Executive Functioning in Young Children with Williams Syndrome: A Longitudinal Study
Jessica L Reeve and Melanie A Porter*
School of Psychology, Herring Road, Macquarie University, North Ryde, NSW, Australia
Submission:July 19, 2023;Published:August 23, 2023
*Corresponding author:Melanie A Porter, School of Psychology, Herring Road, Macquarie University, North Ryde, NSW, Australia, Email: melanie.porter@mq.edu.au
How to cite this article: Jessica L R, Melanie A P. Executive Functioning in Young Children with Williams Syndrome: A Longitudinal Study. Glob J Intellect Dev Disabil. 2023; 12(2): 555834.DOI:10.19080/GJIDD.2023.12.555834
Abstract
Whilst it is generally accepted that Williams syndrome (WS) individuals (at least older children and adults) experience executive functioning difficulties, research has generally focussed on older children and adults, with very limited research on younger children. The present research investigates the emerging profile of executive functioning using an ecologically valid, parent-report questionnaire, tracks the executive abilities of these children over time, and identifies which demographic variables are impacting the development of executive functioning over time. Participants included 18 young WS children, aged between 2 to 5 years at Time 1, who also completed a comprehensive developmental assessment. Parents/guardians also completed the Behavior Rating Inventory of Executive Function (BRIEF), which is commonly used in both research and clinical settings. WS children displayed both impaired and intact functioning relative to the normative sample across different executive functioning components, with, at the group level at least, relative deficits in working memory, planning/organisation, and inhibition, and relative sparing of shifting and emotional control, on average. Preliminary evidence for the developmental trajectory of executive abilities in this syndrome is also evidenced, with increased impairments in shifting, emotional control, and working memory over time and with increased chronological age. Considerable individual variability was apparent, however, confirming the importance of individual assessments and developing individualised management programs for WS children.
Keywords: Williams syndrome; Young children; Executive function; Longitudinal; Intellectual Disability; Neuropsychology; Developmental trajectory
Abbreviations: ADHD: Attention-Deficit Hyperactivity Disorder; ANOVA: Analysis of Variance; ASD: Autism Spectrum Disorder; BRIEF: Behavior Rating Inventory of Executive Functioning; BRIEF-C: Behavior Rating Inventory of Executive Functioning – School aged version; BRIEF-P: Behavior Rating Inventory of Executive Functioning - Preschool version; CA: Chronological age; DAS-II: Differential Ability Scales, Second Edition; DQ: Developmental Quotient; EF: Executive Functioning; ELC: Early Learning Composite; EMI: Emergent Metacognition Index; FISH: Fluorescence in situ hybridization; GCA: General Conceptual Ability; GEC: Global Executive Composite; ISCI: Inhibitory Self-Control Index; FI: Flexibility Index; MSEL: Mullen Scales of Early Learning; NB: Nonbinary; WS: Williams syndrome
Mini Review
Williams syndrome (WS) is a neurodevelopmental disorder caused by a relatively rare sub-microscopic hemizygous deletion on the long arm of chromosome 7 at the location 7q11.23, spanning approximately 26 to 28 genes [1-4]. The prevalence of WS is estimated to be between 1 per 20,000 [5] and 1 per 7,500 [6] live births. Executive functioning (EF) has become an area of increased attention in WS research over the past 15 years due to findings of frontal lobe deficits in WS on structural and functional imaging [7,8] poor performance on EF measures in older children and adults [9,10], and the impact of EF impairment on adaptive, social, and academic outcomes (e.g., [9,11-15]). To date, however, most research on EF in WS has employed a sample spanning only older children and adults with WS, with very limited research on younger children. There has also been no longitudinal research on EF in WS to the best of the authors’ knowledge, and of the research that has been undertaken, most studies have typically only reported on group performance with no, or limited, exploration of individual differences in EF capabilities. Moreover, a lot of WS research has utilised performance-based measures of EF, which are known to lack ecological validity [16-18] and may also not be a valid measure of EF in the WS population given their motor, attention, and other low-level deficits [19-21].
The present study employed a longitudinal design to investigate everyday executive functioning in preschool children with WS aged 2 to 5 years, looking at EF development over approximately a 3.5-year period. Individual variability was also examined. Characterisation of EF in WS individuals at this earliest point in development and gaining an understanding of the early developmental trajectory of real-world EF is critical to: (i) aid in early management; (ii) increase our knowledge of the early developmental trajectory of EF in WS, and (iii) to, ultimately, better understand the links between EF and specific characteristics over time to maximise outcomes through early intervention.
Williams syndrome
As a multisystemic disorder, WS has a distinctive profile of cognitive, physical, behavioural, and psychological characteristics. WS is associated with intellectual disability and a distinct profile of specific cognitive strengths and weaknesses. Full-Scale IQ is typically in the mild to moderate disability range, however intellect can vary from the profoundly impaired to the average range [22]. The cognitive phenotype typically includes a relative strength in verbal abilities, and relative weaknesses in visuospatial construction [23] and select aspects of EF, which may include, in particular, the EF domains of inhibition, initiation, cognitive flexibility, working memory, planning and organisation, and monitoring [9,10,24].
Neuroanatomically, WS includes an overall reduction in brain size and an abnormal cerebral shape [8,25-28]. Structural and functional brain imaging studies also suggest abnormalities in the cerebellum, parts of the frontal lobes (e.g., striatum and dorsolateral prefrontal cortex), the temporal lobes, and the frontoparietal and amygdala-prefrontal circuits [7,29]. Behaviourally, EF difficulties are proposed to manifest with social disinhibition and explain, at least partially, the extreme friendliness seen in this condition, with hypersociability being a hallmark feature of WS [2,13,14,30-32]. Furthermore, WS individuals show a higher prevalence of Attention-Deficit/Hyperactivity Disorder (ADHD) and anxiety disorders, especially specific phobias and generalised anxiety disorder [33-36], conditions which, themselves, are also associated with EF impairments and frontal lobe pathology (e.g., [37,38]).
Executive functioning in the developmental context
EF typically refers to a set of higher-order functions commonly associated with prefrontal circuitry, which are believed to enable independent, purposeful, and goal-directed behaviour [39-41]. EFs are commonly conceptualised as a set of distinct (but partially correlated) components. There are many different theories of EF (e.g., [42,43]), however, in the developmental literature, a multidimensional framework is probably the most widely accepted and components of EF are typically thought to include the broad domains of cognitive and self-control [19,44-50]. Examples of cognitive control include initiation, working memory, planning and organisational abilities, and task-monitoring; selfcontrol (encompassing both emotional and/or behavioural control) includes inhibition, cognitive flexibility/shifting, and emotion regulation [19,20,44,47,49,51,52]. Individuals may have impairments in one or more components of EF.
Components of EF are difficult to assess in young children, especially those with neurodevelopmental conditions and intellectual disability, due to the hierarchical nature of neuropsychological abilities and the impact of lower-level abilities on EF task performance, such as processing speed, attention, and motor skills [19,21,53]. For this reason, and because of higher ecological validity, standardised questionnaires assessing EF have been developed for preschool, school-aged, and adult populations, with the most commonly used being the Behavior Rating Inventory of Executive Function (BRIEF; [49,54-56]) questionnaires. The BRIEF has been used previously with WS individuals, especially with older children and adults (e.g., [9,10,24,57,58]), and, in contrast to performance-based psychometric measures, the BRIEF questionnaires also assess many different domains of the EF construct. To date, the school-aged BRIEF has been most commonly used in the WS literature, and has even been used with adults who have WS based on the rationale that the mental age equivalence of adults with WS is most similar to neurotypical school-aged children.
Executive functioning in williams syndrome
It is now widely accepted that WS individuals experience EF difficulties, especially in older children and adults (e.g., [9,10,57]), which has been the focus of the literature, however, the exact nature of these EF deficits remains somewhat unclear. Using performance-based measures, relative impairments in planning [12,15,59,60], working memory [15,40,118], shifting [15,40], and inhibition [14,30,40,60,61] have been regularly reported in older children and adults with WS relative to typically developing controls. However, there are a number of studies that have found evidence for the preservation of several of these EF processes, like inhibition, categorisation, and shifting, which may perhaps be a reflection of a specific deficit within the verbal modality when compared to the visuospatial domain [12,30 ,59].
Some WS studies have utilised the BRIEF questionnaire, providing further evidence for considerable everyday EF impairment in school-aged children [9] and adults [10] with WS. BRIEF studies have consistently demonstrated impairments in the Working Memory, Initiate, and Task-Monitor clinical scales in comparison to normative samples across both school-aged children and adults with WS, while relative strengths were suggested for Organisation of Materials [9,10,57,58]. However, there have been some mixed results with a few EF processes across the age span. Planning, inhibition, and shifting, for example, which have also been shown as areas of EF weakness in some (but not all) studies utilising performance-based measures [12,30,59], have been reported as a relative strength, at least based on the group average, in various studies utilising the BRIEF (e.g., [9,10,24,58]). It is possible that these mixed results reflect methodological differences (e.g., measures used, comparison groups employed etc.), sample characteristics (e.g., age, co-morbidities etc.), and unspecified individual variability.
While most of the WS research on EF compares WS individuals to normative samples, some studies have used other neurodevelopmental syndromes as a comparison group. Camp et al. [57], for example, found that their WS sample were rated significantly lower on all domains of the school-aged BRIEF (parent form; [49]) compared to Down syndrome individuals matched on chronological age and verbal ability, and compared to mental age matched typically developing controls (aged 4 to 11 years). However, as the study sample spanned the ages of 10 to 26 years, the school-aged BRIEF was administered to the WS adults (and typically developing preschool children) in the study, which may not be appropriate. Indeed, Hocking & Reeve et al. [10] found the adult BRIEF (parent form; [56]) was a more valid measure of EF in WS adults than the school-aged version, suggesting that it is essential to administer age-appropriate real-world EF measures to individuals with neurodevelopmental disorders.
In contrast to Camp et al. [57], in a methodologically strong study with a large sample size, and using the parent form of the BRIEF-2 (i.e., the second edition of the school-aged BRIEF, [55]), Greiner de Magalháes et al. [9] found, on average, considerable weaknesses (i.e., T scores at or above 65) in 308 school-aged children with WS (aged 6 to 17 years) on the Working Memory, Initiate, and Task-Monitor clinical scales, the Cognitive Regulation Index, and the Global Executive Composite. Mild elevations (i.e., T scores between 60 and 64) were also reported for: Inhibit; Self-Monitor; Emotional Control; Shift; Plan/Organise; the Behaviour Regulation Index, and the Emotional Regulation Index. Organisation of Materials was indicated to be relatively spared (normal range), consistent with other WS research [10], although this was on the cusp of falling into the mildly elevated range with a T score of 59.37, with scores ranging from normal to impaired (i.e., T scores of 38 to 81). For the adult WS population, however, Hocking and Reeve et al. [10] reported a different pattern. Using the adult BRIEF (informant form; [56]), their sample of 20 WS adults (aged 18 to 53 years) showed clinically elevated scores for: Working Memory; Initiate; Shift; Plan/Organise; Task- Monitor; the Metacognition Index, and the Global Executive Composite, compared to the normative sample. There was relative sparing reported for: Inhibit; Emotional Control; Self-Monitor; Organisation of Materials, and the Behaviour Regulation Index.
These variations in the group EF profile between WS children and adults potentially indicates age-related changes in EF across the life span, with inhibition, emotional control, and self-monitoring improving into adulthood, on average. Similarly, although not all BRIEF-2 scales (parent form) were included in their analyses, Ng-Cordell et al. [62] reported a moderate effect size suggesting a relationship between improved inhibition and increasing chronological age in their sample of 26 WS individuals spanning both school-aged children and adults (aged 5 to 26 years). An increase in impairment is also evidenced for some EF components, with WS adults performing within the clinical range for Shift in comparison to young and school-aged children (e.g., [9,10,58]). These differences in the group EF profile of WS children and adults perhaps indicate a difference in the development of EF processes between WS and typically developing individuals, with WS individuals having a more protracted development over time. Such improvements in inhibition may also reflect age-related improvements in social functioning [14,63], and aspects of ADHD [60] and anxiety [64]. Longitudinal research is required to better understand the exact mechanisms at play here. Also, demographic variables (such as chronological age and sex) and IQ may partially explain differences in findings, but relationships between these variables and BRIEF scores are also quite mixed [9,10,40,62]. Great individual variability in scores for all clinical scales, indices, and the Global Executive Composite was also noted in these WS studies [9,10,62].
Executive functioning in young children with Williams syndrome: To date, there are only two known studies exploring EF in a young WS sample [24,58]. In his dissertation, Gallo (2009) investigated EF in WS children aged 3 to 7 years (Mage = 5.45 years; SD = 1.24) using both performance-based measures of EF, measuring inhibition and working memory (e.g., A-not-B [65]; Delayed Alternation [66]; Dimensional Change Card Sort [67]; Statue [68]), and the BRIEF-P (parent report; [54]) questionnaire. Findings from performance-based measures of EF indicated impairments in inhibition only age-related improvements within this domain on some tasks were reported. No age-related differences were found on the task assessing working memory and, as such, the authors suggested a protracted period of development for this domain in young WS children.
No relationship was found between EF and the overall developmental quotient (DQ)/IQ, although significant associations were reported between tasks of inhibition and verbal and nonverbal abilities. Due to the age range of the BRIEF-P [54], a subset of 20 parents with WS children aged approximately 3 to 5 years were engaged to complete this questionnaire (the mean age and range of this subset sample was not reported). Gallo [24] found, on average, clinically elevated scores (impairments) in: Working Memory; Inhibit; Plan/Organise; the Emergent Metacognition Index; and the Global Executive Composite; with relative strengths in: Shift; Emotional Control; the Inhibitory Self-Control Index; and the Flexibility Index, in comparison to the normative sample. More specifically, and in contrast to their findings using performancebased measures, the BRIEF-P Working Memory clinical scale had the highest mean T score (76.25; i.e., falling in the clinically impaired range) and the highest percentage of children who were rated in the clinically elevated range (80% of the sample). The next highest areas of difficulty were reportedly the Inhibit and Plan/Organise subscales (both with 50% of the sample). Although the average T score was not elevated, Emotional Control and Shift also had a percentage of WS preschool children who displayed clinically elevated scores (25% and 5%, respectively). At an index level, the Emergent Metacognition Index (which encompasses the Working Memory and Plan/Organise clinical scales) displayed the highest percentage of children in the clinically significant range (75% of sample; T score 73.50), followed by the Inhibitory Self- Control Index (encompassing Inhibit and Emotional Control; 50% and T score of 64.10), and lastly the Flexibility Index (encompassing Shift and Emotional Control; 10% and T score of 56.05). Gallo [24] also looked at correlations between BRIEF-P index scores and chronological age, DQ, and sex. An age-related increase in impairment was indicated for the following indexes and composites: Emergent Metacognition; Inhibitory Self-Control; and the Global Executive Composite (GEC).
A moderate negative effect size suggested a relationship between higher overall DQ and improved performance on the Inhibitory Self-Control Index and the GEC. We note, however, the author did not comment on whether any WS participants displayed floor effects on each subtest of the developmental measure (a common problem for children with intellectual disabilities when utilising the Mullen Scales of Early Learning; [69]) if floor effects were not rectified, it is likely they impacted on these results. No relationship between sex and the BRIEF-P indices and the GEC was found. Individual BRIEF-P clinical scales were not included in any correlational or inferential analyses.
More recently, Kazzi et al. [58] utilised both the BRIEF-P and the school-aged BRIEF (parent forms; combined data) in their sample of WS children aged 3 to 9 years. To align with their study aims, they only included the Inhibit, Shift, Emotional Control, and Working Memory clinical scales, which showed clinically elevated group scores on all EF components, apart from Shift. The mixed results surrounding the clinically elevated EF scales for Emotional Control across both studies with young WS children (i.e., the Gallo et al. [24] study did not find clinically elevated scores on Emotional Control in WS preschool children) is likely due to the broader age range of the Kazzi group sample, as they encompassed both preschool and school-aged children further suggesting possible age-related changes in EF abilities over time. However, these findings may also reflect periods of transition (e.g., a child starting school may experience increased emotional difficulties) or, perhaps, greater parental expectations on older WS children.
Current study
In light of the aforementioned literature, the aims of the present study were three-fold, with predictions also indicated serially with these aims below. The first aim was to investigate the emerging profile of executive functioning in young children with WS aged 2 to 5 years using the BRIEF-P. In line with the multidimensional theories of EF [19,44,46,47], and in line with previous WS research [10], it was expected that not all indices or clinical scales would be impaired in young WS children. More specifically, and in line with previous research on WS preschool children [24], it was predicted that our sample would display elevated levels of executive dysfunction, especially on the BRIEF-P Working Memory, Inhibit, Plan/Organise, Emergent Metacognition Index, Inhibitory Self-Control Index, and the Global Executive Composite in comparison to the normative sample. We did not expect an elevated performance on Shift, Emotional Control, and the Flexibility Index, in line with Gallo [24].
The second study aim was to track the executive abilities of these young WS children over time in a longitudinal study design using parent/guardian ratings on the school-aged BRIEF. In line with BRIEF studies on older children and adults with WS [9,10,58], it was hypothesised that EF impairments would increase over time in young WS children, particularly with shift and emotional control abilities.
Finally, the present study aimed to investigate whether demographic characteristics (i.e., sex and chronological age) and IQ were associated with EF at Time 1, and whether these variables impacted on the development of EF in this WS cohort over time. Based on the current developmental and WS literature, it was hypothesised that several EF difficulties would increase with chronological age, particularly shift and emotional control [9,10,58]. As the association between IQ, sex, and EF remains unclear, no specific hypotheses were generated. Individual variability in everyday EF and in EF developmental trajectories was also examined within the context of these aims.
Method
Participants
Participants consisted of 18 young children (eight males, ten females, and zero nonbinary) with a genetically confirmed diagnosis of WS (fluorescence in situ hybridization [FISH] test; [1,70,71]) and their parents/guardians. All families were recruited through Williams Syndrome Australia Limited or the New Zealand Williams Syndrome Association, and WS children were screened for psychological, neurodevelopmental, neurological, or major sensory impairments that were not a core feature of the syndrome. No child was excluded from the study based on these criteria. Chronological age (CA) at Time 1 ranged from 2 years, 2 months to 5 years, 11 months (M = 4.02, SD = 1.24 years). Males (M = 3.87 years, SD = 1.30) and females (M = 4.14 years, SD = 1.25) did not differ significantly in their chronological age, t(16) = -0.44, p > .05.1 Cognitive ability for each child was assessed using either the Mullen Scales of Early Learning (MSEL; [69]) or the Differential Ability Scales, Second Edition (DAS-II; [72]), depending on their chronological age at Time 1 testing. Overall, WS preschool children performed in the mild to moderate range of disability, with overall developmental quotients (DQ’s) ranging from 28.62 (severe) to 69.13 (mild; M = 54.63, SD = 12.78). The mean DQ score was highly consistent with the mean Full-Scale IQ reported for WS [73], and the wide range of ability levels is consistent with WS cognitive heterogeneity reported in WS [23,74].
At the time of Time 1 testing, WS preschool children were receiving various interventions, including most commonly: Speech Therapy (78%), Occupational therapy (61%), and/or Physiotherapy (56%). Geographic residence for each family was utilised as a measure of socioeconomic status (SES) using the Australian Bureau of Statistics Index of Relative Socio-economic Advantage and Disadvantage [86]. Families were provided with a score from “1” to “5”, with a lower score denoting a greater socioeconomic disadvantage. The mean score for WS families was 3.29 (SD = 1.49) and ranged from 1 to 5, indicating a wide and representative socio-economic spread of the current sample. Descriptive and demographic data for the young WS sample at Time 1 and 2 is shown in Table 1.
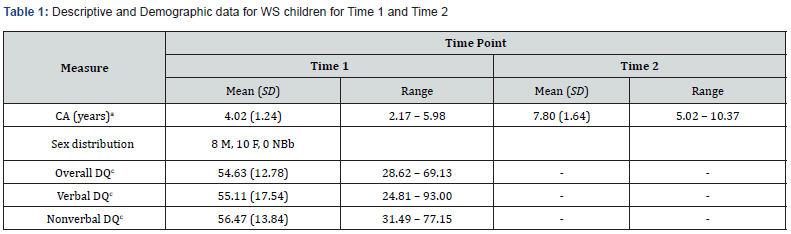
Note: T scores (population M = 50, SD = 10). Standard Scores (population M = 100, SD = 15). a CA = chronological age. b NB = nonbinary. c DQ = developmental quotient as measured by MSEL or DAS-II.
Materials
Executive functioning (as measured by the Behavior Rating Inventory of Executive Functioning Preschool Version [BRIEF-P; {54}]) and DQ (Verbal, Nonverbal, and Global) were assessed for all WS children at Time 1. At Time 2, executive functioning was measured by the Behavior Rating Inventory of Executive Functioning Parent Form (BRIEF-C; [49]).
Executive functioning
The child behavior rating inventory of executive functioning-preschool version (parent form) BRIEF-P: The BRIEF-P is a 63-item questionnaire completed to measure everyday EF behaviours of preschool children aged 2 years, 0 months to 5 years, 11 months, in home and preschool environments [54]. The items map onto five clinical scales (Inhibit, Shift, Emotional Control, Working Memory, and Plan/Organise). These scales combine to form the three indices of Inhibitory Self-Control (ISCI), Flexibility (FI), and Emergent Metacognition (EMI), and one overall composite score, the Global Executive Composite (GEC). Internal consistency is high across all scales, indices, and GEC, with the Cronbach alpha measure ranging from .80 to .97. Test-retest reliability correlation ranged from .78 to .90 over an average interval of 4.50 weeks [54]. Previous studies have also established the validity of the BRIEF-P in neurodevelopmental populations (e.g., [54]).
The child behavior rating inventory of executive functioning (parent form) BRIEF-C: The BRIEF-C is designed to measure everyday EF behaviours of children aged from 5 to 18 years [49]. The questionnaire consists of 86 standardised items, with 72 of those items mapping onto eight clinical scales (Inhibit, Shift, Emotional Control, Initiate, Working Memory, Plan/Organise, Organisation of Materials, and Monitor). The scales combine to form two indices (Behavior Regulation Index [BRI] and the Metacognition Index [MI]), and one composite summary score (Global Executive Composite [GEC]). The BRIEF-C has sound psychometric properties. The internal consistency across all scales, indices, and GEC are high, with the Cronbach alpha measure ranging from .80 to .98. Test-retest reliability correlation ranged from .76 to .85 for a two-week interval [49]. Previous studies have also established the validity of the BRIEF-C in neurodevelopmental populations (e.g., [49,75]). Due to the longitudinal nature of the study, the BRIEF-C was the current edition available when the study began, and it continued to be utilised at time 2 data collection in order to allow for a direct comparison.
The structure of both BRIEF questionnaires is outlined in Appendix A. Each clinical scale and index yield a T score (with population M = 50, SD = 10) based on the individual’s chronological age and sex. Higher scores indicate greater degrees of executive dysfunction, with scores at or above 65 suggesting clinical significance [49,54].
Longitudinal analyses: For the purposes of this research, only the five corresponding clinical subscales (Inhibit, Shift, Emotional Control, Working Memory, and Plan/Organise) and the Global Executive Composite on the two BRIEF questionnaires were utilised for Time 2 analyses. This is in line with other published studies with young children with WS [58]. As the number of items relating to each of the five sub-scales differ between the two measures, T scores were used in our analyses. T scores also most readily signify whether the ratings indicate clinical significance (≥65).
Intellectual functioning
The mullen scales of early learningMSEL: The MSEL [69] is a standardised measure of early cognitive functioning for infants and preschool children from birth through 68 months (US standardisation sample N = 1849). The MSEL consists of a Gross Motor Scale (for children under 33 months), together with four cognitive subtests: Visual Reception; Fine Motor; Receptive Language and Expressive Language [69]. Raw scores on each subtest can be converted to a T score (M = 50, SD = 10). T scores for each of the four cognitive subtests are summed to produce the Early Learning Composite (ELC) standard score (M = 100, SD = 15). The ELC score resembles an overall developmental quotient, where lower ELC scores indicate greater cognitive difficulties. Across each age group, it was reported that the median internal consistency values ranged from .75 to .83 for the five subtests [69]. The internal consistency of the ELC was high, ranging from .83 to .95 (median value was .91). Test-retest reliability correlations ranged from .71 to .96, and interscorer reliability ranged from .91 to .99. As many of the WS preschool children were at floor (standard T score of 20) on individual MSEL cognitive subtests, DQ scores were calculated for each MSEL subtest (utilising the formula DQ = age equivalent scores / chronological age x 100) and averaged to create an overall DQ. This is in line with other published studies with young children with neurodevelopmental disorders, including WS [58,76,77]. Verbal DQ was computed by averaging the DQ scores across the Receptive and Expressive MSEL subtests, and Nonverbal DQ was computed by averaging across the MSEL Visual Reception and Fine Motor.
The differential ability scales, second edition DAS-II: The DAS [72] is an individually administered standardised measure of intelligence designed for children aged from 2 years, 6 months to 17 years, 11 months (US standardisation sample N = 3,475). The DAS-II is divided into two record forms: 1) the Early Years battery, which comprises two levels, the lower level for children aged 2 years, 6 months to 3 years, 5 months, and the upper level for children aged 3 years, 6 months to 6 years, 11 months (and children aged 7 years to 8 years, 11 months who display low ability); and 2) the School-Age battery for children aged 7 years to 17 years, 11 months. The DAS-II yields an overall General Conceptual Ability (GCA) or single g factor, which evaluates an individual’s intellect based on their reasoning and conceptual abilities. The lower level of the Early Years battery has four core subtests that contribute to the GCA and two cluster scores: Verbal Ability and Nonverbal Ability. The upper level Early Years and School-Aged batteries require six core subtests to obtain the GCA and three cluster scores: Verbal Ability, Nonverbal Reasoning Ability, and Spatial Ability. Like other IQ tests, raw scores for each subtest are converted to a T score based on the individuals age (M = 50, SD = 10). The relevant T scores then yield a standard score for each cluster and the GCA (M = 100, SD = 15). On all subtests, clusters, and the GCA, lower scores reflect greater cognitive difficulties (a score of 69 or below is classified as “very low”; [72]). Each subtest, cluster, and the GCA demonstrate adequate internal reliability [72]. Across all ages, the internal reliability coefficient for each core subtest, cluster, and the GCA ranged from .82 to .94 for the Early Years battery, and .68 to .97 for the School-Aged battery. Test-retest coefficients for each core subtest, cluster, and GCA ranged from .63 to .91, and interscorer agreement ranged from .95 to .99. The DAS-II Introductory and Technical Handbook [72] outlines evidence to support both concurrent and construct validity. Previous studies have also established the validity of the DAS-II in the WS population [78].
Combining MSEL and DAS-II scores: In order to reduce the probability of making a Type-II error, the DAS-II cluster (verbal and nonverbal) and GCA scores, and the calculated MSEL DQ scores (all children under 68 months of age at Time 1; WS: n = 17), were combined to create single measures of global, verbal, and nonverbal ability for each WS preschool child. As such, the three DQ scores (i.e., global, verbal, and nonverbal; all children over 68 months of age at Time 1; WS: n = 1) were used for the analyses as a measure of each child’s development or intellect at Time 1 (for details, see [79]). Previous studies have also shown good convergent and concurrent validity for MSEL and DAS-II with young children with neurodevelopmental populations, including WS [58,76,79].
Procedure
This research was part of a wider research study. Ethics approval for this study was gained from the Macquarie University Human Research Ethics Committee (reference numbers: 5200900071 and 52021913524613). Details of the research were then sent to Williams Syndrome Australia Limited and the New Zealand Williams Syndrome Association, who, in turn, forwarded the information onto its members. Researchers were contacted directly by families who were interested in participating. Written consent was obtained from parents/guardians. Face-to-face testing was conducted at a location convenient for each family.
The MSEL and DAS-II was administered by a neuropsychologist in training (first author) who received appropriate instruction and supervision, and who administered the test according to the standardised administration manuals [69,72]. The MSEL Gross Motor subtest was not administered due to the restricted age range. In line with the MSEL administration manual [69], the order of items on this measure was randomised to maintain children’s motivation and avoid any systematic effects.
The BRIEF-P and BRIEF-C were administered to parents/ guardians in accordance with the Examiner’s Manuals [49,54], and were typically completed by the end of each testing session. In cases where parents were unable to complete the questionnaire at the time of face-to-face testing, the questionnaires were mailed back to the investigator within a four-week period from the time of testing.
Analytic Approach
Given the small sample size, corrections for multiple comparisons were not applied due to low power. To minimise the likelihood of a Type-II error, the p value was set was at 0.05 (see [80]), which is in line with other published studies with WS [15,81]. To support the decision to take this approach, moderate to large effect sizes have been reported to demonstrate that the findings are not simply a reflection of Type-I error (in line with recommendations by [82] and [80]) and to assist with interpretation. The effect sizes for r are as follows: <0.1=small; 0.1 to 0.5=medium, >.0.5=large; and the classification of effect sizes for d are as follows: <0.2=small; 0.2 to 0.8=medium, >.0.8=large [83]. Of note, Global DQ scores were found to violate assumptions, and, as such, nonparametric tests were utilised when analysing this data.
In the profile analyses, BRIEF-P profiles were examined at group and individual levels. One sample t-tests were used to compare the mean T scores on the three BRIEF-P indices (Inhibitory Self-Control Index, Flexibility, and Emergent Metacognition) and the Global Executive Composite with the normative sample population mean of 50 (standard deviation of 10). If a t-test analysis reached statistical significance, indicating a significant difference between the corresponding BRIEF-P scales, then a further series of one sample t-tests were employed to compare each of the underlying clinical scales. Repeated measures Analysis of Variance (ANOVA) were carried out to compare the within-group performance of the young WS children on each of the BRIEF-P indices and the clinical scale scores. A series of post hoc comparisons were then carried out to compare each of the BRIEF-P indices and clinical scales in order to examine patterns of performance. Boxplots were utilised to illustrate the variability in everyday EF abilities within the sample.
In the longitudinal analyses, T scores for the five corresponding BRIEF clinical scales at Time 1 (BRIEF-P) and Time 2 (BRIEF-C) were compared using paired-samples t-tests. To determine whether there was a reliable change between Time 1 and Time 2 BRIEF scores, a 90% confidence interval was calculated for each of the five corresponding clinical subscales. A reliable change was determined when there was no overlap between the two boundaries. Lastly, boxplots were utilised to illustrate the change in each group BRIEF clinical scale, index, and Global Executive Composite scores.
Due to the small sample, a series of correlations were used to investigate whether BRIEF-P ratings varied according to sex, chronological age, and DQ (Verbal, Nonverbal, and Global). Change over time in BRIEF scores (calculated as the difference between Time 1 BRIEF-P and Time 2 BRIEF-C scores) were then correlated with sex, chronological age (Time 1), and intellectual ability (Time 1).
Results
Bold font indicates clinically significant group T scores (≥ 65; M = 50, SD = 10). a Percentage of WS children who displayed a reliable change between Time 1 and Time 2 (as measured by the 90% confidence interval) for each of the five corresponding BRIEF-P (Time 1) and BRIEF-C (Time 2) clinical subscale scores [49,54]. Bold typeface indicates group mean and individual T scores which fell in the clinically elevated range.
BRIEF-P profile of preschool children with WS at Time 1
The mean and standard deviation across the BRIEF-P clinical scales, indices, and Global Executive Composite scores are presented in Table 2. On average, preschool children with WS displayed scores in the clinically significant range (T score at or above 65) for the domains of: Inhibit; Working Memory; Plan/ Organise; Inhibitory Self-Control Index; Emergent Metacognition Index; and Global Executive Composite. Table 2 also displays the percentage of WS preschool children in the clinically significant range for each clinical scale, index, and the Global Executive Composite at Time 1. Specifically, the Working Memory scale had the highest mean T score (79.06) and the highest percentage of WS preschool children in the clinically significant range (89%), followed by Plan/Organise with a mean T score of 70.39 (67% in the clinically elevated range), and Inhibit with a mean T score of 68.39 (61% in the clinically elevated range). Shift had the lowest mean T score (57.33) and the lowest percentage of individuals in the clinically significant range (22%). At the index level, the Emergent Metacognition Index had the highest percentage of WS preschool children in the clinically significant range (83%). The percentages of WS preschool children in the clinically significant range for each clinical scale and index paralleled their mean T score ranking.
Comparison of WS Preschoolers to the normative population (M = 50) at Time 1: One sample t-tests revealed that all BRIEF-P indices and the Global Executive Composite for the WS preschool group significantly exceeded the population normative mean of 50: Inhibitory Self-Control Index (t(17) = 5.49, p = .000), Flexibility Index (t(17) = 4.29, p = .000), Emergent Metacognition Index (t(17) = 10.96, p = < .001), and the Global Executive Composite (t(17) = 8.68, p = .000). As such, each of the WS group clinical scale scores were compared to the normative mean, with t-test analyses reaching statistical significance for all BRIEF-P scales: Inhibit, (t(17) = 5.97, p = < .001); Shift, (t(17) = 3.35, p = .004), Emotional Control, (t(17) = 4.03, p = .001); Working Memory, (t(17) = 12.11, p = < .001); and Plan/Organise, (t(17) = 8.28, p = < .001).
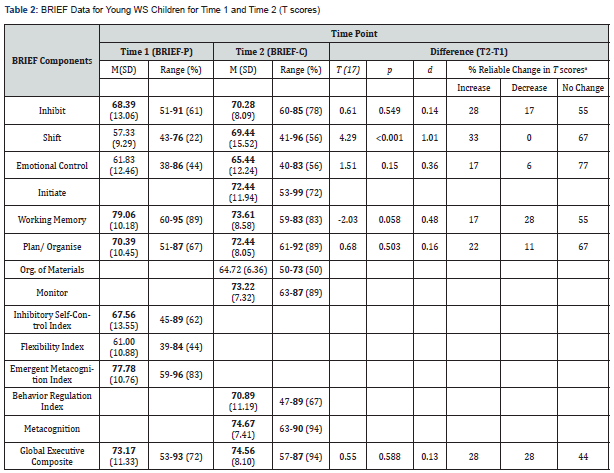
Note. The (%) indicates percentage of children with the clinically significant score of 65 or over on the BRIEF-P.
Within-group patterns of BRIEF-P clinical scales and indices at Time 1: Repeated measures ANOVA’s were conducted to determine whether the group performance of the preschool WS children varied significantly across the three index scores and five clinical scales. For the index scores, there was a main effect revealing significant differences between the indices, F(2, 34) = 22.59, p = < .001. Paired t-tests revealed that the mean Emergent Metacognition Index T score was significantly higher than both the mean of the Flexibility Index and the mean of the Inhibitory Self- Control Index T scores (higher scores indicated worse functioning; both p values < .001), and the mean Inhibitory Self-Control Index T score was significantly higher than the mean Flexibility Index T score (p = .002). For the clinical scales, Mauchly’s test indicated that the assumption of sphericity was not satisfied (2(9) = 20.05, p = .018), and degrees of freedom were corrected using the Greenhouse-Geisser estimate of sphericity (e = .65). Significant differences between the clinical scales were found, F(2.60, 44.16) = 19.54, p = < .001. Post hoc pairwise comparisons revealed that Working Memory T scores, on average, were significantly higher than the mean T scores on all other clinical scales (all p values < .003). The mean Inhibit T score significantly exceeded the mean Shift T score (p = .021). No significant differences were found between the group means for the Inhibit, Plan/Organise or the Emotional Control clinical scales (p = > .05). The mean Plan/ Organise T score was also not significantly different from the mean Emotional Control score (p = .140). Lastly, Shift scores, on average, were significantly lower than all clinical scales (all p values < .03) apart from Emotional Control (p = > .05).
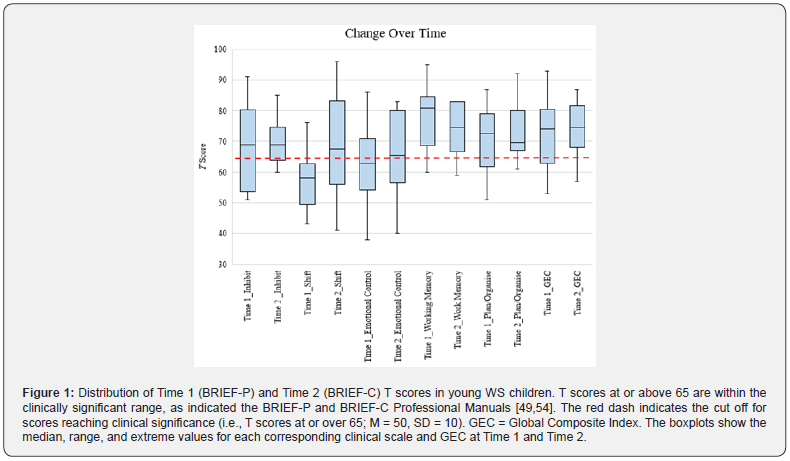
Variability in EF abilities at Time 1: At an individual level, there was variability in BRIEF-P scores with some WS preschool children displaying non-clinical ratings, and others displaying clinically elevated ratings; this occurred across all clinical scales, indices, and the Global Executive Composite (Table 2 and Figure 1).
Developmental course of EF in preschool WS children BRIEF-C profile of WS children at Time 2
Table 2 shows the mean and standard deviations on the BRIEF-C clinical scales, indices, and Global Executive Composite scores at Time 2. At this time point, and in contrast to Time 1, the BRIEF-C Plan/Organise and Monitor clinical scales showed the highest percentage of WS children who were rated in the clinically significant range (both with 89% of the sample), followed by Working Memory (83%), and Inhibit (78%). At the index level, the Metacognition Index had the highest percentage of WS children in the clinically significant range (94%). Although Organisation of Materials had the lowest mean T score and percentage ranking, it was on the cusp of falling in the elevated range (mean T score of 64.72) and half of the WS children fell in the clinically significant range on this scale. At an individual level, there continued to be great variability in BRIEF T scores at Time 2, with some WS children displaying scores in the normal range, and others displaying scores in the clinically elevated ranges across all clinical scales, indices, and the Global Executive Composite (Table 2 and Figure 1).
Change in EF over time: Change in EF (as calculated by the difference in Time 1 and Time 2 EF scores across the five comparable BRIEF clinical scales and Global Executive Composite) varied considerably for WS children, ranging from an increase of 34 and a decrease of 21 points. Paired-samples t-tests indicated that there was only one significant change (an increase) in BRIEF scores, on average, from Time 1 to Time 2, that being for the mean Shift T score (p = < .001). Although all other clinical scales and the Global Executive Composite scores failed to reach significance at the group level (Table 2), medium effect sizes for Emotional Control (an increase) and Working memory (a decrease) were also identified.
Reliable change of EF over time
To explore changes at an individual level, Table 2 shows the percentage of WS preschool children who displayed a significant reliable change in their BRIEF scores from Time 1 to Time 2 (i.e., a significant reliable increase, decrease, or no change at all). A significant reliable change was determined when there was no overlap between the two boundaries of the 90% confidence interval for each of the five corresponding BRIEF clinical subscale scores across the two time-points [49,54]. The Shift clinical scale showed the highest percentage of children with a reliable increase in their scores over time (33% of sample; denoting greater difficulty over time), followed by Inhibit (28%), Plan/Organise (22%), and then Emotional Control and Working Memory (both 17%). A significant reduction in scores (denoting reduced difficulty over time), was most common for the Working Memory clinical scale and the Global Executive Composite (both 28% of sample), followed by the Inhibit (17%), Plan/Organise (11%), and Emotional Control (6%) clinical scales. Shift did not include any WS children with a reliable decrease in difficulty over time. There also seemed to be a trend of more WS children displaying significantly greater difficulties over time in the Inhibit, Emotional Control, and Plan/Organise clinical scales, with an opposite trend seen in the Working Memory scale (i.e., more WS children experienced a significant reduction in difficulties on the latter scale). The Global Executive Composite had 28% of children with a reliable increase and decrease, with 44% showing no change in overall EF abilities. Overall, there was significant change in EF abilities over time in this sample of very young WS children, although substantial within-syndrome variability across the five corresponding BRIEF clinical scales was evident.
Variability in EF abilities at each timepoint
Figure 1 shows boxplots illustrating the distribution of the T scores for each corresponding clinical scale and the Global Executive Composite for Time 1 (as measured by the BRIEF-P) and Time 2 (as measured by the BRIEF-C). Of note, the box represents the interquartile range (containing 50% of the scores), the middle black line across each box represents the median T score, and the whiskers indicate the lowest and highest T scores. Figure 1 illustrates a change in the median T score and range of T scores on Shift (increasing) and Working Memory (decreasing) over time. Specifically, the Shift clinical scale appeared to be a relative strength at Time 1, although there appeared to be a great increase in impairment over time, with a higher median T score and range of T scores at Time 2. Working Memory remained a relative deficit at Time 1 and Time 2 in WS preschool children on average, however, the median T score and range of T scores had reduced over time. Although the median score on Inhibit and on the Global Executive Composite remained stable (falling in the clinically elevated range at Time 1 and at Time 2), the range of T scores appeared to be slightly reduced at Time 2 on both domains. Similarly, a relatively stable median was seen for Emotional Control, however the interquartile range had increased substantially at Time 2, indicating increased impairment over time. Overall, wide variability in EF abilities was observed across most domains for this sample of WS children at each timepoint, although the spread of scores reduced for some domains (e.g., Inhibit) and increased for others (e.g., Shift and Emotional Control) over time.
Relationship between EF and sex, chronological age, and DQ
Time 1: A series of Pearson and Spearman’s Rho correlation coefficients were performed to assess the relationship between the level of executive functioning in preschool children with WS and their respective sex and chronological age DQ score (verbal, nonverbal, and global) at Time 1.
Relationship between sex and EF: T scores did not differ significantly as a function of sex on the BRIEF-P clinical scales, indices, and Global Executive Composite at Time 1 (all p values > .05). There was, however, a positive medium effect size for the correlation between sex and Working Memory (rpb(18) = .32, p = .193), indicating that female WS children experienced increased difficulty with working memory at this young age. The mean BRIEF-P (Time 1) T scores by sex are presented in Appendix B.
Relationship between chronological age and EF: No significant relationships were identified between chronological age and the BRIEF-P clinical scales, indices, and Global Executive Composite (all p values > .05). However, a positive medium effect size was obtained between chronological age and Emotional Control (r(18) = .31, p = .215) indicating that, on average, as age increased WS children experienced greater difficulty controlling their emotions.
Relationship between verbal, nonverbal, and global DQ and EF: There was a significant, negative correlation between Verbal DQ and Flexibility (r(18) = -.48, p = .046). No other significant relationships were identified (all other p values > .05), however there was a negative, medium effect size between: Verbal DQ and Inhibit (r(18) = -.41, p = .091); Shift (r(18) = -.46, p = .057); Emotional Control (r(18) = -.36, p = .138); the Inhibitory Self- Control Index (r(18) = -.41, p = .093); and the Global Executive Composite (rs(18) = -.37, p = .130); as well as Nonverbal DQ and Plan/Organise (r(18) = -.32, p = .192); and Global DQ with both Inhibit (rs(18) = -.37, p = .136) and Shift (rs(18) = -.37, p = .137). These effect sizes suggested a clinically relevant association between DQ, particularly Verbal DQ, and executive functioning in young children with WS (i.e., higher DQ was associated with greater EF abilities). All correlations are reported in Table 3.
The relationship between change over time in EF and sex, chronological age, and DQ:
A series of Pearson and Spearman’s Rho correlation coefficients were performed to assess the relationship between sex, chronological age (Time 1), and DQ score (verbal, nonverbal, and global at Time 1) and the change in EF (calculated as the difference between the corresponding BRIEF clinical scale and global composite scores at Time 1 and Time 2).
The relationship between sex and EF over time: Sex was significantly and positively correlated with the change in Shift (rpb(18) = .51, p = .031), indicating that female WS children had greater difficulty shifting their attention over time. In contrast, a medium negative effect size was found between sex and the change in Working Memory (rpb(18) = -.40, p = .100), suggesting that males had more difficulty with this EF component over time. The mean BRIEF-C (Time 2) T scores by sex are presented in Appendix B.
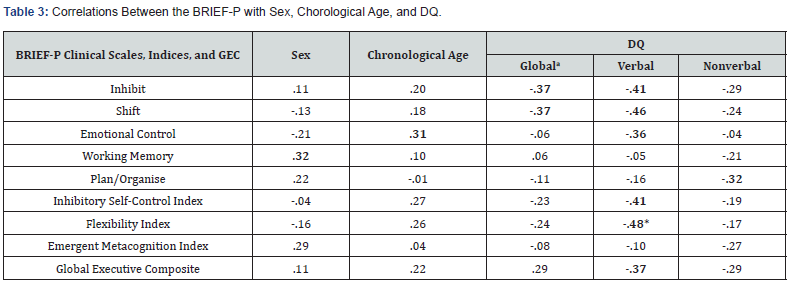
Note. aScores represent Spearman’s Rho correlation coefficient. Bold typeface indicates moderate and large effect sizes. * p < .05. ** p < .01.
The relationship between chronological age and EF over time: Although no significant relationships were identified here (all p values > .05), there was a medium, negative effect size obtained between chronological age at Time 1 and Shift (r(18) = -.35, p = .150), Emotional Control (r(18) = -.45, p = .060), and Working Memory (r(18) = -.39, p = .400) mean T scores over time. These results indicated that difficulties with Emotional Control, Shifting, and Working Memory increased over time in young WS children. However, there was a non-significant trend with a medium, positive effect size obtained between chronological age at Time 1 and change in the Global Executive Composite (r(18) = -.40, p = .102).
Relationship between verbal, nonverbal, and global DQ with EF over time: Significant and negative correlations were revealed between the Shift clinical scale and: Verbal DQ (r(18) = -.50, p = .035); Nonverbal DQ (r(18) = -.59, p = .010) and Overall DQ (rs(18) = -.48, p = .043). No other significant relationships were identified (all other p values > .05), but there was a negative, medium effect size between Emotional Control and intellectual abilities: Verbal DQ (r(18) = -.43, p = .076); Nonverbal DQ (r(18) = -.34, p = .167) and Global DQ (rs(18) = -.40, p = .102). These negative effect sizes suggested increased difficulty with shifting and emotional control over time in young WS children with lower Verbal, Nonverbal, and Global DQs. However, a positive, medium effect size was revealed between Inhibit and intellect: Verbal DQ (r(18) = .38, p = .123); Nonverbal DQ (r(18) = .31, p = .213) and Global DQ (rs(18) = .44, p = .067). A positive, medium effect size was also found between the Global Executive Composite and Nonverbal DQ (r(18) = .33, p = .184). These findings indicated that inhibition and overall EF abilities improved over time with higher DQ within this cohort. All correlations are reported in Table 4.
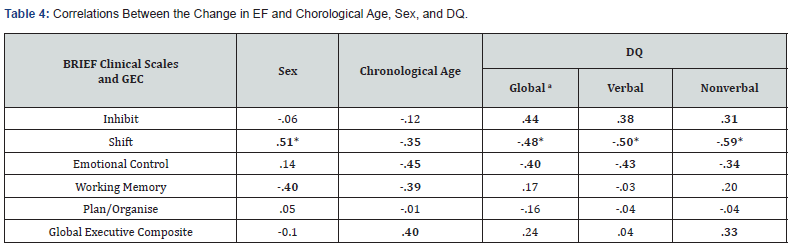
Note. a Scores represent Spearman’s Rho correlation coefficient. The ‘change in EF’ was calculated as the difference between the corresponding BRIEF clinical scale scores at Time 1 and Time 2. Bold typeface indicates moderate and large effect sizes.* p < .05. ** p < .01.
Discussion
This longitudinal study allowed us to track everyday EF abilities in young WS children over time, in terms of both absolute levels of EF and individual profiles of strength and weakness. There were four main findings from this study regarding our research aims, and these main findings are discussed below. Firstly, WS children displayed both impaired and intact functioning relative to the normative sample across different EF components, with, at the group level at least, relative deficits in working memory, planning/organisation, and inhibition, and relative sparing of shifting and emotional control, on average. This EF profile is consistent with Gallo [24]. The second main finding of the current study related to age-related increases in EF impairment, at least on average, for all areas of EF, apart from working memory. In line with previous BRIEF studies on older children and adults with WS (e.g., [9,10,58]), increases in EF difficulties were especially noted for Shift and Emotional Control. The third main finding was that the level of intellectual functioning is associated with EF at Time 1 (especially verbal DQ) and over time (particularly with shift) for WS preschool children. Finally, there was evidence of considerable individual variability, both in absolute levels of EF across and in terms of change in EF abilities over time.
Early profile of EF in WS preschool children (Time 1)
The first aim of the current study was to investigate the emerging profile of executive functioning in young children with WS. As predicted, and in line with the multidimensional theories of EF, the current study found that, on average, WS preschool children exhibited relative weaknesses on the Emergent Metacognition Index (comprised of the Working Memory and Plan/Organise clinical scales) and Inhibitory Self-Control Index (comprised of the Inhibit and Emotional Control clinical scales), with relative sparing of the Flexibility Index (comprised of the Shift and Emotional Control clinical scales), at least at this young age. The within-group profile of everyday EF in WS preschool children revealed the Emergent Metacognition Index was significantly higher than the Inhibitory Self-Control Index, on average. These findings are consistent with Gallo [24]. Greiner de Magalháes et al. [9] and Hocking & Reeve et al. [10] also reported a similar pattern of EF in WS school-aged children and adults.
Again, consistent with Gallo [24], the current study found that, on average, WS preschool children exhibited relative weaknesses on the Working Memory, Plan/Organise, and Inhibit BRIEF-P clinical scales, with relative sparing of Shift and Emotional Control. The within-group profile of everyday EF in WS preschool children revealed working memory was significantly more impaired than all other EF scales on the BRIEF-P at Time 1 testing. These findings suggest, perhaps, that there is a delay in the development of the neural structures involved in some EF constructs. Future longitudinal research is needed to track and compare frontal (brain) and EF development. There was no significant difference between the ratings on the Plan/Organise and Inhibit clinical scales, however both were significantly higher (more impaired) than Emotional Control. The Shift clinical scale was significantly lower than all other BRIEF-P scales, in line with Gallo [24], providing additional evidence that this ability is least impaired for WS individuals at this very young age. Indeed, a similar EF profile was identified in 25 preschool children with ADHD [85]. This finding is not surprising considering the high comorbidity of ADHD among WS individuals [35,36]. It could be that set shifting is developing at a normal rate in comparison to typically developing peers at this young age, and, as shifting abilities develop (rapidly during the school-age years;[86]), the gap in ability widens in WS individuals with age. As such, continued longitudinal research mapping the developmental trajectory of EF components in this population is required.
Consistent with Gallo [24], in the present study, the Emergent Metacognition Index had the highest percentage of WS preschool children in the clinically significant range, followed by the Inhibitory Self-Control Index. In terms specific EF components, Working Memory impairments were the most common EF deficit and were found in the majority of our young WS cohort. The Plan/Organise and Inhibit BRIEF-P clinical scales also displayed high percentages of WS preschool children who fell in the clinically elevated range. These deficits likely contribute towards difficulties with the development of social functioning, academic performance, and adaptive functioning skills. However, future studies examining the association between these areas of daily functioning and EF abilities in the WS preschool population is warranted. In comparison to Gallo [24], the current study found a higher percentage of WS preschool children with difficulties across all BRIEF-P clinical scales. This discrepancy may be explained by heterogeneity in WS and/or demographic and other sampling factors differences across the studies, for example the age differences between the two samples (our sample included children as young as two years of age).
Approximately three-quarters of the WS preschool sample were rated in the clinically significant range on the BRIEF-P Global Executive Composite, indicating overall clinically significant impairments in everyday EF for the majority of young people with WS. Our results support the notion that WS individuals’ experience significant EF difficulties, even at this very young age.
Developmental trajectory of EF in WS preschool children
The second study aim was to track the executive abilities of young WS children over time in a longitudinal study design. With the exception of the BRIEF Working Memory subscale (which saw a reduction in group scores at Time 2), the current findings indicated that young WS children generally displayed higher scores at Time 2, suggesting that the gap in the level of EF ability between WS and typically developing children is growing with age and time. This finding was particularly true for the BRIEF Shift and Emotional Control clinical scales, in line with previous studies on older children and adults with WS [9,10,58]. Although group working memory scores dropped over time, our results indicated that working memory deficits are more likely as WS children get older.
However, the variability in impairment across EF domains at both the group and individual level for young WS children highlights the need to examine potential risk or protective factors driving EF changes in WS individuals over time, as well as underlying biological factors at play. For example, environmental changes (i.e., going to school or work) may impact aspects of EF at different life stages, as the environmental demands placed on these individuals increase. Furthermore, impairments in shift, inhibition, and emotional control have been linked with higher levels of anxiety in older children and adults with WS [62]. For example, Kazzi et al. [58] found a significant relationship between increases in the symptomology for Generalised Anxiety Disorder (GAD) (an anxiety disorder that displays a high comorbidity among WS individuals; [35]) and greater shift, inhibition, working memory and emotional control difficulties, whilst controlling for chronological age and DQ/IQ in young WS children aged 3 to 9 years. Although the direction of these relationships remains unknown, it is possible that, at least partially, anxiety underlies executive dysfunction in some individuals with WS. Likewise, other common comorbidities such as ADHD, or emerging ADHD, must be examined in more detail in future WS research due to the established executive deficits in this disorder [87].
Interestingly, effect sizes between chronological age and the global composite score provided support that, overall, EF abilities [88,89]), one possible explanation is that early development of EF follows a more protracted development in WS. The developmental trajectory of EF is thought to parallel development in this region of the brain [39], thus following the protracted developmental course of the prefrontal cortex [21,90,91]. Empirical studies of neurotypical populations have also suggested the development of EF is a multistage process where different EF processes develop at different times and different rates [21]. During infancy and preschool years, for example, the prefrontal regions typically go through a critical period of rapid growth [53] and research has found typically developing preschool children (3 to 6 years) experience a large increase in their shifting, inhibitory control, and working memory capabilities [92-94].
As such, our results, taken together with the everyday EF profile of WS school-aged children and adults (e.g., [9,10]), lends support for a more protracted, multistage, and perhaps nonlinear, developmental trajectory in individuals with WS, such that, although EF abilities continue to develop over time, it is at a slower rate in comparison to the typically developing population. Thus, instead of increased impairments in each EF domain these findings may reflect a widening of the gap between the development of certain EF skills in WS and the typically developing population at certain timepoints. Again, environment may also be impacting aspects of EF at different life stages. Future longitudinal studies with larger samples over several different time points across an WS individual’s development (early childhood through to adulthood), and preferably in conjunction with brain imaging, will bring clarity to this issue.
EF is associated with sex, chronological age, and IQ
The third aim of the study was to determine whether sex, chronological age, and/or DQ were associated with EF at Time 1, and whether these variables impacted on the development of EF in this WS cohort over time.
Unconfirmed association between EF and sex: In contrast to Gallo [24] and Greiner de Magalháes et al. [9], in the present study, while no significant sex differences were identified in EF abilities, effect sizes suggested that female WS children experienced greater difficulty with working memory at this young age. However, this finding was not replicated when comparing raw scores, indicating that there are no significant sex differences in absolute skill levels on these two scales for the study cohort. As such, we propose two ideas. Firstly, the result may reflect sampling characteristics. Secondly, and in line with Greiner de Magalháes et al. [9], we propose that this result reflects differences in the normative sample (i.e., females typically displaying less difficulty than males in the general population, but perhaps not in WS), resulting in a higher T score being assigned to the same raw score. Overall, the relationship between EF and sex is not yet clear and, as such, warrants further investigation with larger sample sizes.
EF is concurrently associated with chronological age: No significant associations were revealed in the current study between EF and chronological age at Time 1, however, a medium effect size was found with Emotional Control. In line with the typically developing literature [95]. This result provides tentative support for the idea that WS children experience increased difficulties with their ability to regulate their emotions during this early period of development in comparison to same aged peers, possibly due to delays in development of the ventromedial prefrontal cortex and its neural networks [96-98]. Furthermore, as mentioned above, anxiety may also be, at least partially, driving these difficulties for some individuals with WS [58,62]. Of note, this finding contradicts Gallo [24] who found a significant association between chronological age and increased difficulty as reflected on the Inhibitory Self-Control Index, Emergent Metacognition Index, and the overall Global Executive Composite. However, as no associations between chronological age and BRIEF-P clinical scales were reported by Gallo [24], it is difficult to determine which areas of EF may be driving this result.
EF is concurrently associated with intellectual functioning: In line with Greiner de Magalháes et al. [9] and Hocking and Reeve et al. [10], a medium, negative effect size was revealed between overall DQ and the Inhibit and Shift BRIEF-P clinical scales, supporting the idea that lower DQs are associated with EF difficulties in these areas amongst those with WS. Targeted correlations revealed that the verbal developmental quotient was significantly associated with the BRIEF-P Flexibility ratings in this sample of WS preschool children. There were also indications of a relationship between verbal DQ and BRIEF ratings of: Inhibit; Shift; Emotional Control; Inhibitory Self-Control Index, and the Global Executive Composite. These results evidence an association between lowered verbal intelligence (or at least performance on these tasks that comprise verbal DQ) and increased EF deficits in this very young WS population, more specifically EF components of behavioural control. A strong association between verbal abilities and EF has also been reported in typically developing preschool children [99,100]. Indeed, using performance-based measures of EF, Landry et al. [101] also found preliminary evidence for the role verbal development has on cognitive flexibility in schoolaged children with WS. These results are in line with the verbal mediation model [102-104], which stipulates that language mediates executive behavioural control, and supports the notion that, like typically developing children [105], verbal development impacts aspects of EF in WS individuals. Future studies should look at this in more detail. A medium, negative effect size was also revealed between nonverbal DQ and Plan/Organise, providing evidence for the well documented visuospatial difficulties in WS individuals having an impact on the development of planning and organisational skills. Moderate effect sizes were also reported in adult WS individuals [10] between low Full-Scale IQ and poor planning and organisational skills. Overall, these findings suggest a clinically relevant association between IQ, particularly verbal, and executive functioning in very young children with WS (i.e., higher IQ is associated with greater EF abilities). Another possibility, however, is whether poor EF abilities impact or reduce performance on the measures of DQ/IQ.
Change in EF is associated with sex and DQ, over time: Significant associations were found between EF and sex, indicating that very young female WS children experience a greater difficulty with cognitive flexibility, over time. Further, effect sizes indicate that young female WS children experience improvements with working memory over time. Although previous studies have not employed a longitudinal design to explore the relationship between these variables and the changes over time, most WS studies at a single time point have not found evidence of sex related differences in EF abilities [9,24]. Only Gallo [24] reported a trend towards significance indicating female preschool children perform better than males on a performancebased task of inhibitory control. These findings are in line with typically developing children, such that females show improved working memory abilities [106] and males displaying poorer impulse control [107]. Moreover, these findings are in line with other neurodevelopmental disorders, like ADHD and Autism Spectrum Disorder (ASD), which have documented sex differences in the presentation of different EF components across the life span [108,109]. In fact, a recent review by Grissom & Reyes [110] suggested that sex differences in EF performance may be apparent due to the interactions with specific developmental processes involved, which are influenced by the interaction with genotype and/or other underlying biological factors, and environment, and impact EF deficits in specific disorders. A word of caution, however, due to the small sample size, these findings only offer preliminary results and future research should aim to explore these associations further.
Significant correlations were identified between lower DQ (verbal, nonverbal, and overall) and shifting or flexibility (on the BRIEF), over time. Furthermore, medium effect sizes indicated an association between lower DQ with increased impairment with emotional regulation and inhibitory control in young children with WS, over time. Although previous studies have only utilised a Full- Scale IQ score in their analyses, the current findings are consistent with previous research on older children and adults with WS [9,10,40]. More longitudinal research with larger sample sizes is required to further delineate differences between age groups and clarify the relationships between EF and each modality of DQ/IQ across the lifespan.
Heterogeneity of EF in WS preschool children: As predicted, there was considerable individual variability of EF abilities in WS preschool children at a single time point. For example, there was a lot of variability in terms of whether a WS child fell into the clinical range (or not) in each EF domain, and each individual profile of EF strengths and weaknesses. There was also significant individual variability in how EF abilities changed over time, with a proportion of WS children displaying both significant increases and reductions in difficulties in each EF domain at Time 2 follow-up testing, and how an individual’s EF profile changed over time. This finding of EF heterogeneity in young WS children is commensurate with the cognitive variability previously reported in WS individuals [22,23,74] and with biopsycho- social models such as that described by Dennis et al. [111], which states that biological, cognitive, and environmental events are all considered to impact an individual’s cognitive phenotype. As suggested in typically developing children [112], it is very likely that the developmental trajectory of EF processes varies across WS individuals. However, as the prefrontal cortex is dependent on an extensive network of neural connections to many different regions of the brain [113], a disruption at any level or stage of development may result in an impairment that resembles executive dysfunction [114]. As such, a possible explanation for individual variabilities in everyday EF found in the current study may be associated with their social environment (e.g., family dynamics, temperament; either risk or protective factors) or biological characteristics (e.g., genetics; either risk and protective factors) as reported by studies in typically developing individuals [115] and other neurodevelopmental disorders such as ASD and ADHD [116].
Clinical Implications
As EF plays a major role in a child’s academic, social, and adaptive development [19,112,117], it is essential for clinicians to not only understand the unique profile of EF in preschool children with WS, but also to understand how EF abilities develop (or fail to develop properly) over time in this young population. As such, assessment of executive functions in very young children with WS is advised in order to provide early targeted intervention. Furthermore, the findings may direct thoughts to ways that EF impairments could be minimised in WS preschool children through interventions that are targeted to their specific deficits, as well as ways to minimise any functional impairments associated with EF delays or dysfunction [118].
While everyday EF development in WS preschoolers is clearly atypical relative to their typically developing peers (as evident by the uneven EF profile), longitudinal examination of individual clinical scales and indices on the BRIEF lends some support that EF development may be delayed rather than atypical (i.e., the psychometric deficits seen on testing indicate a widening of the gap between the abilities of typically developing children), at least for some EF domains.
Also, findings from the current study suggest that EF can be differentiated as evidenced by relative strengths and weaknesses of individual EF domains, and the changes in EF functioning over time, providing further support for multidimensional models of EF [19,44,47]. Lastly, given the wide individual variability in EF abilities across this sample of young WS children, in line with the bio-psycho-social model (described above; [111], it is imperative that environmental contributions to these EF deficits be understood and incorporated into early intervention programs to improve outcomes in this population.
Limitations and future research
A unique aspect of this study was the longitudinal design, which allowed exploration of the development of EF components across time, as well as their association with chronological age, sex, and IQ in a sample of WS preschool children. Nonetheless, the current study had several limitations that should be considered when interpreting the study findings and that need to be addressed in future research. Firstly, although it is comparable to most WS studies to date, the sample size was relatively small (N = 18) and, as such, this limited statistical power. Although the significance levels and effect sizes were usually indicative of adequate power, a larger sample size would produce more robust results, less sampling error and improve the generalisability of the findings. Unfortunately, due to the rarity of the syndrome, it is very difficult to recruit large numbers, particularly for longitudinal studies and for a restricted age range. To overcome this, however, it is recommended that future work engage in a more collaborative, multisite, and even international research approach. This would be particularly useful for allowing further examination of the impact of not only demographic, but also cultural factors on the development of EF in WS preschool children. Another issue pertains to the reliance on caregiver reports of everyday EF deficits, which may lead to retrospective or subjective rater bias. To overcome this issue, it is recommended that future studies incorporate data from multiple informants. As self-report measures are not appropriate for such a young cohort, future research should combine performance-based measures of EF and informant reports from multiple sources (i.e., caregivers and preschool teachers) to assess EF in WS preschool children, with the view that, although the level of agreement between these measures is usually low [19,24], each form of assessment provides unique and specialised information. As EF deficits have been shown to impact adaptive functioning, social functioning, and academic outcomes in WS individuals, it is recommended that future studies examine the role EF plays in the early development of these difficulties. Finally, future longitudinal research is needed, and it is recommended that this research includes other neurodevelopmental conditions as a comparison, to determine whether the developmental trajectory identified in WS preschool children is syndrome-specific, or perhaps, characteristic to preschool children with syndromes associated with intellectual disabilities. Ideally, brain imaging may be utilised as well as neuropsychological measures, in addition to the collection of a wide range of data to help tease out environmental and biological contributions.
Conclusion
In summary, the current study highlights particular weaknesses in working memory, planning/organisation, and inhibition, and relative strengths in shifting and emotional control, in young children with WS. Preliminary evidence for the developmental trajectory of EF abilities in this syndrome is also evidenced, with increased impairments in shifting, emotional control, and working memory over time and chronological age in this young cohort. The present study indicates that EF abilities need to be assessed and tracked from an early age in those with WS, with important implications for intervention and improving daily functioning in all aspects of life. Considerable individual variability was evidenced in EF abilities over time, confirming the importance of individual assessments and developing individualised management programs. The current study extends previous research by examining, for the first time, the early patterns of change in everyday EF in WS.
Acknowledgements
This research was part of a wider research study. It was supported financially by an Australian Government Research Training Program (RTP) scholarship, Australian Postgraduate Award (APA; 2014 to 2017), and the Macquarie University Postgraduate Research scholarship (2016) awarded to Jessica Reeve, and a research grant from Williams Syndrome Australia Limited awarded to Melanie Porter. We are grateful to the families and WS children who participated in the research, and for the continuing support from Williams Syndrome Australia Limited and the New Zealand Williams Syndrome Association, without whom this research would not have been possible.
Appendix A: Behaviour Rating Inventory of Executive Function
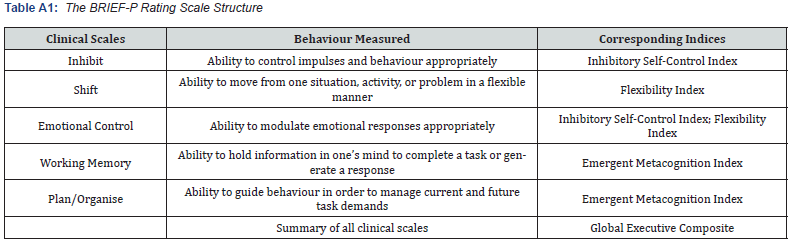
Note. Adapted from the “Behavior Rating Inventory of Executive Function-Preschool Version” by [54]
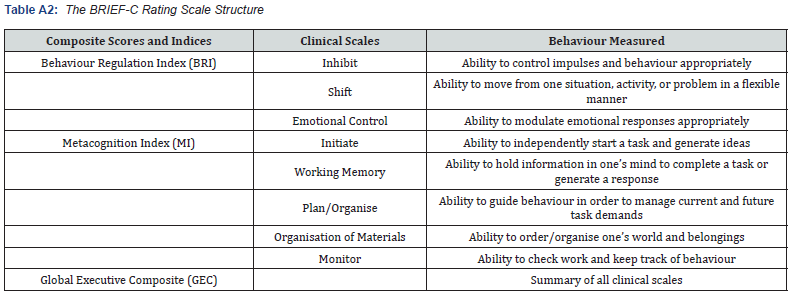
Note. BRI = sum of Initiate, Shift, and Emotional Control; MI = sum of Initiate, Working Memory, Plan/Organise, Organisation of Materials, and Monitor; GEC = sum of all clinical scales. Adapted from the “Behavior Rating Inventory of Executive Function” by [49]
Appendix B
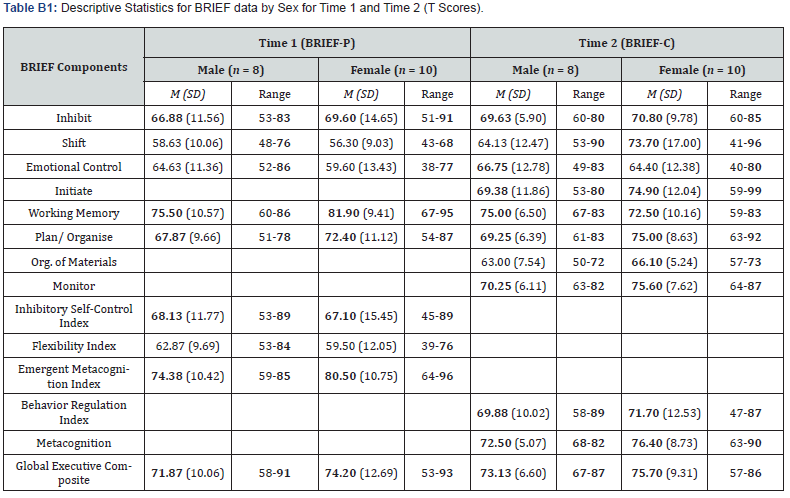
Note. T scores (population M = 50, SD = 10). Bold typeface indicates group mean and individual scores that fall within the clinically impaired range (T score at or over 65; for further details see [49,54]).
References
- Fryssira H, Palmer R, Hallidie-Smith KA, Taylor J, Donnai, D, et al. (1997) Flourescent in Situ Hybridisation (FISH) for hemizygous deletion at the elastin locus in patients with isolated supravalvar aortic stenosis. Journal of Medical Genetics 34(4): 306-308.
- Porter MA, Dobson-Stone C, Kwok JB, Schofield PR, Beckett W, et al. (2012) A role for transcription factor GTF2IRD2 in executive function in Williams-Beuren syndrome. PloS ONE 7(10): e47457.
- Schubert C (2009) The genomic basis of the Williams-Beuren syndrome. Cellular and Molecular Life Sciences 66(7): 1178-1197.
- Tassabehji M (2003) Williams-Beuren syndrome: A challenge for genotype-phenotype correlations. Human Molecular Genetics 12 (2): 229-237.
- Hillier LW, Fulton RS, Fulton LA, Graves TA, Pepin KH, et al. (2003) The DNA sequence of human chromosome 7. Nature 424(6945): 157-164.
- Strømme P, Bjømstad PG, Ramstad K (2002) Prevalence estimation of Williams syndrome. Journal of Child Neurology 17(4): 269-271.
- Jackowski AP, Rando K, de Araújo CM, Del Cole CG, Silva I, et al. (2009) Brain abnormalities in Williams syndrome: A review of structural and functional magnetic resonance imaging findings. European Journal of Paediatric Neurology 13(4): 305-316.
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, et al. (2004) An experiment of nature: Brain anatomy parallels cognition and behavior in Williams syndrome. Journal of Neuroscience 24(21): 5009-5015.
- Greiner de Magalhães C, Pitts CH, Mervis CB (2022) Executive function as measured by the Behavior Rating Inventory of Executive Function‐2: Children and adolescents with Williams syndrome. Journal of Intellectual Disability Research 66(1-2): 94-107.
- Hocking DR, Reeve J, Porter MA (2015) Characterising the profile of everyday executive functioning and relation to IQ in adults with Williams syndrome: Is the BRIEF adult version a valid rating scale? PloS ONE 10(9): e0137628.
- Brawn G, Porter M (2014) Adaptive functioning in Williams syndrome and its relation to demographic variables and family environment. Research in Developmental Disabilities 35(12): 3606-3623.
- Menghini D, Addona F, Costanzo F, Vicari S (2010) Executive functions in individuals with Williams syndrome. Journal of Intellectual Disability Research 54(5): 418-432.
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, et al. (2005) Neural correlates of genetically abnormal social cognition in Williams syndrome. Nature Neuroscience 8(8): 991-993.
- Porter MA, Coltheart M, Langdon R (2007) The neuropsychological basis of hypersociability in Williams and Downs syndrome. Neuropsychologia 45: 2839-2849.
- Rhodes SM, Riby DM, Park J, Fraser E, et al. (2010) Executive neuropsychological functioning in individuals with Williams syndrome. Neuropsychologia 48(5): 1216-1226.
- Burgess PW, Alderman N, Evans JON, Emslie H, Wilson BA (1998) The ecological validity of tests of executive function. Journal of the International Neuropsychological Society 4(6): 547-558.
- Burgess PW, Alderman N, Forbes C, Costello A, Laure MC (2006) The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. Journal of the International Neuropsychological Society 12(2): 194-209.
- Isquith PK, Roth RM, Gioia G (2013) Contribution of rating scales to the assessment of executive functions. Applied Neuropsychology: Child 2(2): 125-132.
- Anderson P (2002) Assessment and development of executive function (EF) during childhood. Child Neuropsychology 8(2): 71-82.
- Pennington BF, Ozonoff S (1996) Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry 37(1): 51-87.
- Welsh MC, Pennington BF (1988) Assessing frontal lobe functioning in children: Views from developmental psychology. Developmental Neuropsychology 4(3): 199-230.
- Miezah D, Porter M, Batchelor J, Boulton K, Veloso GC (2020) Cognitive abilities in Williams syndrome. Research in Developmental Disabilities 104: 103701.
- Martens MA, Wilson SJ, Reutens DC (2008) Research review: Williams syndrome: A critical review of the cognitive, behavioral, and neuroanatomical phenotype. The Journal of Child Psychology and Psychiatry 49(6): 576-608.
- Gallo FJ (2009) Executive functions in young children with Williams syndrome. The University of Wisconsin, Milwaukee.
- Eckert MA, Tenforde A, Galaburda AM, Bellugi U, Korenberg JR, et al. (2006) To modulate or not to modulate: Differing results in uniquely shaped Williams syndrome brains. Neuroimage 32(3): 1001-1007.
- Gothelf D, Searcy YM, Reilly J, Lai PT, Lanre‐Amos T, et al. (2008) Association between cerebral shape and social use of language in Williams syndrome. American Journal of Medical Genetics Part A 146(21): 2753-2761.
- Marenco S, Siuta MA, Kippenhan JS, Grodofsky S, Chang WL, et al. (2007) Genetic contributions to white matter architecture revealed by diffusion tensor imaging in Williams syndrome. Proceedings of the National Academy of Sciences 104(38): 15117-15122.
- Rae C, Karmiloff-Smith A, Lee MA, Dixon RM, Grant J, et al. (1998) Brain biochemistry in Williams syndrome: Evidence for a role of the cerebellum in cognition? Neurology 51(1): 33-40.
- Meyer-Lindenberg,A, Mervis CB, Faith Berman K (2006) Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nature Reviews Neuroscience 7(5): 380-393.
- Carney DP, Brown JH, Henry LA (2013) Executive function in Williams and Down syndromes. Research in Developmental Disabilities 34(1): 46-55.
- Jones W, Bellugi U, Lai Z, Chiles M, Reilly J, et al. (2000) II. Hypersociability in Williams syndrome. Journal of Cognitive Neuroscience 12(1): 30-46.
- Dodd HF, Porter MA (2009) Psychopathology in Williams syndrome: The effect of individual differences across the life span. Journal of Mental Health Research in Intellectual Disabilities 2(2): 89-109.
- Dykens EM (2003) Anxiety, Fears, and Phobias in persons with Williams syndrome. Developmental Neuropsychology 23(1-2): 291-316.
- Einfeld SL, Tonge BJ, Florio T (1997) Behavioural and emotional disturbance in individuals with Williams syndrome. American Journal on Intellectual and Developmental Disability 102(1): 45-53.
- Kozel BA, Barak B, Kim C, Mervis CB, Osborne LR, et al. (2021) Williams syndrome. Nature Reviews Disease Primers 7(1): 1-22.
- Leyfer OT, Woodruff-Bordon J, Klein-Tasman BP, Fricke JS, Mervis CB (2006) Prevalence of psychiatric disorders in 4 to 6-year-olds with Williams syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 141B(6): 615-622.
- Ursache A, Raver CC (2014) Trait and state anxiety: Relations to executive functioning in an at-risk sample. Cognition & Emotion 28(5): 845-855.
- Willcutt, EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF (2005) Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry 57(11): 1336-1346.
- Anderson V (1998) Assessing executive functions in children: Biological, psychological, and developmental considerations. Neuropsychological Rehabilitation 8(3): 319-349.
- Osório A, Cruz R, Sampaio A, Garayzábal E, Martínez-Regueiro R, et al. (2012) How executive functions are related to intelligence in Williams syndrome. Research in Developmental Disabilities 33(4): 1169-1175.
- Waid-Ebbs JK, Wen PS, Heaton SC, Donovan NJ, Velozo C (2012) The item level psychometrics of the behaviour rating inventory of executive function-adult (BRIEF-A) in a TBI sample. Brain Injury 26(13-14): 1646-1657.
- Baddeley AD (1986) Working memory. Oxford: Oxford University Press.
- Norman DA, Shallice T (1986) Attention to action: Willed and automatic control of behavior. In RJ Davidson, GE Schwartz, D Shapiro (Eds.), Consciousness and self-regulation: Advances in Research and Theory New York: Plenum Press 4: 1-18.
- Anderson PJ (2008) Towards a developmental model of executive function. In V Anderson, R Jacobs, PJ Anderson (Eds.), Executive functions and the frontal lobes: A lifespan perspective p: 3-22.
- Lanfranchi S, Jerman O, Dal Pont E, Alberti A, Vianello R (2010) Executive function in adolescents with Down syndrome. Journal of Intellectual Disability Research 54(4): 308-319.
- Metcalfe J, Mischel W (1999) A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review 106(1): 3-19.
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter, A, et al.(2000) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology 41(1): 49-100.
- Rowe J, Lavender A, Turk V (2006) Cognitive executive function in Down's syndrome. British Journal of Clinical Psychology 45(1): 5-17.
- Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000): Behavior Rating Inventory of Executive Function. Child Neuropsychol 6(3):235-238.
- Zelazo PD, Qu L, Müller U (2005) Hot and cool aspects of executive function: Relations in early development. In W Schneider, R Schumann-Hengsteler, B Sodian (Eds.), Young children's cognitive development: Interrelationships among executive functioning, working memory, verbal ability, and theory of mind. Lawrence Erlbaum Associates Publishers. P:71-93.
- Grattan LM, Eslinger PJ (1989) Higher cognition and social behavior: Changes in cognitive flexibility and empathy after cerebral lesions. Neuropsychology 3(3): 175-185.
- Willner P, Bailey R, Parry R, Dymond S (2010) Evaluation of executive functioning in people with intellectual disabilities. Journal of Intellectual Disability Research 54(4): 366-379.
- Anderson PJ, Reidy N (2012) Assessing executive function in preschoolers. Neuropsychology Review 22(4): 345-360.
- Gioia GA, Espy KA, Isquith P (2003) Behavior Rating Inventory of Executive Function-Preschool Version. Lutz, FL: Psychological Assessment Resources, Inc.
- Gioia GA, Isquith PK, Guy SC, Kenworthy L (2015) BRIEF-2: Behavior Rating Inventory of Executive Function. Lutz, FL, Psychological Assessment Resources.
- Roth RM, Isquith PK, Gioia GA (2005) Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A). Lutz, FL: Psychological Assessment Resources.
- Camp JS, Karmiloff-Smith A, Thomas MS, Farran EK (2016) Cross-syndrome comparison of real-world executive functioning and problem solving using a new problem-solving questionnaire. Research in Developmental Disabilities 59: 80-92.
- Kazzi C, Porter M, Zhong Q, Veloso G, Reeve J (2021) The relationship between anxiety and executive functioning in children with Williams syndrome. Global Journal of Intellectual & Developmental Disabilities 8(4): 1-6.
- Costanzo F, Varuzza C, Menghini D, Addona F, Gianesini T, et al. (2013) Executive functions in intellectual disabilities: A comparison between Williams syndrome and Down syndrome. Research in Developmental Disabilities 34(5): 1770-1780.
- Mobbs D, Eckert MA, Mills D, Korenberg J, Bellugi U, et al. (2007) Frontostriatal dysfunction during response inhibition in Williams syndrome. Biological Psychiatry 62(3): 256-261.
- Greer J, Riby DM, Hamiliton C, Riby LM (2013) Attentional lapse and inhibition control in adults with Williams Syndrome. Research in Developmental Disabilities 34(11): 4170-4177.
- Ng-Cordell E, Hanley M, Kelly A, Riby DM (2018) Anxiety in Williams syndrome: the role of social behaviour, executive functions and change over time. Journal of Autism and Developmental Disorders 48(3): 796-808.
- Little K, Riby DM, Janes E, Clark F, Fleck R, et al. (2013) Heterogeneity of social approach behaviour in Williams syndrome: the role of response inhibition. Research in Developmental Disabilities 34(3): 959-967.
- Green T, Avda S, Dotan I, Zarchi O, Basel‐Vanagaite L, et al. (2012). Phenotypic psychiatric characterization of children with Williams syndrome and response of those with ADHD to methylphenidate treatment. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 159(1): 13-20.
- Diamond A (1988) Abilities and neural mechanisms underlying AB performance. Child development 59(2):523-527.
- Espy KA, Kaufmann PM, McDiarmid MD, Glisky ML (1999) Executive functioning in preschool children: Performance on A-not-B and other delayed response format tasks. Brain and cognition 41(2): 178-199.
- Zelazo PD (2006) The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nature protocols 1(1): 297-301.
- Korkman M, Kirk U, Kemp SL (1998) NEPSY. A developmental neuropsychological assessment. San Antonio, TX: Harcourt Brace.
- Mullen EM (1995) Mullen scales of early learning. Circle Pines, MN: American Guidance Service 1:6.
- Korenberg JR, Chen XN, Hirota H, Lai Z, Bellugi U, et al. (2000). VI. Genome structure and cognitive map of Williams syndrome. Journal of cognitive neuroscience 12(Supplement 1): 89-107.
- Tassabehji M, Metcalfe K, Fergusson WD, Carette MJ, Dore JK, et al. (1996) LIM-kinase deleted in Williams syndrome. Nature Genetics 13(3): 272-273.
- Elliott CD (2007) Differential Ability Scales-Second Edition: Introductory and technical handbook. San Antonio: Harcourt Assessment.
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP, et al. (2000) The Williams syndrome cognitive profile. Brain and Cognition 44(3): 604-628.
- Porter MA, Coltheart M (2005) Cognitive heterogeneity in Williams syndrome. Developmental Neuropsychology 27(2): 275-306.
- Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE (2002) Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychology 8(4): 241-248.
- Farmer C, Golden C, Thurm A (2016) Concurrent validity of the differential ability scales, with the Mullen Scales of Early Learning in young children with and without neurodevelopmental disorders. Child Neuropsychology 22(5): 556-569.
- Rogers SL, Estes A, Lord C, Visara L, Winter J, et al. (2012) Effects of a brief Early Start Denver Model (ESDM)-Based parent intervention on toddlers at risk for Autism Spectrum Disorders: A randomized control trial. Journal of the American Academy of Child & Adolescent Psychiatry 51(10): 1052-1065.
- Mervis CB, Pitts CH (2015) Children with Williams syndrome: Developmental trajectories for intellectual abilities, vocabulary abilities, and adaptive behavior. American Journal of Medical Genetics Part C: Seminars in Medical Genetics 169(2): 158-171.
- Bishop S, Guthrie W, Coffing M, Lord C (2011) Convergent validity of the Mullen Scales of Early Learning and the Differential Ability Scales in children with autism spectrum disorders. American Journal on Intellectual and Developmental Disabilities 116(5): 331-343.
- Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1(1): 43-46.
- Porter MA, Dodd H, Cairns D (2009) Psychopathological and behavior impairments in Williams-Beuren syndrome: The influence of gender, chronological age, and cognition. Child Neuropsychology 15(4): 359-374.
- Nakagawa S (2004) A farewell to Bonferroni: The problems of low statistical power and publication bias. Behavioral Ecology 15(6): 1044-1045.
- Cohen J (1988) Statistical power analysis for the behavioural sciences. Hillsdale, NJ: Erlbaum.
- Australian Bureau of Statistics (2016) Census of population and housing: Socio-Economic Indexes for Areas (SEIFA), Australia.
- Mahone EM, Hoffman J (2007) Behavior ratings of executive function among preschoolers with ADHD. The Clinical Neuropsychologist 21(4): 569-586.
- Luciana M, Nelson CA (1998) The functional emergence of prefrontally-guided working memory systems in four-to eight-year-old children. Neuropsychologia 36(3): 273-293.
- Lawrence V, Houghton S, Douglas G, Durkin K, Whiting K, et al. (2004) Executive function and ADHD: A comparison of children’s performance during neuropsychological testing and real-world activities. Journal of Attention Disorders 7(3): 137-149.
- Casey BJ, Giedd JN, Thomas KM (2000) Structural and functional brain development and its relation to cognitive development. Biological Psychology 54(1-3): 241-257.
- Casey BJ, Tottenham N, Liston C, Durston S (2005) Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences 9(3): 104-110.
- Benes FM (2001) The development of prefrontal cortex: The maturation of neurotransmitter systems and their interactions. In CA Nelson & M Luciana (Eds.), Handbook of developmental cognitive neuroscience pp: 79-105.
- Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology 387(2): 167-178.
- Garon N, Bryson SE, Smith IM (2008) Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin 134(1): 31-60.
- Montgomery DE, Koeltzow TE (2010) A review of the day-night task: The Stroop paradigm and interference control in young children. Developmental Review 30(3): 308-330.
- Zelazo PD, Carlson SM, Kesek A (2008) The development of executive function in childhood. In C. A Nelson & M Luciana (Eds.), Handbook of developmental cognitive neuroscience pp. 553-574.
- Orgeta V (2009) Specificity of age differences in emotion regulation. Aging and Mental Health 13(6): 818-826.
- Casey BJ, Heller AS, Gee DG, Cohen AO (2019) Development of the emotional brain. Neuroscience Letters 693: 29-34.
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, et al. (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences 110(39): 15638-15643.
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, et al. (2013) A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. Journal of Neuroscience 33(10): 4584-4593.
- Carlson SM (2005) Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology 28(2): 595-616.
- Hughes C (1998) Executive function in preschoolers: Links with theory of mind and verbal ability. British Journal of Developmental Psychology 16(2): 233-253.
- Landry O, Russo N, Dawkins T, Zelazo PD, Burack JA (2012) The impact of verbal and nonverbal development on executive function in Down syndrome and Williams syndrome. Journal on Developmental Disabilities 18(2).
- Luria AR (1961) The role of speech in the regulation of normal and abnormal behavior. New York: Liveright.
- Luria AR, Wertsch JV (1981) Language and cognition. Washington, DC: V.H. Winston.
- Vygotsky LS (1962) Thought and language. Cambridge, MA.: MIT Press.
- Joseph RM, McGrath LM, Tager-Flusberg H (2005) Executive dysfunction and its relation to language ability in verbal school-age children with autism. Developmental Neuropsychology 27(3): 361-378.
- Lejbak L, Vrbancic M, Crossley M (2009) The female advantage in object location memory is robust to verbalizability and mode of presentation of test stimuli. Brain and Cognition 69(1): 148-153.
- Barnett JH, Jones PB, Robbins TW, Müller U (2007) Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Molecular Psychiatry 12(5): 502-509.
- Gurvich C, Rossell SL (2015) Dopamine and cognitive control: sex-by-genotype interactions influence the capacity to switch attention. Behavioural Brain Research 281: 96-101.
- White EI, Wallace GL, Bascom J, Armour AC, Register‐Brown K, et al. (2017) Sex differences in parent‐reported executive functioning and adaptive behavior in children and young adults with autism spectrum disorder. Autism Research 10(10): 1653-1662.
- Grissom NM, Reyes TM (2019) Let’s call the whole thing off: evaluating gender and sex differences in executive function. Neuropsychopharmacology 44(1): 86-96.
- Dennis M, Landry SH, Barnes M, Fletcher JM (2006) A model of neurocognitive function in spina bifida over the life span. Journal of the International Neuropsychological Society 12(2): 258-296.
- Isquith PΚ, Gioia GA, Espy KA (2004) Executive function in preschool children: Examination through everyday behavior. Developmental Neuropsychology 26(1): 403-422.
- Heyder K, Suchan B, Daum I (2004) Cortico-subcortical contributions to executive control. Acta Psychologica 115(2-3): 271-289.
- Alexander MP, Stuss DT (2000) Disorders of frontal lobe functioning. In Seminars in neurology. Semin Neurol 20(4): 427-437.
- Fay‐Stammbach T, Hawes DJ, Meredith P (2014) Parenting influences on executive function in early childhood: A review. Child development Perspectives 8(4): 258-264.
- Greene CM, Braet W, Johnson KA, Bellgrove MA (2008) Imaging the genetics of executive function. Biological Psychology 79(1): 30-42.
- Bull R, Scerif G (2001) Executive functioning as a predictor of children's mathematics ability: Inhibition, switching, and working memory. Developmental Neuropsychology 19(3): 273-293.
- Rhodes SM, Riby DM, Fraser E, Campbell LE (2011) The extent of working memory deficits associated with Williams syndrome: Exploration of verbal and spatial domains and executively controlled processes. Brain and Cognition 77(2): 208-214.






























