Abstract
Globally, the main cause of death is cancer. Most of the doctors treat it using methods such as surgery, chemotherapy, and radiation. These treatments help improve health, but they also destroy our healthy body tissues along with cancerous cells during therapies, such as toxicity, a weakened immune system, and resistance to treatments. Due to several issues, most cancer patients end up malnutrition, also called cancer cachexia, a condition in which patients lose weight and muscle, and it affects 60% of those having advanced cancer which causes difficulty and leads to worsen outcomes. Recent research advancements have been focused on personalized nutrition, were doctors design custom plans using genomics. Certain diet plans are also being tested to make current cancer treatments work effectively. An example of cutting down calories and fasting for a short duration makes cancer cells more sensitive to treatment. Furthermore, the ketogenic diet uses methods that prevent cancer cells from getting energy and kill cancer cells more easily. Arginine and Probiotics can help fix problems with cancer, according to early results by using Patient Generated Subjective Global Assessment (PGSGA), diet changes can affect patients, and it would be difficult to follow these plans. Consistent measures with Randomized Controlled Trials (RCTs) should be the priority. Oncologists and Supportive care, need to work together closely on the patient get care.
Keywords: Cancer; Nutrition; Dietary Intervention; Management; Ketogenic Diet
Abbrivation: PGSGA: Patient Generated Subjective Global Assessment; RCTs: Randomized Controlled Trials; IARC: International Agency for Research on Cancer; MNA®: Mini Nutritional Assessment; PNI: Prognostic Nutritional Index; KD: Ketogenic Diet; CR: Calorie Restriction; DSS: Differential Stress Sensitization; DSR: Differential Stress Resistance; Gln: Glutamine; QoL: Quality of Life; CRF: Cancer-Related Fatigue; ROS: Reactive Oxygen Species; LOS: Length of Stay; TPN: Total Parenteral Nutrition
Introduction
Cancer is described as the multifactorial uncontrolled development and growth of body cells. There are more than 100 varieties of cancer, which are divided by the type of tissue or organ affected by the human body including genomic and environmental interactions [1,2]. The characteristic features of the cancer include unrestrained proliferation, lack of responsiveness to growth factors, sustained angiogenesis, anti-apoptotic properties, and invasion of other tissues [3]. Global cancer burden estimates (GLOBOCAN) are provided by agencies such as International Agency for Research on Cancer (IARC) [3-6] The types of cancers are treated either with standard remedies, i.e., surgery, radiation therapy, and chemotherapy, or using nonconventional or complementary or traditional treatment, i.e., hormone therapy, immunotherapy, nano-therapy, etc. [7] The choice of effective cancer treatment method is based on the nature of detected malignancies, their growth stage, age, the frequency, dosage of drugs, and the health of the patients. [3,8-12] Chemotherapy damages rapidly dividing cells but harms normal tissues, manifested by side effects such as myelosuppression, nausea, and loss of hair. Moreover, it is hindered by drug resistance [8,13]. Radiotherapy uses ionizing radiation to produce local tumor damage but can damage nearby healthy tissues, leading to fibrosis, dysfunction of an organ, or secondary malignancies [9,14-16]. On the whole, these traditional treatments cause collateral damage to normal somatic tissues often inducing malnutrition, cachexia and metabolic imbalances which negatively affect immune competence and worsen prognosis [17,18].
The dietary impact on cancer metabolism is one of the modern academic research topics. Though the word prevention might be slightly exaggerated. Healthy habits are linked to reducing cancer risk and nutritional modulation has shown promise in decimation of cancer growth in preclinical models [19,20-23]. Proper nutritional care helps maintain strength, boost immune system and improve tolerance to therapy, and enhance quality of life [17]. Evidence suggests that diets rich in fruits, vegetables, wholegrains, and omega-3 fats can enhance treatment outcomes by regulating inflammation and oxidative stress [24-26].
By Incorporating evidence-based, customized nutrition plans, which can regulate fundamental intracellular pathways involved in tumor development; clinicians can mitigate undesirable treatment outcomes, enhanced efficacy, and promote longterm survivorship [26]. This mini review is focused to elaborate nutrition in cancer management, recent advancements and its integration of nutrition with conventional cancer therapies.
Overview of Nutrition in Cancer Management
Cancer patients have a high rate of malnutrition that is closely associated with poor clinical outcomes. Mini Nutritional Assessment (MNA®) is commonly used to individuals with cancer who may suffer from older age, but it is questionable whether it has any predictive validity in terms of patient prognosis. One of the common occurrences in cancer patients is malnutrition, a phenomenon that has the capability to affect the course of the disease as well as the survival [27]. Recent evidence shows that Prognostic Nutritional Index (PNI) is a statistically significant prognostic determinant of survival as shown in univariate and multivariate analyses. Further, pre-operative serum albumin was observed to have poorer outcomes, which work as autonomous prognostic markers in various studies [28,29].
Cancer cachexia is a multidimensional wasting syndrome involving progressive weight loss that significantly contributes to cancer morbidity and mortality, and although reduced dietary intake is common and strongly correlated with the degree of weight loss, many patients fail to achieve the recommended dietary intake even after nutritional counseling [30-32]. This condition is metabolically distinct from simple starvation, as tumors actively affect neural mechanisms regulating appetite and energy expenditure while promoting catabolism and wasting of peripheral tissues, including cardiac and skeletal muscle, adipose tissue, and bone. Defining effective and multimodal interventions for cachexia remains challenging due to a lack of consensus on definitions, low strength of evidence from clinical trials, and a scarcity of robust, rigorous, and mechanistic studies [30]. Malnutrition is also a prevalent and challenging health concern in older adults, linked to increased mortality, morbidity, physical decline, and reduced quality of life [33]. In oncology, the prevalence of malnutrition (or risk of it) increases with the stage of the disease, affecting about 60% of advanced cancer patients, and critically, malnutrition defined by involuntary weight loss (>5% in 3–6 months) is a potent predictor of poor outcome, with weight loss before or during chemotherapy linked to worse overall survival, though conversely, weight gain in patients with non-small cell lung cancer has been reported to be associated with a significant improvement of overall survival [34-37].
The resolution of clinical decisions in practice still involves diagnosing and treating a given condition through the assessment of the signs and symptoms, the pathophysiology of the organ systems, the underlying cause(s), and the corresponding therapy [38].
The Food and Nutrition Board of the Institute of Medicine has established the Recommended Dietary Allowance of certain nutrients 22 and Dietary Reference Intakes from the applications of evidence-based reviews conducted by expert panels [39-41].
Recommendations from IARC and WCRF/AICR/NCI are consistent and streamlined, advising individuals to maintain their weight on the lower end of the healthy range (between 18.5 and 25 kg/m2), to avoid gaining more than 5 kg during adulthood, and to lose 5-10% of body weight if already overweight or obese, along with moderate activities like brisk walking or cycling for 30 minutes on most days of the week, for a reduction in chronic disease (coronary heart disease and diabetes) and for overall health. For those who do drink alcohol, consumption should be restricted to <5% of total energy for men and <2.5% of total energy for women, or fewer than 2 drinks per day for men and one for women [42-44]. According to recommendation of IARC, consuming at least 400 g/d of a variety of fruits and vegetables, including fiber, and NCI suggests 5–9 servings/d [43].
Recent Advancements in Dietary Interventions
Indeed, the future direction of cancer care is more personalized and precision nutrition that is no longer based on a standard diet due to the high degree of cancer classification, patient-specific environment and lifestyle [26]. The essence of the proposed project is the creation of individualized nutrition plans that enhance patient outcomes. The rationale behind this method is that dietary manipulation affects the amount of metabolites accessible to the tumor cell in its microenvironment and, thus, the way in which cancer cells use nutrients to grow, develop, and survive [45]. Other tools like genetics and genomics that are necessary in comprehending the genetic predisposition (for example, particular sub-types of breast cancer in women of African descent) and deriving the genomic insights are crucial to the prognosis (as in ovarian cancer, progression-free survival) are also incorporated into precision care. Moreover, metabolomics is also used as a state-of-the-art analytical technique to monitor the metabolic alteration and identify mechanistic connections among the host, the disease, and the gut microbiome. The role of the gut microbiota in cancer susceptibility is that nutritional factors can regulate the gut flora to promote the development of tumorsuppressing or carcinogenic bacteria [46,47].
Nutrigenetics involves the study of the impact of nutrition on the gene level, with its focus being on the effect and the way in which specific genetic variants are affected by nutrient interactions. It incorporates environmental exposure, nutrition, with the human genome, with the focus on the gene [48,49].
Nutrigenomics examines how nutrients alter genomic and transcriptomic profiles and then examines the resultant effects on proteomics and metabolomics. In this method, the nutrients are studied holistically regarding the way they alter gene expression and transcript patterns and, thus, affect proteomic and metabolomic mechanisms [50].
The overall objective of the integration of nutrigenetics and nutrigenomics is to use personalized medicine and customized cancer therapy, such as considering of genomic profile and nutrient intake of a patient or adjuvant nutrition support of cancer treatment, apoptosis regulation or angiogenesis prevention [51- 53]. Refer below to the Table 1 that provides information about the key components in nutrients and its approximate Energy Distribution in cancer therapy.
Ratio: 3:1 or 4:1 (Fat: Carbohydrates + Protein) [54] Ketogenic diet (KD) has become one of the leading methods that seek to attack the metabolic processes of the cancer cells [55]. In comparison to traditional dietary interventions, KD is theorized as a metabolic therapy that aims to provide an inhospitable environment to the malignant cell [56]. The main aim of the KD is to force the organism to change its metabolic state of utilizing glucose to produce ATP to the oxidation of lipid substances to produce ketone body and put the organism in a ketonic state [57]. The basis of the application of KD is the exploitation of the underlying metabolic differences between normal and cancerous cells which is often known as the Warburg effect [58,59].
It has been demonstrated that dietary intake affects many pathways involved in carcinogenesis and tumor progression. Dietary guidelines have recommended the use of a plant-based diet as a way of preventing cancer [56,60-62] (Figure 1).
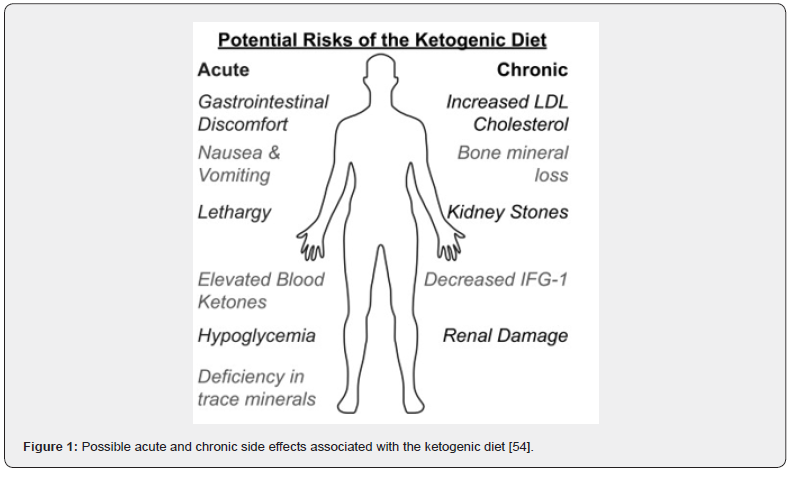
Integration of Nutrition with Conventional Cancer Therapies
Dietary strategies are employed to enhance the effectiveness of conventional cancer therapies by exploiting metabolic differences between cancer and normal cells or providing protective effects [63]. Calorie Restriction (CR) and short-term fasting are researched interventions because they induce differential stress sensitization (DSS) in cancer cells, increasing their susceptibility to chemotherapy and radiation, while simultaneously promoting differential stress resistance (DSR) in normal cells, shielding them from toxic damage [64,65]. Other than the regulation of dietary intake, additional intake of particular nutrients could be beneficial. As an example, glutamine (Gln) might alleviate the undesirable radiational effects and promote the effectiveness of chemotherapy because of diminishing the harmful side effects. In addition, omega fatty acids (ω-3) could be used to enhance the efficacy of chemotherapy in preventing the growth of cancerous cells at the same time they are used alongside chemotherapy. The Ketogenic Diet (KD) is thought to be a way to change how your body works to fight cancer by making things challenging for cancer cells. [23,66] The manipulation of the gut microbiota is extremely important as evidenced. Nucleotide synthesis by intestinal bacteria can also change the reaction of rectal cancer patients to pre-operative chemo-radiotherapy, which means that the adjustment of the microbiota may enhance treatment outcomes [67].
Efficient and carefully designed nutritional support is invaluable to neutralizing the negative impact of treatment plans, promoting immune efficiency, and enhancing the Quality of Life (QoL). Optimal nutrition care provision requires that there is an energy intake of between 25-30 kcal per kilogram of body weight per day with a protein intake of above 1g/kg/day and ideally above 1.5g/kg/day, to protect lean body mass, tissue repair, and prevent the development of sarcopenia [68,69] Much has been done in the control of common toxicities that affect the eating habits. Nausea and vomiting (N/V) are now better controlled using advanced anti-emetic regimens, including 5-HT3 antagonists for immediate N/V, neurokinin receptor antagonists for delayed nausea, and the inclusion of corticosteroids and olanzapine. To address mucositis damage to the mucosal lining’s strategies, include oral cryotherapy (ice chips), keratinocyte growth factor (palifermin), and glutamine, which protects the intestines and can reduce chemotherapy-induced complications like GI-mucositis and diarrhea [70,71]. Although systematic reviews suggest that nutrition therapy alone has no definitive overall effect on QoL or Cancer-Related Fatigue (CRF), there is preliminary evidence that plant-based dietary patterns (anti-inflammatory diets high in fruits, vegetables, fish, nuts, and seeds) may offer improvements in CRF. Importantly, interventions that successfully utilized nutritional assessment tools like the Patient-Generated Subjective Global Assessment (PGSGA) to improve nutritional status often correlated with subsequent improvements in patient quality of life [72-74] (Figure 2).
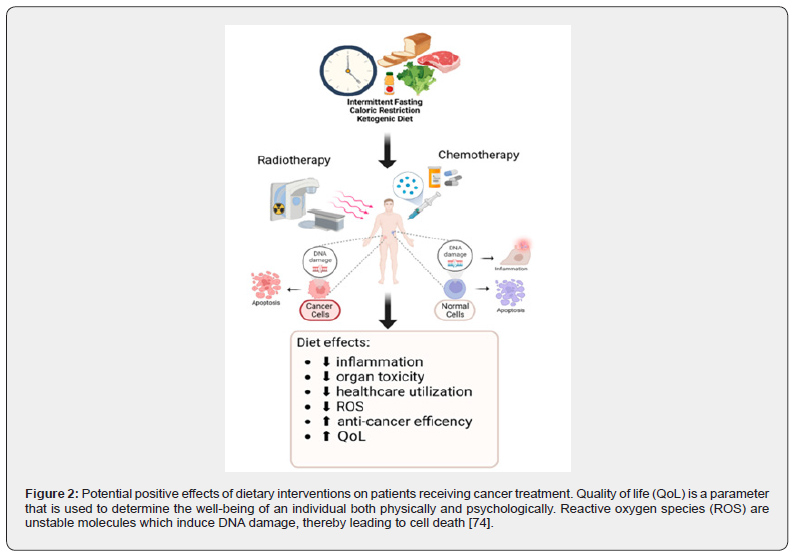

Immuno-nutrition consists of modulating the immunological responses and fostering the rehabilitation of tissues by the oral, enteral, or parenteral administration of immune system-boosting nutrients [75]. These nutrients include arginine, glutamine, omega-3 fatty acids, and nucleotides [76]. Recommended timeframes include the postoperative period and postoperative care of patients after upper gastrointestinal cancer surgery. Immune system boosts during the postoperative period have been documented to improve safety and efficacy by lowering complication rates, especially infectious ones, and shortening hospital length of stay (LOS) [75]. Specifically, postoperative, Glnenriched total parenteral nutrition (TPN) improves nutritional status, nitrogen balance, and diminishing inflammatory status (IL-6 and CRP) [77]. Dysbiosis induced by cancer therapy, exacerbating gastrointestinal (GI) mucositis and malnutrition, necessitates explicit attention to modulation of Gut Microbiota (GM). Probiotics and symbiotic have clinical benefits and in one study, the authors describe reduced infectious complications postoperatively in patients with colorectal cancer [78]. Another study in patients undergoing neoadjuvant chemotherapy for esophageal cancer documented a reduction of chemotherapyassociated diarrhea, thus lowering the need for prophylactic antibiotics. Specific nutrients, such as omega-3 fatty acids and arginine, are known to beneficially alter certain in-body processes [79].
Challenges and Limitations
Results don’t seem to be materializing as our integration of good nutrition into therapeutic plans still encounters severe hurdles. Some hurdles like differentiating level of implications and effects inflicted on and by different types of cancer. Other hurdles, like a lack of uniform guidelines in clinical and crosssectional literature, and therefore a lack of conclusive analyses on the therapeutic impacts of nutrition on cancer, are to be used in studies on Cancer-Related Fatigue (CRF) and Quality of Life (QoL) [80]. Cancer patients shut down on compliance and on limits set by practical considerations. Many conventional strategies for Cancer care cause distressing toxicities [81]. It is the toxicities like nausea and mucositis that literally make eating on un-impacted days of the cancer care cycle a considerable emotional and physical challenge as well as a cause of shame for the patient. It is these unconsumed calories that fuel the patient’s decline and worsen prognostic outcomes [82].
Conclusion and Future Perspectives
In conclusion, while the overall pooled data continues to show no conclusive value of nutrition therapy on CRF or QoL, certain focused approaches show potential promise, especially where Patient-Generated Subjective Global Assessment (PGSGA) tools have been designed to drive personalized assistance and where plant-centric or anti-inflammatory eating patterns have been utilized. Personalized nutrition and precision nutrition research with tools like metabolic profiling and genomics must be the focus of upcoming studies. To secure credible data, the field requires more large-scale Randomized Controlled Trials (RCTs) that incorporate reliable and standardized instruments to measure CRF and QoL, determine the most strategic implementation of an intervention (pre-, during- or post-treatment), and address hypermetabolic energy needs, dietitians, and palliative care teams in anticipatory, predictable supportive care. For clinical outcomes, interdisciplinary interaction should be highlighted to deal with the toxicities through proactive therapeutic relationships involving oncologists, dietitians, and palliative care teams to facilitate predictable and anticipatory supportive care.
References
- Weinberg RA (1996) How cancer arises. Scientific American 275(3): 62-70.
- Ullah M, Uzma AA, Abdul M, Muhammad N, Jin WY, et al. (2025) Cancer nanomedicine: Smart arsenal in the war against cancer. Inorganic Chemistry Communications 174(2): 114030.
- Chandra PMS, Dey A, Swamy MK (2022) Introduction to cancer and treatment approaches, in Paclitaxel. Elsevier p. 1-27.
- Barrera S, Demark W (2009) Nutrition during and after cancer therapy. Oncology (Williston Park, NY) 23(2 Suppl): 15-21.
- Ferlay J, Murielle C, Isabelle S, Donald MP, Marion P, et al. (2021) Cancer statistics for the year 2020: An overview. International journal of cancer 149(4): 778-789.
- Ahmed Z, Muneeb U, Danish Z, Shahid UK, Fawad A, et al. (2025) Exploring the tumor microenvironment in solid cancer: From biology to therapy. Methods in cell biology 198: 359-385.
- Bailar JC, Gornik HL (1997) Cancer undefeated. New England Journal of Medicine 336(22): 1569-1574.
- Anand U, Abhijit DB, Arvind KSCC, Rupa Sd, Amarnath M, et al. (2023) Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes & diseases 10(4): 1367-1401.
- Liu B, Hongyu Z, Licheng T, Kin THS, Xin YG, et al. (2024) Exploring treatment options in cancer: tumor treatment strategies. Signal transduction and targeted therapy 9(1): 175.
- Zafar A, Summaiya K, Muhammad JK, Junaid A, Aisha N, et al. (2025) Advancements and limitations in traditional anti-cancer therapies: a comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discover oncology 16(1): 607.
- Khan U (2025) Nanotechnology in Cancer Therapy: Current Trends, Challenges, and Future Prospects. International Journal of Basic Medical Sciences and Pharmacy (IJBMSP) 12(1).
- Safdar M, Zoya A, Muneeb U, Abdul W, Nurhasni H, et al. (2025) Cancer stem cell analysis and targeting. Methods in cell biology 198: 251-271.
- Min HY, Lee HY (2021) Mechanisms of resistance to chemotherapy in non-small cell lung cancer. Archives of pharmacal research 44(2): 146-164.
- Webster M, Alexander P, Fiona L, Yuwei Z, Hyunuk J, et al. (2025) New approaches in radiotherapy. Cancers 17(12): 1980.
- Wang K, Tepper JE (2021) Radiation therapy‐associated toxicity: Etiology, management, and prevention. CA: a cancer journal for clinicians 71(5): 437-454.
- Saifi Z (2025) Formulation approaches and pharmaceutical applications of chitosan and thiolated chitosan nanoparticles. Journal of Carbohydrate Chemistry 44(1-3): 1-30.
- Rock CL, Cynthia AT, Kristen RS, Carol LH, Lawrence HK, et al. (2022) American Cancer Society nutrition and physical activity guideline for cancer survivors. CA: a cancer journal for clinicians 72(3): 230-262.
- Chapek MA, Martindale RG (2021) Nutrition in cancer therapy: overview for the cancer patient. Journal of Parenteral and Enteral Nutrition 45(S2): S33-S40.
- Nawaz K (2025) Role of Nutrition in the Management of Inflammatory Bowel Disease. Recent Progress in Nutrition 5(1): 1-76.
- Gray A, Brian ND, Theodore BM, Roger C, Peter P, et al. (2020) A review of nutrition and dietary interventions in oncology. SAGE Open Medicine 8.
- Lévesque S, Jonathan GP, Gladys F, Lorenzo G, Laurence Z, et al. (2019) Trial watch: dietary interventions for cancer therapy. Oncoimmunology 8(7): e1591878.
- Taylor SR (2022) Developing dietary interventions as therapy for cancer. Nature Reviews Cancer 22(8): 452-466.
- Zeb F, Aqsa M, Huma N, Muneeb U, Afraa W, et al. (2024) Nutrition and dietary intervention in cancer: gaps, challenges, and future perspectives. Nutrition and Dietary Interventions in Cancer 191: 281-307.
- Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, et al. (2021) Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines 5. ESMO open 6(3): 100092.
- Nagy S (2023) The impact of modern dietary practices on cancer risk and progression: a systematic review. Cureus 15(10): e46639.
- Mayne ST, Playdon MC, Rock CL (2016) Diet, nutrition, and cancer: past, present and future. Nature reviews clinical oncology 13(8): 504-515.
- Torbahn G, Strauss T, Sieber CC (2020) Nutritional status according to the mini nutritional assessment (MNA®) as potential prognostic factor for health and treatment outcomes in patients with cancer -a systematic review. BMC Cancer
- Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C (2017) Clinical Value of Nutritional Status in Cancer: What is its Impact and how it Affects Disease Progression and Prognosis? Nutr cancer 69(8): 1151-1176.
- Aftab M (2025) Recent Advancements in Inorganic Based Nanomaterials for Wound Healing; Challenges and Future Opportunities. Journal of Cluster Science 36(6): 1-33.
- Nasrah R, Der BCV, Kanbalian M, Jagoe RT (2020) Defining barriers to implementation of nutritional advice in patients with cachexia. J Cachexia Sarcopenia Muscle 11(1): 69-78.
- Baracos VE, Lisa M, Murray K, Denis CG, Kenneth CHF, et al. (2018) Cancer-associated cachexia. Nature reviews Disease primers 4(1): 1-18.
- Osman A (2025) Sugar nanocluster adhesive boosts wound healing in diabetic mice. Carbohydrate Polymer Technologies and Applications 11: 100933.
- Norman K, Haß U, Pirlich M (2021) Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 13(8): 2764.
- Laviano ADLL, Koverech A (2018) Nutrition support and clinical outcome in advanced cancer patients. Proceedings of the Nutrition Society 77(4): 388-393.
- Lu Z, Yang L, Yu J (2014) Change of body weight and macrophage inhibitory cytokine-1 during chemotherapy in advanced gastric cancer: what is their clinical significance? Plos one 9(2): e88553.
- Patel J, Pereira JR, Chen J (2016) Relationship between efficacy outcomes and weight gain during treatment of advanced, non-squamous, non-small-cell lung cancer patients. Ann Oncol 27(8): 1612-1619.
- Amin Z (2025) Trojan Horses: A Secret Route for Nanomedicines. Current Pharmaceutical Biotechnology.
- Go VLW, Wong DA, Wang Y, Wilkerson L, Butrum RR, et al. (2004) Diet and Cancer Prevention: Evidence-based Medicine to Genomic Medicine. J Nutr 134(12 suppl): 3513S-3516S.
- Washington DC, National Academy Press (1989) In: National Research Council Recommended Dietary Allowances, (10th), USA.
- (2004) Institute of Medicine Food and Nutrition Board Dietary Reference Intakes.
- Aftab M (2025) Advancement of 3D Bioprinting Towards 4D Bioprinting for Sustained Drug Delivery and Tissue Engineering from Biopolymers. Journal of Manufacturing and Materials Processing 9(8): 285.
- Maki KC (2014) Limitations of observational evidence: implications for evidence-based dietary recommendations. Advances in nutrition 5(1): 7-15.
- Murphy JL, Girot EA (2013) The importance of nutrition, diet and lifestyle advice for cancer survivors-the role of nursing staff and interprofessional workers. Journal of Clinical Nursing 22(11-12): 1539-1549.
- Ullah M Introduction to Gastrointestinal Inflammation and Gut Microbiota, in Gastrointestinal Inflammations and Gut Microbiota. CRC Press p. 1-13.
- Reglero C, Reglero G (2019) Precision nutrition and cancer relapse prevention: a systematic literature review. Nutrients 11(11): 2799.
- Guo H, Liu Y, Wan T, Song D, Palanisamy CP, et al. (2024) Toward personalized cancer management: Role of precision nutrition-diet interventions.
- Guo H (2024) Toward personalized cancer management: Role of precision nutrition-diet interventions. Journal of Functional Foods 123: 106584.
- Farhud D, Yeganeh MZ (2010) Nutrigenomics and nutrigenetics. Iranian journal of public health 39(4): 1-14.
- Aftab M (2025) Biomedical Applications of Carbon Dots: Advances in Antimicrobial Therapy and Targeted Delivery Systems. Biomedical Materials & Devices p. 1-22.
- Kang JX (2013) Nutrigenomics and cancer therapy. Journal of nutrigenetics and nutrigenomics 6(3): 1-2.
- Ardekani AM, Jabbari S (2009) Nutrigenomics and cancer. Avicenna journal of medical biotechnology 1(1): 9-17.
- Caradonna FIC, Luparello C (2022) Nutrigenetics, nutrigenomics and phenotypic outcomes of dietary low-dose alcohol consumption in the suppression and induction of cancer development: evidence from in vitro studies. Critical reviews in food science and nutrition 62(8): 2122-2139.
- Aftab M (2025) Recent Trends and Future Directions in 3D Printing of Biocompatible Polymers. Journal of Manufacturing and Materials Processing 9(4): 129.
- Allen BG, Sudershan KB, Carryn MA, Julie MEG, Zita AS, et al. (2014) Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox biology 2: 963-970.
- Komal, Shanker N (2025) Role of Therapeutic Diets in Management of Diseases, in Revolutionizing Agri-food Systems: Sustainability, Health, and Innovation. Springer p. 225-240.
- Shah UA, Iyengar NM (2022) Plant-based and ketogenic diets as diverging paths to address cancer: a review. JAMA oncology 8(8): 1201-1208.
- Winters N, Kelley JH (2017) The Metabolic Approach to Cancer: Integrating Deep Nutrition, the Ketogenic Diet, and Nontoxic Bio-Individualized Therapies. Chelsea Green Publishing.
- Allen BG, Bhatia SK, Anderson CM, Eichenberger GJM, Sibenaller ZA, et al. (2014) Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol 2: 963-970.
- Talib WH, Mahmod AI, Kamal A, Rashid HM, Alashqar AMD, et al. (2021) Ketogenic Diet in Cancer Prevention and Therapy: Molecular Targets and Therapeutic Opportunities. Curr Issues Mol Biol 43(2): 558-589.
- Mukherjee AK (2001) Advances in cancer therapy with plant based natural products. Current medicinal chemistry 8(12): 1467-1486.
- Kaczmarzyk IM, Nowak P, Woźniak M (2024) Promising approaches in plant-based therapies for thyroid cancer: An overview of in vitro, in vivo, and clinical trial studies. International Journal of Molecular Sciences 25(8): 4463.
- Ullah M, Juho L, Nurhasni H, Lukman H, Dongmin K, et al. (2024) Clindamycin-loaded polyhydroxyalkanoate nanoparticles for the treatment of methicillin-resistant Staphylococcus aureus-infected wounds. Pharmaceutics 16(10): 1315.
- Isenring E (2013) Updated evidence‐based practice guidelines for the nutritional management of patients receiving radiation therapy and/or chemotherapy. Nutrition & Dietetics 70(4): 312-324.
- Donaldson SS, Lenon RA (1979) Alterations of nutritional status. Impact of chemotherapy and radiation therapy. Cancer 43(S5): 2036-2052.
- Ullah M (2024) Carbon dots: new rising stars in the carbon family for diagnosis and biomedical applications. Journal of Nanotheranostics 6(1): 1.
- Plotti F, Corrado T, Daniela L, Martina B, Giuseppe M, et al. (2020) Diet and chemotherapy: the effects of fasting and ketogenic diet on cancer treatment. Chemotherapy 65(3-4): 77-84.
- Zahra A, Melissa AF, Emyleigh O, Kranti AM, Sudershan KB, et al. (2017) Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the University of Iowa experience of two phase 1 clinical trials. Radiation research 187(6): 743-754.
- Golant M, Altman T, Martin C (2003) Managing cancer side effects to improve quality of life: a cancer psychoeducation program. Cancer nursing 26(1): 37-44.
- Doyle C, Lawrence HK, Tim B, Kerry SC, Wendy DW, et al. (2006) Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA a cancer journal for clinicians 56(6): 323-353.
- Caro MMM, Laviano A, Pichard C (2007) Nutritional intervention and quality of life in adult oncology patients. Clinical nutrition 26(3): 289-301.
- Ravasco P (2004) Cancer: disease and nutrition are key determinants of patients’ quality of life. Supportive Care in Cancer 12(4): 246-252.
- Uster A, Ursula R, Maya R, Miklos P, Marco S, et al. (2013) Influence of a nutritional intervention on dietary intake and quality of life in cancer patients: a randomized controlled trial. Nutrition 29(11-12): 1342-1349.
- Rosenbaum E, Holly G, Pat F, Eric N, Bernadette F, et al. (2004) Cancer supportive care, improving the quality of life for cancer patients. A program evaluation report. Supportive Care in Cancer 12(5): 293-301.
- Mercier BD, Eemon T, Errol JP, Qianhua F, Ziyi H, et al. (2022) Dietary interventions in cancer treatment and response: a comprehensive review. Cancers 14(20): 5149.
- Mattavelli E, Francesco A, Alice T, Francesca DS, Lorenzo P, et al. (2025) Nutritional status, immunonutrition, and gut microbiome: a coming of age for immunotherapy? Frontiers in Immunology 16: 1612567.
- Kaźmierczak SK, Agnieszka D, Marcin F, Wojciech M, Lebiedzińska A, et al. (2020) Immuno-nutritional support as an important part of multidisciplinary anti-cancer therapy. Central European Journal of Immunology 45(4): 454-460.
- Frąk M, Anna G, Paweł K, Janusz M, Ewa K, et al. (2022) Interactions between dietary micronutrients, composition of the microbiome and efficacy of immunotherapy in cancer patients. Cancers 14(22): 5577.
- Raczyńska A (2025) The impact of immunomodulatory components used in clinical nutrition-a narrative review. Nutrients 17(5): 752.
- Lee KA, Heather MS, Veronique B, Paul N, Tim DS, et al. (2020) Role of the gut microbiome for cancer patients receiving immunotherapy: Dietary and treatment implications. European Journal of Cancer 138: 149-155.
- Ebert MP (2006) Advances, challenges, and limitations in serum-proteome-based cancer diagnosis. Journal of proteome research 5(1): 19-25.
- Zugazagoitia J, Cristiano G, Santiago P, Irene F, Sonia MP, et al. (2016) Current challenges in cancer treatment. Clinical therapeutics 38(7): 1551-1566.
- Ravasco P (2019) Nutrition in cancer patients. Journal of clinical medicine 8(8): 1211.






























