Abstract
The effect of nutrition on human health begins before conception and continues throughout life. Pregnancy, childhood, and adolescence are critical stages for growth and development. Diets during these stages must balance environmental sustainability and health outcomes. Integrated approaches such as integrated (multi) OMICS represents new trends in human health and personalized nutrition (PN). The genetic, phenotypic, medical, nutritional, and other relevant information for individuals provides tailored healthy eating and nutritional guidance based on specific needs. The PN concept involves a deep understanding of the complex molecular interplay between genetic makeup and environmental (episomal) factors including nutrition, metabolism, and diet, in an individual or group of consumers. Multi-OMICS represent contemporary trends in human health and PN.
Keywords: personalized and precision medicine (PPM); Obesity; Diabetes Mellitus; Multi OMICS; Microbiota
Abbreviations: CVDs: Cardiovascular Diseases; PPM: Personalized and Precision Medicine; PN: Personalized Nutrition; NCD: Non-Communicable Diseases
Editorial
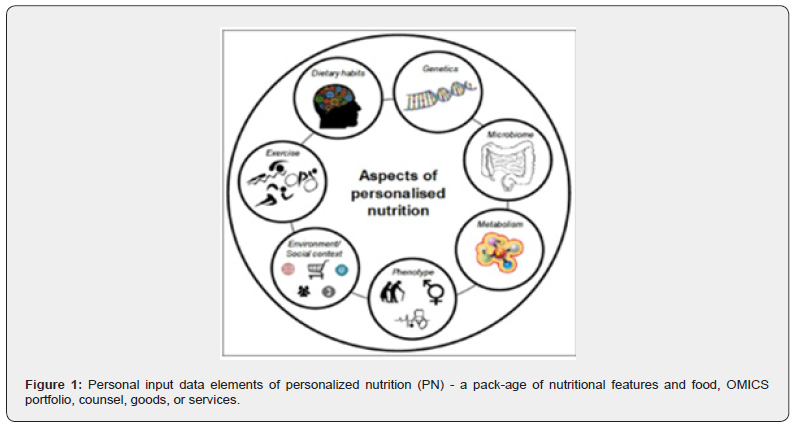
The rising prevalence of many chronic diseases like obesity, diabetes, and cardiovascular diseases (CVDs), reflects the consequences of an unhealthy diet and lifestyle. These nutritional disorders are global health issues and require a new biomarkerdriven target approach. PPM focuses on the individual seeking the most appropriate and specific treatment according to the intrinsic conditions of their health status and living conditions. Compared to traditional nutrition with a generic approach based on population groups, personalized nutrition (PN) uses specific information about the individual to prescribe more appropriate and precise nutritional management (advice, products, and ser-vices) to benefit their health status. The use of OMICS technologies, among which genomics and nutrigenomics stand out, provides valuable information on both the phenotype and genotype alongside other information (Figure 1).
NP assumes that everyone’s unique phenotype and genotype affect their inter-actions with different dietary constituents and nutrients. Many disorders are susceptible to the effects of multiple genes and the environment, with each gene having a moderate or modest impact. Furthermore, diseases emerge from the intricate connection between genetic predisposition and the external environment. In this specific framework, the applications of advanced multi-OMICS technologies supported by bioinformatics can be promising, including gut microbiome analysis and comprehensive phenotyping, which have the potential to identify heritable factors and gene-environment interactions.
Notably, many of the risk factors - gut dysbiosis, chronic immune dysfunction, and low-level inflammation - are hypothesized to be shared across multiple non-communicable diseases (NCD). Advancing preventive measures require a comprehensive comprehension of disease mechanisms, encompassing pivotal molecules and pathways. While molecular diagnostics focuses primarily on the detection of specific biomarkers, precision pathology, through the analysis of genetic mutations, gene expression patterns, epigenetic modifications, and protein alterations, studies and interprets molecular and genetic changes in tissues and cells to facilitate the diagnosis, pathogenesis, prognosis, and treatment of diseases, and to understand the mechanisms underlying disease development and progression. Epigenetic interaction in early life significantly affects the development of adult tissues. Human microbiota can broadly influence human genome expression through mechanisms such as DNA methylation, histone modification, noncoding RNA, and chromatin remodeling, generating a wide range of physiological impacts.
While OMICS data are biomolecular, epigenomic, and exposome data are environmental, biometric, and medical metadata sources. Environmental and bio-metric data, highly diversified but easily accessible, can be collected and processed by sharp sensor networks embedded in new mobile platforms (wearable devices, smartphones, or watches), being analyzed from the point of care for monitoring and diagnosis. Currently, it is a biophysical challenge in systems biology to collect biomolecular data from intra- and intercellular systems at the genome to microbiome scale, requiring specific and individualized tools to identify biomolecules with particle resolution and unique binding sites.
Interestingly, while multi-OMICS data (microbiome, metabolomics, genomics, etc.) translational to PN through precision food omics is a cutting-edge and developing area, several validated biomarkers and algorithmic platforms exist. However, most algorithms are not yet clinically approved for humans.
Additionally, with OMICS-technologies, precision food omics and food design, biodata analysis and data technology, PN are increasingly a reality. We may use genomic data to tailor PN for specific nutritive supplements, giving rise to the interdisciplinary science called nutrigenetics and integrating microbiomics and metabolomics. Recent advancements in OMICS-powered tools and related techniques are applicable in the nutrition science field. In addition, advances in multi-OMICS technology will enable the establishment of objective biomarkers of food intake and health status. These advances include the capability to make PN recommendations based on their principles and food design and monitor food intake. PPM and PPM-guided nutrition aim to individualize medical practice and its care services with a personalized approach based on genetic testing, individualized biomarkers, and the development of specific treatments and diets guided by the identified data.
In this context, PN and PF approaches are becoming essential instruments to assess an individual’s optimal metabolic space. The latter is crucial to recognize specific gene-metabolite, dietmetabolite and gene–diet connections. This information is indispensable for data-driven decision-making on the nutritional aspects of food and sustainable diets. In this framework, we adapt dietary recommendations according to genotype and critical measures in subjects with genetic defects, helping them efficiently improve their health status, especially those patients with metabolic and nutritional disorders.
PN considers all the details of individual characteristics to create a package of individualized nutritional recommendations, products, or services. Nonetheless, it is essential to consider that achieving sustainable health improvements through large-scale PN requires affordable and accessible approaches being based on principles and philosophy of PF and food design.
PN offers considerable potential, but individualized intervention methods are often less accessible to low-income individuals. These services are rarely covered by health insurance or by trained nutritionists. Furthermore, to ensure a lasting intervention, it is essential to assess patient progress periodically and refine and improve the intervention. These unresolved issues leave the target majority of the population without coverage. Although there is consensus in nutritional recommendations across different continents and regions on the need to consume a variety of foods in balanced proportions, including fruits and vegetables, whole grains, legumes, and moderate consumption of animal products, and the limitation of sugary and fatty foods with high energy con-tent, the primary objective of personalized, population-wide, and planetary nutrition for public health should focus on combating the etiology and detrimental health con-sequences of the triple burden of malnutrition, promoting nutritional science, research, and international cooperation at the global level. This PN should reflect the specific physiological requirements of each life-cycle stage, taking into account cultural and socioeconomic differences across regions, countries, and areas, as well as individualized clinical and phenotypic characteristics (psychological and personality patterns, ethnic variabilities, food allergies and intolerances, medication side effects, dietary preferences, lifestyle, and environmental factors). Furthermore, new genetic and transcriptomic biomarker development and nutrigenomic and metagenomic tools for nutrient metabolism are a priority.
The advancement of PN depends on a solid theoretical foundation development that establishes the most relevant individual traits for personalization. Integrative nutritional biomarkers play a crucial role in shaping PN, enhancing our ability to tailor dietary recommendations to individual needs. An adequate design is vital to show the effectiveness and profitability of intervention and family planning studies. An appropriate regulatory framework is needed to protect the public from personalized and private information. This approach fosters trust in health professionals and policymakers. Currently, we face several challenges related to the acceptance of PN, including the definition of the health-disease continuum, the identification of biomarkers, changes in their regulation, and accessibility and measurement of their success. While PN approaches hold promise for public health, more holistic approaches are needed to address the accuracy of dietary intake assessment, the use and standardization of more systemic approaches, and the application and communication of evidence.






























