Bacteriospermia among Asymptomatic Infertile Males, a Trigger for Morphological and Physiological Deterioration of Spermatozoa
Shakeel Waqqar1,3*, Isaac Asher2, Sonia Riaz3, Rabia Shakeel3, Moh'd Nawaz 3 and Shahid Hussain Sheikh 3
1Center for Research in Molecular Medicine, The University of Lahore, Pakistan
2Pakistan Institute of Medical Institute, Pakistan
3NIDS Treatment & Research Center, Pakistan
Submission: June 30, 2017; Published: July 28, 2017
*Corresponding author: Shakeel Waqqar, Center for Research in Molecular Medicine, NIDS Treatment & Research Center, Pakistan Institute of Medical Institute, Islamabad, The University of Lahore, Lahore, Pakistan, Email: shakeel.waqqar@imbb.uol.edu.pk
How to cite this article: Shakeel W, Isaac A, Sonia R, Rabia S, Moh'd N, et al.Bacteriospermia among Asymptomatic Infertile Males, a Trigger for Morphological and Physiological Deterioration of Spermatozoa. Glob J Intellect Dev Disabil. 2017; 2(2): 555584. DOI: 10.19080/GJIDD.2017.02.555584
Abstract
Male genitourinary tract can harbor different pathogens yet most individuals are asymptomatic with bacteriospermia. Leukospermia and bacteriospermia are often associated with the male infertility but clear definition and role of these conditions is not very clear. A total of 510 individuals participated in this study. Cultures from Oligospermic infertile male were compared with normal health fertile males. Data analysis showed that infection rates among infertile males with oligospermia were greater compared with controls. We also found that presence of different bacterial species can alter quality of semen to a variable extent. We found that bacterial isolates are associated with semen changes and types of bacterial pathogen and their association with semen changes could be different in different settings. We feel that duration of bacteriospermia shell be considered and large data sets are required with follow up of the participants to determine true effects and extent of the damage caused by bacteriospermia.
Introduction
Male can harbor a number of pathogens in their genital tract without producing significant signs and symptoms i.e. the infections remain sub-clinical. Role of urogenital tract infections and bacteriospermia is being considered as a leading cause of the male infertility. Urogenital infections by viruses and bacteria are thought to be the major etiological agents among the infertile male population [1].
Patients with two years of attempts to conceive without using any contraceptives are considered as sub fertile. Chances of achieving pregnancy for a healthy couple during the first six month are 60 percent which increases to 92 percent by the second year [2]. Infertility is greater in the under developed countries as a result of the lesser resources to the diagnostics and treatment [3]. Infertility affects 13-15 percent of the couples, in these infertile couples, male factor is responsible for 60 percent either directly or indirectly [4].
Asymptomatic bacteriospermia could be a greater risk factor, infection of the accessory sex glands, bacteriospermia is associated with a number of pathological and physiological changes, like structural changes, reduced motility, compromised spermatozoa cell integrity, initiation of autoimmune processes and deteriorated spermatogenesis [1,5,6].
Members of the Enterobacteriaceae family, which belongs to gram negative enteric bacteria and staphylococcus which belongs to gram positive bacteria are associated with semen changes [7]. Other bacterial and viral pathogens involved in infections of the male genital tract are N. gonorrhea C.trachomatis, Cytomegalo virus (CMV), Hepatitis B virus (HBV), Epstein-Barr virus (EBV), Human papilloma virus (HPV) and Herpes simplex virus (HSV) are believed to be present in the semen of infertile males with leukospermia [8,9]. Leukospermia or pyosemia are the terms used to indicate the number of presence of white blood cells in the semen in excessive numbers i.e. greater than 106 WBC's per ML of semen (WHO 1999).
Treatment for the leukospermia is controversial [10] because presence of WBC's in the semen showed contradictory results [11,12]. But in contrast C. trichomatis showed strong association with the leukospermia, when nested PCR techniques were used [13]. Many viruses are considered as the etiological agents of the male genital tract but HSV1, CMV IgM sera-positivity and EBM showed increased activity of the WBC in the semen [14].
Aim and Objectives
Asymptomatic infections of the male genital tract can lead to infertility, in this study we determined bacterial pathogens in semen samples of the asymptomatic infertile patients compared with the control males. We also determined level of morphological changes and motility reduction of spermatozoa.
Materials and Methods
A total of 510 individuals were included in this study. Out of the respondents 310 oligospermic cases were selected to be included in this study. Our case group candidates were infertile male, who were married for past three years or more, with no anatomical morbidity, previously labeled as oligospermic during their investigations. All the oligospermic cases included in this study were responsible for infertility while their female partners were found fit for conception. Remaining 200 respondents were normal healthy individuals who had at least one child and were normospermic. All the participants were guided to wash their hand and genitalia with soap. Samples were collected by ejaculation in a wide mouth sterile container Samples were immediately transferred to the microbiology laboratory where samples were processed according to Cheesbrough 1984 and Bailey & Scott's 2002. Standard WHO guidelines were used for semen analysis. All the samples were inoculated (0.001ml semen) on Blood agar, Mc Conky agar, Chocolate agar and Saba rod's dextrose agar, incubated at 37 0C for 48 to 72 hrs. Growth>10,000 CFU/M) was considered significant. Positive cultures were gram stained for initial identification scheme, divided into gram negative and gram positive groups. For species level identification API 20E, API NH, API Staph, API Strep and API 20C AUX, biochemical identification batteries from Biomerieux- USA, were used. Antibiotic sensitivity was determined using standard disc diffusion method.
Results
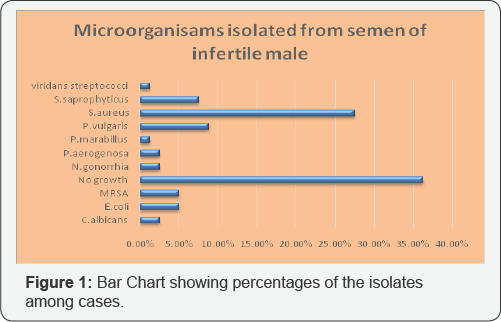
Study groups were divided into case and control groups. 310 oligospermic cases were cultured using defined culture batteries, 112 (36.25%) Semen samples yield no growth or non-significant growth, while 198 (63.8%) yielded significant growth of bacteria or fungus. Staphylococcus aureus was leading isolate, present in 27.50% of the samples, out of which 15 isolates were methicillin resistant Staphylococcus aureus (MRSA). 23 (7.50%) isolates were identified as S saprophyticus. 31 (10%) of the isolates were identified as Proteus spp, of which 8.75% isolates were confirmed as P vulgaris and 1.25% were labeled as P marabillus Ecoli and P aerogenosa were present in 5% and 2.50% of the samples respectively, i.e 15 samples yielded E.coli and 8 samples were positive for P aerogenosa. N. gonorrhea was also present in 8 cases i.e. P aerogenosa and N. gonorrhea were present in equal numbers. Viridans streptococci were present in 1.25% of the samples. Candida albicans was isolated as pure significant growth in 2.50% of the cases (Figure 1).
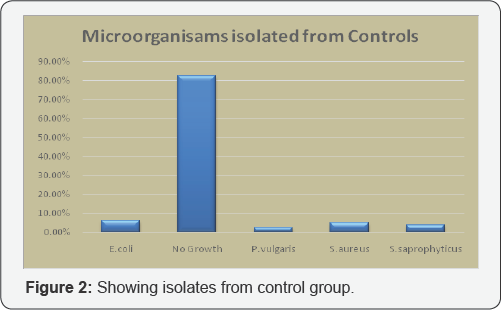
200 respondents were placed in control group, of which 165 (82.50%) yielded no growth. Only 35 samples were found positive for bacteriospermia, of which 10 (5%) yielded S aureus, 12 (6.25%) were carrying E coli, P vulgaris was isolated from 5 (2.50%) samples and S seprophyticus was present in 7 (3.75%) samples (Figure 2).
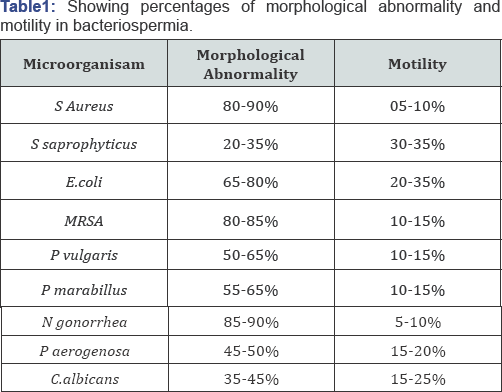
We also determined the level of morphological abnormalities and compared it with the motility of spermatozoa. We found that S aureus and N. gonorrhea alter spermatozoa morphology by 90% and causes a significant lowering of motility i.e. below 10%. Other pathogens also cause morphological changes and result in decreased motility of the spermatozoa (Table 1).
Aminoglycoside Amikacin was sensitive for 70% of the isolates. Cephalosporins, Ceftazidime and Cefotaxime showed sensitivity in 77% and 73% of the cases, respectively Ciprofloxacin and levofloxacin were sensitive in 58% of the isolates, while Ofloxacin was sensitive in 61 percent cases. Combination of Amoxicillin+Clavulanic acid was least sensitive, it showed activity against 13% of the cases. Erythormycin (18%) and Tetracyclin 17% were slinglty more effective then Amoxicillin+Clavulanic acid. We found that combination of Piperacillin+Tazobactam was effective against 90 percent of the isolated.
Discussion
Infections of the male genitourinary tract play an important role in the fertility. Various bacteria, fungus, viruses and protozoa are linked with semen quality and infertility [1,15,16]. Bacteriospermia do not correlate with leukospermia and leukospermia do not show correlation with semen parameters [17]. From literature review, we found that absence of leukospermia does not warrant absence of infection (data not shown for leukocytes count) hence we ignored this parameter and emphasis to the presence of bacterial and fungal pathogens present in significant numbers.
Rodin et al showed that Staphylococcus aureus is the most common isolate from semen of infertile male, 25.4% of all patients. We found that 27.50% of the patients were infected with the S. aureus. Thus our findings go hand in hand with the previous studies. But in contrast to the Rodin's observation we found that Staphylococcus aureus is associated with greater morphological deterioration and motility reduction, compared with the other pathogens.
On the other hand Sangeeta et al. [18] reported E faecalis as the dominating pathogen in bacteriospermia, 30% in this case, followed by Staphylococcus aureus in 20% of the cases. From these studies we can assume that in different populations or depending upon the size of study group, dominant isolate could be different but S. aureus still finds its place among leading pathogens.
Quality of semen in presence of bacteriospermia is still a question of investigation. Some studies showed that it has no effect on semen parameters [19]. E Cottel et al. [11] claimed that semen isolates are of merely any importance and labeled these organisms as contaminants. But our study found that there is a clear difference of numbers, of isolates from sub fertile and normal healthy population controls. We agree to the point that these bacteria are present in otherwise health and fertile male but their proportion is significantly lesser compared with sub fertile population. Other demonstrated that bacterial isolates from fertile and non-fertile male can be in equal proportion making it debatable, if bacteriospermia should be treated or not [20,21]. Isaiah et al. [22] and Golshani et al. [23] reported that in presence of bacteriospermia semen morphology and motility are strongly effected, our findings of reduced motility and increased morphological abnormality are consistent with previous reports.
Other pathogens like E coli, Proteus vulgaris and Proteus merabilis are reported in previously conducted studies. Our findings in term of E coli are similar to those already reported but in our study we find slightly greater load of the Proteus spp. It could be because of the sample size variation as most of the studies, we went through, included small sample size. A few reports showed presence of Candida species as a cause of semen changes, some only considered bacterial pathogens in their study. Those who reported Candida albicans isolates, our study show consistent results with their findings [18,20,21,24].
To conclude, we found that bacterial isolates are associated with semen changes and types of bacterial pathogen and their association with semen changes could be different in different settings. We feel that duration of bacteriospermia shell be considered and large sample size to be included to reach valuable conclusion. In almost all the studies who reported asymptomatic bacteriospermia not all the points were considered like years of infertility, correlation with female flora, exclusion of other parameters like hormonal changes or previously treated with antibiotics or not. In our study we also missed these points due to financial constraints. We recommend that large scale studies with follow up out comes shall be considered, to provide evidence that these asymptomatic oligospermics need antibiotic therapy or not, to improve the quality of semen.
Acknowledgement
We are thankful to Dr MH Qazi for his support at Center of Research in Molecular Medicine.
References
- Keck C, Gerber Schafer C, Clad A, Wilhelm C, Breckwoldt M (1998) Seminal tract infections: impact on male fertility and treatment options. Hum Reprod 4(6): 891-903.
- Kamel RM (2010) Management of the infertile couple: an evidence- based protocol. Reprod Biol Endocrinol 8: 21-28.
- Cates W, Farley TM, Rowe PJ (1985) Worlwide patterns of infertility: is Africa different? Lancet 2: 596-598.
- Esteves SC, Miyaoka R, Agarwal A (2011) An update on the clinical assessment of the infertile male. Clinics 66(4): 691-700.
- Bukharin OV, Kuzmin MD, Ivanov IB (2003) The role of the microbial factor in the pathogenesis of male infertility. Zhurnal Microbiologii Epidemiologii I Immunobiologii 2: 106-110.
- Elnhar A (2005) Male genital tract infection: the point of view of the bacteriologist. Gynecol Obstet Fertil 33(9): 691-697.
- Esfandiari N, Saleh RA, Abdoos M, Ruozrokh A, Nazemian Z (2002) Positive bacterial culture of semen from infertile men with asymptomatic leukocytosspermia. Int J Fertil Womens Med 47(6): 265270.?
- Dejucq N, Jegou B (2001) Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev 65(2): 208-231.
- Hamdad Daoudi F, Petit J, Eb F (2004) Assessment of Chlamydia trachomatis infection in asymptomatic male partners of infertile couples. J Med Microbiol 53: 985-990.
- Thomas J, Fishel SB, Hall JA, Green S, Newton TA, et al. (1997) Increased polymorphonuclear granulocytes in seminal plasma in relation to sperm morphology. Hum Reprod 12(11): 2418-2421.
- Cottell E, Harrison RF, Mc Caffrey M, Walsh T, Mallon E, et al. (2000) Are seminal fluid microorganisms of significance or merely contaminants? Fertil Steril 74(3): 465-470.
- Erel CT, Senturk LM, Demir F, Irez T, Ertungealp E (1997) Antibiotic therapy in men with leukocytospermia. Int J Fertil Womens Med 42(3): 206-210.
- Hosseinzadeh S, Eley A, Pacey AA (2004) Semen quality of men with asymptomatic chlamydial infection. J Androl 25(1): 104-109.
- Krause W, Herbstreit F, Slenzka W (2002) Are viral infections the cause of leukocytospermia? Andrologia 34(2): 87-90.
- Diemer T, Huwe P, Ludwig M, Hauck EW, Weidner W (2003) Urogenital infection and sperm motility Andrologia 35(5): 283-287.
- Ochsendorf FR (2008) Sexually transmitted infections: impact on male fertility. Andrologia 40: 72-75.
- Rodin DM, Larone D, Goldstein M (2003) Relationship between semen cultures, leukospermia, and semen analysis in men undergoing fertility evaluation. Fertil Steril 79(Suppl 3): 1555-1558.
- Vilvanathan S, Kandasamy B, Jayachandran AL, Sathiyanarayanan S, Vijayalakshmi TS, et al. (2016) Bacteriospermia and Its Impact on Basic Semen Parameters among Infertile Men. Interdisciplinary Perspectives on Infectious Diseases 2016(2016): 1-6.
- T Domes, KC Lo, ED Grober, JBM Mullen, T Mazzulli, et al. (2010) The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertility and Sterility 97(5): 10501055.
- Ombelet W, Bosmans E, Janssen M, Cox A, Vlasselaer J, et al. (1997) Semen parameters in a fertile versus sub fertile population: a need for change in the interpretation of semen testing. Hum Reprod 12(5): 987993.
- Ahmad S, Wasim S, Tiwari N, Verma V, Gupta N, et al. (2016) Evaluation of Bacteriospermia as Etiology for Oligospermia: An Analysis. Ijss 4(2): 194-197.
- N Isaiah, BT Nche, IG Nwagu, II Nnanna (2011) Current studies on bacteriospermia the leading cause of male infertility: a protege and potential threat towards mans extinction. N Am J Med Sci 3(12): 562564.
- Golshani M, Taheri S, Eslami G, Suleimani Rahbar AA, F Fallah, et al. (2006) Genital tract infection in asymptomatic infertile men and its effect on semen quality. Iranian Journal of Public Health 35(3): 81-84.
- WHO (1999) laboratory manual for the examination of human semen and sperm-cervical interaction. World health organization, Germany, Switzerland.






























