by measuring responses to 500 intermixed auditory and visual stimuli presented 1.5 seconds apart. The task involves clicking the mouse when the target stimuli, represented by an auditory or visual “1,” appear, while refraining from clicking for the foil stimuli, represented by an auditory or visual “2.” Quotient scores for all IVA-2 scales are reported as standard scores, with a mean of 100 and a standard deviation of 15 as reported in table 2.
Evaluation of the blood samples
Sample collection: Blood samples were collected from both the study and control groups by drawing 7ml blood from the antecubital vein. No specific timing or fasting requirements were imposed for the blood collection process. The collected blood was placed in standard biochemistry tubes and then centrifuged at a speed of 1,000× g for a duration of 25 minutes. After centrifugation and washing, the serum samples were separated and promptly transferred to a freezer set at -80°C. These samples were stored under the same conditions for both the patients and the blood doners (control group).
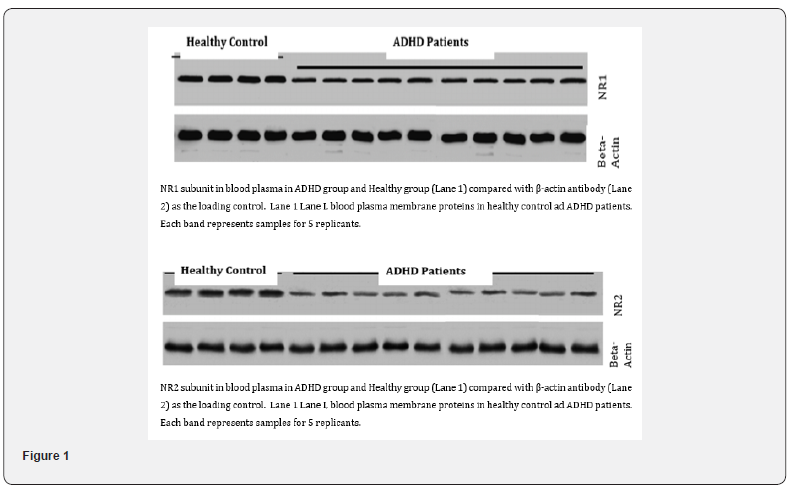
Quantifying NR1 and NR 2 receptors: The stored serum samples were utilized for quantifying the expression levels of NR1 and NR2 receptors using Western blotting. Briefly, Plasma proteins were separated on a 7% reducing SDS gel and transferred to PVDF (polyvinylidene difluoride) membrane. The membrane was incubated with Beta-Actin antibodies, followed by washing steps to remove unbound antibodies. The protein bands corresponding to NR1 and NR2 receptors was visualized using fluorescence. The intensity of these bands was then be quantified using computerassisted imaging analysis software (ImageJ) [20], to determine the relative abundance of NR1 and NR2 receptors in the blood samples from both the study and control groups (Figure 1).
Result
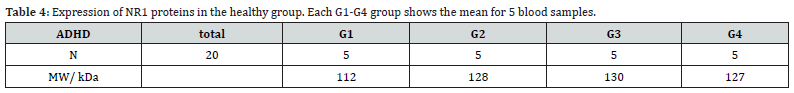
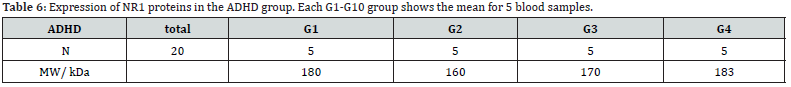
All analysis was performed with SPSS software version 26 [20]. The mean expression of NR1 receptors in the experimental group (ADHD) was 97.7 (Table 3 and 4). While the mean expression of NR1 receptors in the healthy control group was 124.25 (Table 5 and 6). The mean expression of NR2 receptors in the ADHD group was 154.6, compared to the mean expression of NR2 receptors in the healthy control group, which was 173.25. This implies a difference in the mean expression levels of NR2 receptors between the ADHD and healthy control groups.
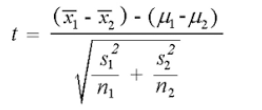
Using the following formula, the t-value is -19.32149. The p-value is < .00001. The result is significant at p < .05 (Table 4)
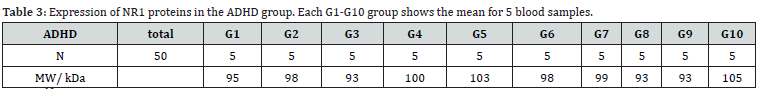
N1: 50, df1 = N - 1 = 50 - 1 = 49, M1: 97.7
SS1: 810.5, s21 = SS1/ (N - 1) = 810.5/(50-1) = 16.54
N2: 20, df2 = N - 1 = 20 - 1 = 19, M2: 124.25
SS2: 1023.75, s22 = SS2/ (N - 1) = 1023.75/ (20-1) = 53.88
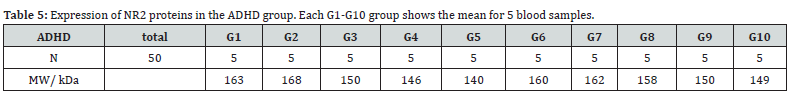
N1: 50, df1 = N - 1 = 50 - 1 = 49, M1: 154.6
SS1: 3532, s21 = SS1/ (N - 1) = 3532/(50-1) = 72.08
N2: 20, df2 = N - 1 = 20-1 = 19, M2: 173.25, SS2: 1633.75
s22 = SS2/ (N - 1) = 1633.75/ (20-1) = 85.99
The t-value is -8.08756. The p-value is < .00001. The result is significant at p < .05 with a p < 0.05, we reject the null hypothesis and conclude that there is a significant difference in the mean molecular weight of NR1 receptors between the ADHD and healthy control groups.
Discussion
The present study uncovered a significant association between ADHD and abnormal expression of NMDA glutamate receptors, specifically the NR1 and NR2 subunits, in the blood plasma of individuals with ADHD compared to healthy controls. These findings highlight a potential role for NMDA receptors in the pathophysiology of ADHD, suggesting that impaired NMDA receptor function may contribute to the development of this neurodevelopmental disorder [21]. Dysfunction of NMDA receptors can have complex effects on the levels of neurotransmitters like dopamine and epinephrine in the brain, which can contribute to ADHD symptoms [22,23].
NMDA receptors play a crucial role in regulating the release of dopamine in the brain, as demonstrated in numerous studies associated with reward, motivation, and attention [19,23]. When NMDA receptors do not function properly, it can disrupt the balance of dopamine release [22,24]. This dysregulation can lead to both excessive and insufficient dopamine levels in different brain regions [19]. The excessive dopamine release in some areas of the brain can result in hyperactivity, impulsivity, and difficulties in regulating attention, which are hallmarks of ADHD [25,26]. Additionally, low dopamine levels can result in difficulties with motivation, focus, and reward processing, contributing to the inattentive symptoms seen in ADHD [26].
Furthermore, when NMDA receptors are disrupted, it can lead to an overactive stress response, causing increased release of epinephrine. This heightened stress response can exacerbate ADHD symptoms, particularly the hyperactivity and impulsivity components [27]. However, it’s important to note that while the study establishes a correlation, it does not determine causation. Further research is needed to elucidate the exact mechanisms and causal relationships between reduced NR1/NR2 receptor expression and the development of ADHD, as well as to investigate potential therapeutic interventions targeting NMDA receptors in the management of ADHD symptoms [28-39].
The implications of the study’s results are far-reaching and carry significant importance for the field of ADHD research and clinical practice. Firstly, these findings advance the understanding of ADHD by highlighting the involvement of NMDA glutamate receptors, adding a new dimension to the established dopaminecentric model. This complexity underscores the need for a more holistic view of ADHD’s neurobiological underpinnings, prompting researchers to explore the intricate interplay between various neurotransmitter systems. Secondly, the results offer potential therapeutic avenues, suggesting that interventions targeting NMDA receptor function may hold promise in managing ADHD symptoms. This could diversify treatment options, potentially minimizing side effects and enhancing treatment efficacy [40-49].
Thirdly, the study contributes to the emerging field of personalized medicine in ADHD, where specific genetic or environmental factors linked to reduced NMDA receptor expression may guide tailored treatment approaches. Lastly, beyond ADHD, these findings have broader implications for understanding brain function, particularly in neuropsychiatric disorders influenced by glutamatergic signaling. In essence, these results matter as they deepen our comprehension of ADHD, offer therapeutic potential, pave the way for personalized care, and contribute to our broader understanding of brain function in neuropsychiatric contexts [50-77].
Limitations
Limitations: This study has several limitations that need to be considered. Firstly, one limitation is the relatively small sample size, which may affect the generalizability of the findings. Secondly, the recruitment of participants was done through convenience sampling, which means that the participants were selected based on their easy accessibility rather than using a random sampling method. It is important to note that random sampling is generally preferred in order to obtain a representative sample and minimize potential biases. Therefore, the findings should be interpreted with caution, considering the limitations of the study design and the sampling method employed.
Suggestion for future research
To build upon these findings, future research avenues should delve into several key areas. Firstly, longitudinal studies could help elucidate whether reduced NR1/NR2 receptor expression precedes the onset of ADHD or is a consequence of the disorder. This would provide critical insights into the causal relationship. Secondly, investigations into the genetic and environmental factors influencing NMDA receptor expression in ADHD could identify atrisk populations and inform targeted interventions. Furthermore, experimental studies exploring the effects of NMDA receptor modulation in animal models of ADHD could help establish causation and assess potential therapeutic approaches. Lastly, a more comprehensive exploration of the interaction between the glutamatergic and dopaminergic systems in ADHD could provide a nuanced understanding of the disorder’s neurobiology.
Conclusion
On the basis of findings of this study, we conclude that individuals with ADHD have significantly less NR1/NR2 proteins, and therefore potentially fewer functional NMDA receptors in their blood plasma. Impaired synaptic plasticity can disrupt the normal communication between brain regions involved in attention and impulse control, potentially leading to attention deficits and hyperactivity.
Additionally, reduced expression of NR1/NR2 impairs the dopaminergic system which is closely associated with the regulation of attention and reward processing and further contributing to the symptoms of ADHD.
Additionally, While the findings from our research have indicated a link between NR1/NR2 receptor dysfunction and ADHD symptoms, the precise nature of this relationship and the directionality of the causation have yet to be fully elucidated and it remains uncertain whether ADHD leads to a decrease in NR1/ NR2 receptor expression or if the reduced expression of NR1/ NR2 receptors contributes to the manifestation of ADHD and associated neurodevelopmental disorders.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nature Reviews Neuroscience 14(6): 383-400.
- Greger IH, Mayer ML (2019) Structural biology of glutamate receptor ion channels: towards an understanding of mechanism. Curr Opin Struct Biol 57: 185-195.
- Mayer ML, Armstrong N (2004) Structure and function of glutamate receptor ion channels. Annual review of physiology66: 161-181.
- Bear M, Connors B, Paradiso MA (2020)Neuroscience: exploring the brain, enhanced edition: exploring the brain. Jones & Bartlett Learning
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, et al. (2010) Glutamate receptor ion channels: Structure, regulation, and function. Pharmacological Reviews 62(3): 405-496.
- Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, et al. (2021) Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacological Reviews73(4): 298-487.
- Liu J, Chang L, Song Y, Li H, Wu Y (2019) The role of NMDA receptors in Alzheimer's disease. Frontiers in neuroscience13: 43.
- Coyle JT, Tsai G, Goff D (2003) Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Annals of the New York Academy of Sciences1003: 318-327.
- Sanacora G, Treccani G, Popoli M (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology62(1): 63-77.
- Dang YH, Ma XC, Zhang JC, Ren Q, Wu J, et al. (2014) Targeting of NMDA receptors in the treatment of major depression. Current Pharmaceutical Design, 20(32), 5151-5159.
- Chang JP, Lane HY, Tsai GE (2014) Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Current pharmaceutical design20(32): 5180-5185.
- Tariq S, Sidhu J, O Brien AT (2020) Selective NMDA Receptor Antagonists for Depression: A Systematic Review and Meta-Analysis. Frontiers in psychiatry 11: 662.
- Kuś J, Saramowicz K, Czerniawska M, Wiese W, Siwecka N, et al. (2023) Molecular Mechanisms Underlying NMDARs Dysfunction and Their Role in ADHD Pathogenesis. Int J Mol Sci 24(16): 12983.
- Franco R, Rivas-Santisteban R, Casanovas M, Lillo A, Saura CA, et al. (2020) Adenosine A2Areceptor antagonists affects NMDA glutamate receptor function. potential to address neurodegeneration in Alzheimer's disease. Cells 9(5): 1075.
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. 5th Ed (DSM-5) American Psychiatric Association, Washington DC.
- Etikan I, Musa SA, Alkassim RS (2016) Comparison of convenience sampling and purposive sampling.American Journal of Theoretical and Applied Statistics 5(1): 1-4.
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U (2000) K-SADS-PL.J Am Acad Child Adolesc Psychiatry 39(10): 1208.
- Tinius TP (2003) The integrated visual and auditory continuous performance test as a neuropsychological measure. Arch Clin Neuropsychol18(5): 439-454.
- Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75(3): 889-907.
- Bourne R, Bourne R (2010) ImageJ.Fundamentals of digital imaging in medicine 185-188.
- Burnashev N, Szepetowski P (2015) NMDA receptor subunit mutations in neurodevelopmental disorders. Current opinion in pharmacology 20: 73-82.
- Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, et al. (2019) Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol39(1): 31-59.
- Whitton PS (1997) Glutamatergic control over brain dopamine release in vivo and in vitro. Neurosci Biobehav Rev 21(4): 481-488.
- Homayoun H, Stefani MR, Adams BW, Tamagaz GD, Moghaddam B (2004) Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology29(7): 1259-1269.
- Madden DR (2002) The structure and function of glutamate receptor ion channels. Nature reviews. Neuroscience3(2): 91-101.
- Schachar R (2009) Attention deficit hyperactivity disorder in children, adolescents, and adults.Continuum: Lifelong Learning in Neurology 15(6): 78-97.
- Reiner A, Levitz J (2018) Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron98(6): 1080-1098.
- Anbarasan D, Kitchin M, Adler LA (2020) Screening for adult ADHD. Current Psychiatry Reports 22: 1-5.
- Andersen CH, Thomsen PH, Nohr EA, Lemcke S (2018) Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. Eur Child Adolesc Psychiatry 27: 139-148.
- Arnsten AF (2009) The emerging neurobiology of attention deficit hyperactivity disorder: The key role of the prefrontal association cortex. J pediatr 154(5): I-S43.
- Balazs J, Kereszteny A (2017) Attention-deficit/hyperactivity disorder and suicide: A systematic review. World Journal of psychiatry 7(1): 44-59.
- Bellato A, Arora I, Hollis C, Groom MJ (2020) Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neuroscience and Biobehavioral Reviews 108: 182-206.
- Blum K, Chen AL, Braverman ER, Comings DE, Chen TJ, et al. (2008) Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatric Disease and Treatment 4(5): 893-918.
- Charney DS, Buxbaum JD, Sklar P, Nestler EJ (2013) Neurobiology of mental illness. Oxford University Press, Incorporated.
- Cortés-Albornoz MC, García-Guáqueta DP, Velez-van-Meerbeke A, Talero-Gutiérrez C (2021) Maternal nutrition and neurodevelopment: A scoping review. Nutrients 13(10): 3530.
- Dark C, Homman-Ludiye J, Bryson-Richardson RJ (2018) The role of ADHD associated genes in neurodevelopment. Developmental Biology 438(2): 69-83.
- Driga AM, Drigas, A (2019) ADHD in the early years: Pre-natal and early causes and alternative ways of dealing. International Journal of Online & Biomedical Engineering 15(13).
- Drover SS, Villanger GD, Aase H, Skogheim TS, Longnecker MP, et al. (2019) Maternal thyroid function during pregnancy or neonatal thyroid function and attention deficit hyperactivity disorder: A systematic review. Epidemiology 30(1): 130-144.
- Eilertsen EM, Gjerde LC, Reichborn-Kjennerud T, Ørstavik RE, Knudsen GP, et al. (2017) Maternal alcohol use during pregnancy and offspring attention-deficit hyperactivity disorder (ADHD): A prospective sibling control study. International Journal of Epidemiology 46(5): 1633-1640.
- Faraone SV, Larsson H (2019) Genetics of attention deficit hyperactivity disorder. Molecular Psychiatry 24(4): 562-575.
- Forns J, Stigum H, Høyer BB, Sioen I, Sovcikova E, et al. (2018) Prenatal and postnatal exposure to persistent organic pollutants and attention-deficit and hyperactivity disorder: A pooled analysis of seven European birth cohort studies. International Journal of Epidemiology 47(4): 1082-1097.
- Garcia-Martínez I, Sánchez-Mora C, Soler Artigas M, Rovira P, Pagerols M, et al. (2017) Gene-wide association study reveals RNF122 ubiquitin ligase as a novel susceptibility gene for attention deficit hyperactivity disorder. Scientific Reports 7(1): 5407.
- Gehricke JG, Kruggel F, Thampipop T, Alejo SD, Tatos E, et al. (2017) The brain anatomy of attention-deficit/hyperactivity disorder in young adults: A magnetic resonance imaging study. PloS One 12(4): e0175433.
- Green BC, Johnson KA, Bretherton L (2014) Pragmatic language difficulties in children with hyperactivity and attention problems: An integrated review. International Journal of Language & Communication Disorders 49(1): 15-29.
- Grimm O, Kranz TM, Reif A (2020) Genetics of ADHD: What Should the Clinician Know? Current Psychiatry Reports 22(4): 18.
- Halperin D, Stavsky A, Kadir R, Drabkin M, Wormser O, et al. (2021) CDH2 mutation affecting N-cadherin function causes attention-deficit hyperactivity disorder in humans and mice. Nature Communications 12(1): 6187.
- Hamshere ML, Langley K, Martin J, Agha SS, Stergiakouli E, et al. (2013) High loading of polygenic risk for ADHD in children with comorbid aggression. Am J Psychiatry 170(8): 909-916.
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, et al. (2017) Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. The Lancet Psychiatry 4(4): 310-319.
- Huang J, Mauche N, Rullmann M, Ulke C, Becker GA, et l. (2022) Association between individual Norepinephrine Transporter (NET) availability and response to pharmacological therapy in adults with attention-deficit/hyperactivity disorder. Brain sciences 12(8): 965.
- Kleberg JL, Frick MA, Brocki KC (2023) Eye-movement indices of arousal predict ADHD and comorbid externalizing symptoms over a 2-year period. Scientific Reports 13(1): 4767.
- Klein B, Damiani-Taraba G, Koster A, Campbell J, Scholz C (2015) Diagnosing attention-deficit hyperactivity disorder (ADHD) in children involved with child protection services: are current diagnostic guidelines acceptable for vulnerable populations? Child: Care, Health and Development 41(2): 178-185.
- Knopik VS, Marceau K, Bidwell LC, Palmer RH, Smith TF, et al. (2016) Smoking during pregnancy and ADHD risk: A genetically informed, multiple‐rater approach. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 171(7): 971-981.
- Kaufman J, Schweder AE (2004)The Schedule for Affective Disorders and Schizophrenia for School-Age Children: Present and Lifetime version (K-SADS-PL).
- Maher GM, Dalman C, O Keeffe GW, Kearney PM, McCarthy FP, et al. (2020) Association between preeclampsia and attention-deficit hyperactivity disorder: a population-based and sibling-matched cohort study. Acta psychiatrica Scandinavica 142(4): 275-283.
- Mill J, Xu X, Ronald A, Curran S, Price T, et al. (2005) Quantitative trait locus analysis of candidate gene alleles associated with attention deficit hyperactivity disorder (ADHD) in five genes: DRD4, DAT1, DRD5, SNAP‐25, and 5HT1B. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 133(1): 68-73.
- Mitchell JT (2010) Behavioral approach in ADHD: Testing a motivational dysfunction hypothesis. Journal of Attention Disorders 13(6): 609-617.
- Myers L, Anderlid BM, Nordgren A, Willfors C, Kuja-Halkola R, et al. (2017) Minor physical anomalies in neurodevelopmental disorders: Atwin study. Child and Adolescent Psychiatry and Mental Health 11: 57.
- Nakao T, Radua J, Rubia K, Mataix-Cols D (2011) Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry 168(11): 1154-1163.
- Pagnin D, Zamboni Grecco ML, Furtado EF (2019) Prenatal alcohol use as a risk for attention-deficit/hyperactivity disorder. European Archives of Psychiatry and Clinical Neuroscience 269(6): 681-687.
- Pievsky MA, McGrath RE (2018) The neurocognitive profile of attention-deficit/hyperactivity disorder: A review of meta-analyses. Archives of Clinical Neuropsychology 33(2): 143-157.
- Pinto S, Correia-de-Sá T, Sampaio-Maia B, Vasconcelos C, Moreira P, et al. (2022) Eating Patterns and Dietary Interventions in ADHD: A Narrative Review. Nutrients 14(20): 4332.
- Rakesh M (2023) Fearful versus loving parents: A cross-sectional study in Mysuru city to assess influence of parenting style on ADHD. International Journal of Community Medicine and Public Health 10(8): 2908.
- Riglin L, Leppert B, Dardani C, Thapar AK, Rice F, et al. (2021) ADHD and depression: Investigating a causal explanation. Psychological Medicine, 51(11), 1890-1897.
- Rowland J, Wilson CA (2021) The association between gestational diabetes and ASD and ADHD: A systematic review and meta-analysis. Scientific Reports 11(1): 5136.
- Salari N, Ghasemi, H, Abdoli, N, Rahmani, A, Shiri, M. H, Hashemian, A. H, Akbari, H, & Mohammadi, M (2023) The global prevalence of ADHD in children and adolescents: A systematic review and meta-analysis. Italian journal of pediatrics, 49(1): 48.
- San Martin Porter M, Maravilla JC, Betts KS, Alati R (2019) Low-moderate prenatal alcohol exposure and offspring attention-deficit hyperactivity disorder (ADHD): Systematic review and meta-analysis. Archives of Gynecology and Obstetrics 300: 269-277.
- Shahinuzzaman M, Siddique MNEA, Hassan M, Liza M, Alia SS, et al. (2022) Parenting practice, Matrimonial Instability, and children's attention deficit hyperactivity disorders. International Journal of Indian Psychology 10 (3): 857-866.
- Si FF, Liu L, Li HM, Sun L, Cao QJ, et al. (2021) Cortical morphometric abnormality and its association with working memory in children with attention-deficit/hyperactivity disorder. Psychiatry Investigation 18(7): 679-687.
- Skogheim TS, Weyde KVF, Aase H, Engel SM, Surén P, et al. (2021) Prenatal exposure to per-and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environmental Research 202: 111692.
- Song P, Zha M, Yang Q, Zhang Y, Li X, et al. (2021) The prevalence of adult attention-deficit hyperactivity disorder: A global systematic review and meta-analysis. Journal of Global Health 11: 04009.
- Thapar A, Stergiakouli E (2008) An overview on the genetics of ADHD. Xin li xue bao. Acta psychologica Sinica 40(10): 1088-1098.
- Torvik FA, Eilertsen EM, McAdams TA, Gustavson K, Zachrisson HD, et al. (2020) Mechanisms linking parental educational attainment with child ADHD, depression, and academic problems: A study of extended families in the norwegian mother, father and child cohort study. Journal of Child Psychology and Psychiatry 61(9): 1009-1018.
- Wannapaschaiyong P, Penphattarakul A, Rojmahamongkol P, Sutchritpongsa S (2023) The Relationship between primary caregivers’ psy-chosocial factors and self-esteem in children and adolescents with ADHD: An exploratory cross-sectional study.Siriraj Medical Journal SMJ
- Werenberg Dreier J, Nybo Andersen AM, Hvolby A, Garne E, Kragh Andersen P, et al. (2016) Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. Journal of Child Psychology and Psychiatry, and Allied Disciplines 57(4): 540-548.
- Willard SS, Koochekpour S (2013) Glutamate, glutamate receptors, and downstream signaling pathways. International Journal of Biological Sciences9(9): 948-959.
- Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clinical Psychology Review 26(4): 379-395.
- Won DC, Guilleminault C, Koltai PJ, Quo SD, Stein MT, et al. (2017) It is just attention-deficit hyperactivity disorder…or Is it? J Dev Behav Pediatr 38(2): 169-172.






























