Abstract
Introduction: Data on the real-world use of Lyumjev®, ultra-rapid-acting insulin lispro, as a mealtime insulin for people with diabetes in Germany are limited. We aimed to describe children and adults with diabetes receiving Lyumjev® in routine care in Germany.
Materials and Methods: This retrospective cohort study used data on patients with type 1 or type 2 diabetes who initiated Lyumjev® treatment between June 14, 2020, and December 31, 2023, from the multicenter diabetes perspective follow-up registry. Patients were grouped into cohorts by age and diabetes type. Demographics, clinical characteristics, comorbidities, details of treatment at Lyumjev® initiation, and antidiabetic treatment history were described by cohort at baseline. Selected characteristics and treatment details were described for cohorts and follow-up time points at which data for ≥25 patients were available.
Results: In total, 485 patients were included: 106 children and 291 adults with type 1 diabetes and 88 adults with type 2 diabetes. These cohorts receiving Lyumjev® were generally similar to the respective diabetes Lyumjev® phase III clinical trial populations in terms of age, disease duration, body mass index, and glycated hemoglobin. In adults with type 1 diabetes followed up for 12-18 months, glycated hemoglobin was stable without an increase in prandial and overall insulin dose or hypoglycemic events.
Conclusion: These data suggest that the clinical utility of Lyumjev® demonstrated in phase III clinical trials is reflected in a real-world setting in Germany.
Keywords: Insulin; Type 1 Diabetes; Type 2 Diabetes; Ultra-Rapid Lispro
Abbreviations: BMI: Body Mass Index; CT: Conventional Insulin Therapy; DPV: Diabetes Prospective Follow-up registry; eGFR: Estimated Glomerular Filtration Rate; GLP-1 RA: Glucagon-Like Peptide-1 Receptor Agonist; HbA1c: Glycated Hemoglobin; HDL-C: High-Density Lipoprotein Cholesterol; ICT: Intensive Conventional Insulin Therapy; LDL-C: Low-Density Lipoprotein Cholesterol; M: Month; N/A: Not Applicable; OAD: Oral Anti diabetes Drug; PPG: Postprandial Glucose; SD: Standard Deviation; SDS: Standard Deviation Score; SIT: Supplementary Insulin Therapy at Mealtimes; T1D: Type 1 Diabetes; T2D: Type 2 Diabetes
Introduction
Diabetes is a common, chronic, and progressive disease. Several studies have shown that the prevalence of diabetes in Germany has increased significantly in recent years [1–5]. In Germany in 2023, the estimated number of people with documented diabetes was almost 9 million (372,000 with type 1 diabetes and 8.9 million with type 2 diabetes), and an estimated 2 million people had unreported diabetes [6].
Insulin therapy has advanced significantly over the past few decades to include recombinant insulin analogs that more effectively mimic the basal insulin response between meals and the rapid insulin response after meals in people without diabetes (e.g., insulin lispro, as part, and glulisine) [7,8]. These rapid-acting insulins offer a more rapid onset and shorter duration of action than regular human insulin and are associated with less hypoglycemia and lower HbA1c [8]. The 2023 American Diabetes Association Standards of Care in Diabetes guideline recommended rapid-acting insulin analogs in most individuals with type 1 diabetes to decrease hypoglycemia risk [8].
However, despite the availability of many basal and mealtime insulin analogs, a considerable number of people with diabetes still do not achieve glycemic goals [9-11]. Elevated postprandial glucose (PPG) is a persistent challenge to diabetes management among both people with type 1 diabetes and those with type 2 diabetes [12,13], and inadequately controlled PPG is associated with unfavorable clinical outcomes for people with diabetes [14- 16].
One of the primary limiting factors for exogenous insulin response is the rate of absorption; therefore, recent rapid-acting insulin analog development has focused on approaches to address PPG control by speeding up the absorption of insulin into the bloodstream [17]. Ultra-rapid-acting insulin lispro (Lyumjev®, Eli Lilly, Indianapolis, IN, USA) is a novel insulin lispro that aims to mimic physiological prandial insulin secretion for PPG control [18]. Lyumjev® uses two excipients to accelerate the absorption of insulin lispro at the injection site: a microdose of treprostinil, a prostacyclin analog that increases local vasodilation, and citrate, which enhances local vascular permeability [19-21]. Clinical trials have confirmed the superiority of ultra-rapid-acting over rapid-acting insulin analogs in controlling PPG excursions and shown a safety profile that is comparable to that of the rapid-acting insulins [22].
In phase III clinical trials (PRONTO-T1D [23] and PRONTO-T2D [24]), Lyumjev® achieved noninferior reduction in HbA1c compared with Humalog® at 26 weeks in adults with type 1 diabetes (mean age 44.1 years, BMI 26.6 kg/m2, HbA1c 8.04%, and duration of diabetes 18.8 years) or type 2 diabetes (mean age 60.2 years, BMI 32.1 kg/m2, HbA1c 8.30%, and duration of diabetes 16.4 years). Lyumjev® also demonstrated superior PPG control at 1 and 2 hours after meals [23,24]. Notably, in adults with type 1 diabetes (PRONTO-T1D continuous glucose monitoring sub-study), Lyumjev® improved daytime time in range compared with Humalog® [25].
On March 24, 2020, Lyumjev® received a marketing authorization from the European Medicines Agency as a mealtime insulin for subcutaneous injection for adults with diabetes [26]. Lyumjev® subsequently became available in Germany in September 2020 [27]. The indication for Lyumjev® 100 units/ml was expanded to include adolescents and children aged ≥1 year in November 2022 [28]. To date, limited real-world evidence is available for Lyumjev®. The objective of this study was to describe patients with type 1 or type 2 diabetes receiving Lyumjev® treatment in real-world clinical practice in Germany, including demographics and clinical characteristics over the course of Lyumjev® treatment.
Materials and Methods
Data source
This retrospective cohort study used data from the diabetes perspective follow-up (DPV) initiative database. The DPV registry was established in 1995 and is a standardized, prospective, multicenter, computer-based registry of diabetes care and outcomes [29,30]. Data from 511 pediatric and adult diabetes care centers, located predominantly in Germany but also in Austria, Luxembourg, and Switzerland, are recorded locally and transferred to the registry database twice a year for central analysis after anonymization.
Patient consent and ethics approval
Data collection and analysis of anonymized routine data for benchmarking and diabetes research within the DPV initiative were approved by the ethics committee of Ulm University (number 314/21) and by local review boards of the participating centers.
Study design
Patients were indexed to the study between June 14, 2020, and December 31, 2023. A patient’s index date was defined as the date when Lyumjev® treatment was started. Baseline was defined as the 3-month period preceding the patient’s index date. Patients were followed from index until the end of the study period (December 31, 2023), discontinuation of Lyumjev® treatment, or loss to follow-up, whichever occurred first. Depending on the date on which Lyumjev® treatment was started, the length of follow-up could vary between 1 day and 24 months. Index +3M, index +6M, and index +9M were defined as the index date plus 3, 6, or 9 months, respectively, plus or minus 1.5 months. Index +12M was defined as the index date plus 12 months, minus 1.5 months and plus 3 months. Index +18M and index +24M were defined as the index date plus 18 or 24 months, respectively, plus or minus 3 months. The study index, pre-index, and follow-up periods are illustrated in Figure S1 in the Supplementary materials.
Study population
Patients diagnosed with type 1 or type 2 diabetes who received Lyumjev® treatment outside a clinical trial for which treatment initiation was documented in the DPV registry were included. Patients were excluded if they had a diagnosis of gestational diabetes or had received another prandial insulin (analog or human insulin) or a premixed insulin during treatment with Lyumjev®.
Study patients were divided into four cohorts based on age and diabetes type. Cohort 1 included children with type 1 diabetes aged ≥1 year and <18 years at the start of Lyumjev® treatment, and Cohort 2 included adults with type 1 diabetes aged ≥18 years at the start of Lyumjev® treatment. Cohort 3 included children with type 2 diabetes aged ≥1 year and <18 years at the start of Lyumjev® treatment, and Cohort 4 included adults with type 2 diabetes aged ≥18 years at the start of Lyumjev® treatment.
Study variables
To evaluate the use of Lyumjev® in routine clinical care, demographics, clinical characteristics, comorbidities, details of treatment at Lyumjev® initiation, and antidiabetic treatment history were described by cohort at baseline. The record closest to the index date was used for baseline descriptions. Selected characteristics (HbA1c, BMI, and number of patients with severe hypoglycemic events) and treatment details (Lyumjev® dose and insulin comedication) were described across the follow-up period, if available. As sample size markedly decreased across the follow-up time points, baseline characteristics were also described for the subgroup of patients with follow-up data available at each respective time point. The record closest to the end of the respective time period was used. Follow-up results were only described for cohorts, variables, and time points at which data for at least 25 patients were available.
Statistical analyses
Descriptive statistics were implemented for each study cohort. The number of patients with missing data were reported for each measured variable in the study. For categorical variables, results were presented as number, number missing, frequency, and percentage. For continuous variables, results were presented as number, number missing, mean, and SD. No comparative analyses were performed. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Patients
Initially, 637,188 people with type 1 or type 2 diabetes from the DPV registry were confirmed as eligible for study participation based on predefined inclusion and exclusion criteria (Figure 1). Of those, 485 patients treated with Lyumjev® were included in the study: 106 children and 291 adults with type 1 diabetes were included in Cohorts 1 and 2, respectively; the number of children with type 2 diabetes was less than five (Cohort 3), and 88 adults with type 2 diabetes were included in Cohort 4. As the sample size of Cohort 3 was less than five, no further description of this cohort is provided.
The mean (SD) duration of follow-up was short: 0.1 (0.2) years in Cohort 1, 0.5 (0.7) years in Cohort 2, and 0.6 (0.9) years in Cohort 4. Figure 1 describes the number of patients available for follow-up at each post-index time point in each cohort. Most patients, especially children, initiated Lyumjev® treatment late during the study period, which strongly limited the duration of available follow-up data. In Cohorts 1, 2, and 4, when the follow-up period ended, 86.8%, 84.2%, and 76.1% of patients, respectively, were still receiving Lyumjev® treatment; 13.2%, 15.8%, and 23.9% of patients, respectively, stopped Lyumjev® treatment during the study period. The reason for treatment discontinuation is not recorded within the DPV registry.
Baseline characteristics
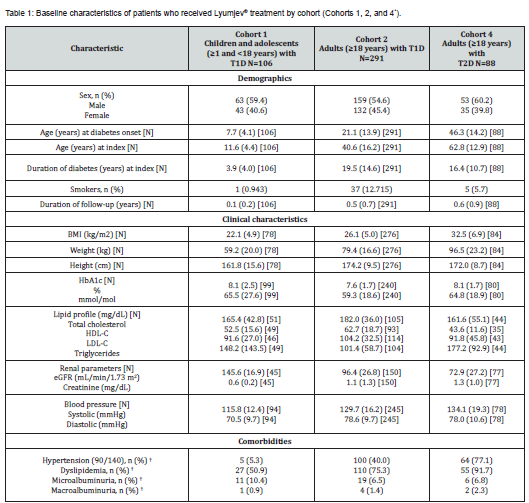
The baseline demographics, clinical characteristics, and comorbidities of patients who received Lyumjev® treatment are summarized by cohort in Table 1. In Cohort 1, children with type 1 diabetes (59.4% male) had a mean (SD) age of 11.6 (4.4) years at index, BMI 22.1 (4.9) kg/m2 (BMI standard deviation score [SDS] 0.7 [1.2]), HbA1c 8.1 (2.5) %, and duration of diabetes of 3.9 (4.0) years. In this cohort, 50.9% of children had dyslipidemia, 10.4% had microalbuminuria, 5.3% had hypertension, and 0.9% had macroalbuminuria. Whether patients received medication for comorbid conditions (e.g., lipid-lowering or antihypertensive treatments) was not analyzed.
All data reported are mean (SD) unless described otherwise.
N values in italics represent the number of patients with data available for continuous variable at baseline.
*As the sample size in Cohort 3 was small, no description of this cohort is provided.
†Percentage calculated with number of patients with non-missing data in denominator.
BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; n, number of patients in categorical variable category at baseline; N, total number of patients in cohort; SD, standard deviation; T1D, type 1 diabetes; T2D, type 2 diabetes.
In Cohort 2, adults with type 1 diabetes (54.6% male) had a mean (SD) age of 40.6 (16.2) years at index, BMI 26.1 (5.0) kg/ m2, HbA1c 7.6 (1.7) %, and duration of diabetes of 19.5 (14.6) years. In this cohort, 75.3% of adults had dyslipidemia, 40.0% had hypertension, 6.5% had microalbuminuria, and 1.4% had macroalbuminuria.
Adults with type 2 diabetes in Cohort 4 (60.2% male) had a mean (SD) age of 62.8 (12.9) years at index, BMI 32.5 (6.9) kg/ m2, HbA1c 8.1 (1.7) %, and duration of diabetes of 16.4 (10.7) years. In this cohort, 91.7% of adults had dyslipidemia, 77.1% had hypertension, 6.8% had microalbuminuria, and 2.3% had macroalbuminuria.
Details of treatment at Lyumjev® initiation and antidiabetic treatment history


In Cohort 1, the mean (SD) Lyumjev® (prandial) dose at index was 29.0 (22.7) IU per day for children with type 1 diabetes (n=106), and 22 (20.8%) patients received Lyumjev® directly at initial diagnosis. In total, 40% of children received basal insulin as comedication (Figure 2A). The mean (SD) overall insulin dose was 46.0 (31.6) IU (n=106) and 0.9 (0.4) IU/kg per day (n=103). In this cohort, 64.2% of children were on a pump insulin regimen, and 34.0% were on an intensive conventional insulin therapy (ICT) regimen (Figure 2A). Of the 70 children in Cohort 1 for whom data on prior medication were available, all had previously received insulin therapy, with 44.3% receiving basal insulin and 84.3% receiving prandial insulin (Figure 4).


In Cohort 2, the adults with type 1 diabetes received a mean (SD) Lyumjev® (prandial) dose at index of 30.1 (33.4) IU (n=282), and eight (2.7%) patients received Lyumjev® directly at initial diagnosis. A total of 63% of adults received basal insulin as comedication (Figure 2B). The mean (SD) overall insulin dose was 52.5 (39.5) IU (n=290) and 0.7 (0.5) IU/kg per day (n=272). In this cohort, 37.1% of adults were on an ICT insulin regimen, 28.9% were on a pump insulin regimen, and 25.4% were on a conventional insulin therapy (CT) regimen (Figure 2B). Of the 118 adults in Cohort 2 for whom data on prior medication were available, all had previously received insulin therapy, with 68.6% receiving basal insulin and 79.7% receiving prandial insulin (Figure 6).

In adults with type 2 diabetes in Cohort 4, the mean (SD) Lyumjev® (prandial) dose at index was 37.1 (32.3) IU (n=84), and five (5.7%) patients received Lyumjev® directly at initial diagnosis. In total, 83% of adults received basal insulin as comedication (Figure 2C). The mean (SD) overall insulin dose was 62.0 (43.8) IU (n=86) and 0.7 (0.6) IU/kg per day (n=80). The majority of adults in this cohort (56.8%) were on an ICT regimen, and 25.0% were on a CT regimen (Figure 2C). In this cohort, 78.4% of adults with type 2 diabetes received an oral antidiabetic drug (OAD) or glucagon-like peptide 1 receptor agonist (GLP-1 RA) as comedication with Lyumjev® (Figure 2C). Of the 37 adults in Cohort 4 for whom data on prior medication were available, 81.1% had previously received insulin therapy, 43.2% had received a prandial insulin, and 86.5% had received an OAD or GLP-1 RA (Figure 3C).


Characteristics of patients over the course of Lyumjev® treatment
Because the sample sizes in Cohorts 1 and 2 were small, follow-up data (including clinical characteristics, details of Lyumjev® treatment, and antidiabetic comedication) are presented and described until index +3 months for children with type 1 diabetes (Cohort 1) (Table 2) and until index +18 months for adults with type 1 diabetes (Cohort 2) (Table 3). Results are descriptive only; no comparative statistical tests were conducted between follow-up time points. Data were available for fewer than 25 patients at all follow-up time points in cohort 4; therefore, no description of patient characteristics over the course of Lyumjev® treatment is provided for adults with type 2 diabetes.


All data are presented as mean (SD) unless described otherwise.
BMI, body mass index; HbA1c, glycated hemoglobin; N/A, not applicable; SD standard deviation.
In Cohort 1, mean (SD) HbA1c decreased from baseline at index +3 months (6.9 [1.1] vs. 9.1 [3.2] %), whereas mean (SD) BMI increased from baseline (22.1 [4.9] vs. 21.6 [4.9] kg/m2; BMI-SDS 0.8 [1.1] vs. 0.6 [1.3]) among children with type 1 diabetes (Table 2). The mean (SD) Lyumjev® (prandial) dose decreased from baseline (25.5 [19.8] vs. 27.9 [19.7] IU), the proportion of children receiving basal insulin as comedication to Lyumjev® treatment decreased from baseline (54.3 vs. 56.5%), and the mean (SD) overall insulin dose decreased from baseline (41.5 [30.3] vs. 43.7 [30.3] IU) at index +3 months. One of the 46 children with type 1 diabetes in Cohort 1 with follow-up data available at index +3 months had one or more severe hypoglycemic event during the first 3 months of treatment.

In Cohort 2, mean (SD) HbA1c varied between 6.9 (0.9) and 7.2 (0.9) % at follow-up time points until index +18 months among adults with type 1 diabetes (Table 3). Generally, values at follow-up time points were slightly lower than at baseline for the respective patient subgroups. Mean (SD) BMI varied between 26.4 (5.1) and 28.2 (6.0) kg/m2 over the follow-up time points to index +18 months. At all follow-up time points, the mean (SD) BMI was marginally higher than at baseline. The mean (SD) Lyumjev® (prandial) dose and overall insulin dose varied little across follow-up time points until index +18 months (between 34.3 [37.6] and 39.0 [52.2] IU and between 58.8 [48.5] and 63.3 [52.8] IU, respectively). Generally, the proportions of adults receiving basal insulin as comedication to Lyumjev® treatment at follow-up time points were slightly higher than at baseline for the respective subgroups. Of the 92 adults with type 1 diabetes in Cohort 2 with follow-up data available at index +3 months, one had one or more severe hypoglycemic event during the first 3 months of treatment. No severe hypoglycemic events were reported during any of the other follow-up periods.
Discussion
The objective of this study was to describe the population of patients with diabetes receiving Lyumjev® treatment in everyday clinical practice in Germany since the ultra-rapid-acting insulin analog became available, using real-world data derived from the DPV initiative database. Given the superiority of ultra-rapid-acting over rapid-acting insulin analogs in controlling PPG excursions demonstrated in clinical trials, many people with diabetes may benefit from these treatments in terms of prandial glycemic control [22]. However, ultra-rapid-acting insulin analogs are not explicitly addressed in current type 2 diabetes management guidelines or consensus recommendations for clinical practice issued by medical societies such as the American Diabetes Association or the European Association for the Study of Diabetes [8,31]. Furthermore, in the equivalent type 1 diabetes professional guidelines and recommendations, ultra-rapid-acting insulin analogs are only mentioned as an alternative equivalent to rapid-acting insulin analogs [8,32]. Therefore, real-world data on the use of Lyumjev®, and the populations of people with diabetes for whom ultra-rapid-acting insulin analogs may be beneficial, provide additional evidence and are important to inform healthcare professionals and improve patient care. This study showed that the real-world populations with type 1 or type 2 diabetes receiving Lyumjev® treatment in Germany were generally similar to those of the adult diabetes phase III clinical trial populations in which Lyumjev® demonstrated superiority in terms of PPG control and non-inferiority in HbA1c reduction versus insulin lispro [23,24], thereby supporting the generalizability of these results to everyday clinical practice.
Adults with type 1 diabetes in this study were slightly younger (mean age 40.6 vs. 44.1 years) but had a similar disease duration (mean 19.5 vs. 18.8 years), a similar BMI (mean 26.1 vs. 26.6 kg/m2), and slightly lower HbA1c (mean 7.58 vs. 8.04%) when initiating Lyumjev® treatment than those who were enrolled in the PRONTO-T1D trial [23]. These findings were mirrored when comparing the characteristics of children with type 1 diabetes in this study with those in the phase III pediatric Lyumjev® clinical trial population [33]. Children with type 1 diabetes in this study were slightly younger (mean 11.6 vs. 12.1 years) but had a slightly higher BMI (mean 22.1 vs. 20.5 kg/m2), a marginally shorter disease duration (mean 3.9 vs. 4.5 years), and a marginally higher HbA1c (mean 8.15 vs. 7.91%) when initiating Lyumjev® treatment than those in the PRONTO-Peds trial [33]. Adultswith type 2 diabetes in this study were slightly older (mean age 62.8 vs. 60.2 years) but had the same average disease duration (mean 16.4 vs. 16.4 years), a similar BMI (mean 32.5 vs. 32.1 kg/m2), and marginally lower HbA1c (mean 8.1 vs. 8.3%) when initiating Lyumjev® treatment than those who were enrolled in the PRONTO-T2D trial [24].
This study had strong sample size limitations for the follow-up period, especially for Cohorts 1 and 4. Importantly, the short follow-up time was driven by patients starting Lyumjev® treatment close to the end of the study period. Most patients were still on Lyumjev® treatment at the end of the study period: 13.2% of patients in Cohort 1, 15.8% in Cohort 2, and 23.9% in Cohort 4 discontinued Lyumjev® during the study period. In this study, HbA1c, reflecting glycemic control, was still stable after 12/18 months among adults with type 1 diabetes, without an observed increase in prandial and overall insulin dose or an increase in the risk of hypoglycemic events.
The results of this real-world Lyumjev® study are in line with the results of the PRONTO-T1D clinical trial [23], which showed noninferior reduction in HbA1c compared with Humalog® at 26 weeks, even though the duration of action of Lyumjev® is shorter and the onset of action is faster [34]. However, the superiority of ultra-rapid insulin lispro, which is only expected post-prandially [23,24], could not be assessed in this study because of sample size limitations for continuous glucose monitoring measures. For children with type 1 diabetes, an improvement in glycemic control reflected by HbA1c could be observed with a reduction in daily Lyumjev® dose and overall insulin dose after 3 months of treatment. Only one patient reported experiencing a severe hypoglycemic event. These results are in line with those reported for the PRONTO-Peds clinical trial [33]. The follow-up data for patients with type 2 diabetes who received Lyumjev® in this study was insufficient to enable us to draw conclusions. However, our findings can serve as a basis for future research on the potential benefits of Lyumjev® treatment seen in clinical trials and how they could translate outside of the clinical trial setting.
Limitations
The main study limitations were the descriptive nature of the study, the baseline sample size restriction in Cohort 3 (children with type 2 diabetes), and the follow-up sample size restrictions in Cohorts 4 (adults with type 2 diabetes) and 1 (children with type 1 diabetes), most likely due to the date that Lyumjev® became available for children in Germany. Therefore, the described characteristics of patients over the course of Lyumjev® treatment should be interpreted with caution. Information on nutrition habits or individual socioeconomic status was not available in the DPV registry; therefore, residual confounding factors could not be completely ruled out. However, the multicenter, prospective data collection within the DPV registry enabled the longitudinal follow-up of individuals with diabetes of several age groups on both a national and an international level using large sample sizes. The DPV registry captured more than 85% of children and adolescents with type 1 diabetes in Germany [35], making the results representative for type 1 diabetes care in Germany. Moreover, the DPV registry could be regarded as representative for routine diabetes specialist care in Germany for adults with type 1 or type 2 diabetes. However, adults with diabetes treated in primary care services were underrepresented.
Conclusions
These findings collectively contribute to the growing body of evidence supporting the clinical utility of Lyumjev® in real-world settings and the generalizability of the phase III Lyumjev® clinical trials to everyday clinical practice in Germany. However, further research is required to understand the real-world use of Lyumjev® in routine care.
References
- Heidemann C, Scheidt NC (2017) Prevalence, incidence and mortality of diabetes mellitus in adults in Germany - a review in the framework of the Diabetes Surveillance. J Health Monit 2: 98-121.
- Goffrier B, Schulz M, Bätzing FJ (2017) Administrative prevalence and incidence of diabetes mellitus from 2009 to 2015. Healthcare Atlas Report 17/03. Berlin.
- Heidemann C, Du Y, Paprott R, Haftenberger M, Rathmann W, et al. (2016) Temporal changes in the prevalence of diagnosed diabetes, undiagnosed diabetes and prediabetes: findings from the German Health Interview and Examination Surveys in 1997–1999 and 2008–2011. Diabet Med 33: 1406-1414.
- Boehme MW, Buechele G, Frankenhauser MJ, Mueller J, Lump D, et al. (2015) Prevalence, incidence and concomitant co-morbidities of type 2 diabetes mellitus in South Western Germany -a retrospective cohort and case control study in claims data of a large statutory health insurance. BMC Public Health 15: 855.
- Spieker J, Vetter VM, Drewelies J, Spira D, Steinhagen TE, et al. (2023) Diabetes type 2 in the Berlin Aging Study II: Cross-sectional and longitudinal data on prevalence, incidence and severity over on average seven years of follow-up. Diabet Med 40: e15104.
- Deutscher Gesundheitsbericht. Diabetes 2024: The Inventory.
- Wilson LM, Castle JR (2020) Recent advances in insulin therapy. Diabetes Technol Ther 22(12): 929-936.
- El Sayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, et al. (2023). Pharmacologic approaches to glycemic treatment: Standards of Care in Diabetes-2023. Diabetes Care 46: S140-S157.
- Centers for Disease Control and Prevention (2020) National diabetes statistics report: estimates of diabetes and its burden in the United States 2020.
- Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, et al. (2019) State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther 2019 21: 66-72.
- McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, et al. (2015) Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med 32: 1036-1050.
- Brod M, Nikolajsen A, Weatherall J, Pfeiffer KM (2016) The economic burden of post-prandial hyperglycemia (PPH) among people with type 1 and type 2 diabetes in three countries. Diabetes Ther 7: 75-90.
- Pfeiffer KM, Sandberg A, Nikolajsen A, Brod M (2018) Postprandial glucose and healthcare resource use: a cross-sectional survey of adults with diabetes treated with basal-bolus insulin. J Med Econ 21: 66-73.
- Lefever E, Vliebergh J, Mathieu C (2021) Improving the treatment of patients with diabetes using insulin analogues: current findings and future directions. Expert Opin Drug Saf 20: 155-169.
- Riddle MC (2017) Basal glucose can be controlled, but the prandial problem persists - It's the next target! Diabetes Care 40: 291-300.
- Madsbad S (2016) Impact of postprandial glucose control on diabetes-related complications: how is the evidence evolving? J Diabetes Complications 30: 374-385.
- Heise T, Piras de OC, Juneja RRA, Chigutsa F, Blevins T, Blevins T (2022) What is the value of faster acting prandial insulin? Focus on ultra rapid lispro. Diabetes Obes Metab 24: 1689-1701.
- Leohr J, Dellva MA, LaBell E, Coutant DE, Arruble J, et al. (2024) Ultra rapid lispro (Lyumjev®) shortens time to recovery from hyperglycaemia compared to Humalog® in individuals with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Obes Metab 26: 215-223.
- Michael MD, Zhang C, Siesky AM, Cox AL, Sperry AE, et al. (2017) Exploration of the mechanism of accelerated absorption for a novel insulin lispro formulation (968-P). Diabetes 66: A250.
- Paavola CD, Cox AL, Sperry AE, Hansen RJ, Li S, et al. (2017) A stable, hexameric, ultra-rapid insulin formulation containing citrate (979-P). Diabetes 66: A254.
- Pratt E, Leohr J, Heilmann C, Johnson J, Landschulz W, Landschulz W (2017) Treprostinil causes local vasodilation, is well tolerated, and results in faster absorption of insulin lispro (975-P). Diabetes 66: A253.
- Giorgino F, Battelino T, Bergenstal RM, Forst T, Green JB, et al. (2025) The role of ultra-rapid-acting insulin analogs in diabetes: an expert consensus. J Diabet Sci Technol 19(2): 452-469.
- Klaff L, Cao D, Dellva MA, Tobian J, Miura J, et al. (2020) Ultra rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: Results from the 26-week PRONTO-T1D study. Diabetes Obes Metab 22: 1799-1807.
- Blevins T, Zhang Q, Frias JP, Jinnouchi H, Chang AM, et al. (2020) Randomized double-blind clinical trial comparing ultra-rapid lispro with lispro in a basal-bolus regimen in patients with type 2 diabetes: PRONTO-T2D. Diabetes Care 43: 2991-2998.
- Malecki MT, Cao D, Liu R, Hardy T, Bode B, et al. (2020) Ultra-rapid Lispro improves postprandial glucose control and time in range in type 1 diabetes compared to Lispro: PRONTO-T1D Continuous Glucose Monitoring Substudy. Diabetes Technol Ther 22(11): 853-860.
- European Medicine Agency (EMA) [website]. Lyumjev (previously Liumjev). Last updated 30 April 2024.
- Lilly [website]. Pressemitteilung. Innovative Weiterentwicklung von Humalog®. Neues Insulin lispro Lyumjev® in Deutschland verfügbar. August 2020.
- European Commission (EC). Commission implementing decision of 18.11.2022 amending the marketing authorisation granted for Decision C (2020) 1943 (final) for “Lyumjev – Insulin lispro”, a medicinal product for human use. Brussels, 18.11.2022.
- Bohn B, Karges B, Vogel C, Otto KP, Marg W, et al. (2016) 20 years of pediatric benchmarking in Germany and Austria: age-dependent analysis of longitudinal follow-up in 63,967 children and adolescents with type 1 diabetes. PLoS One 11: e0160971.
- Bohn B, Kerner W, Seufert J, Kempe HP, Jehle PM, et al. (2016) Trend of antihyperglycaemic therapy and glycaemic control in 184,864 adults with type 1 or 2 diabetes between 2002 and 2014: analysis of real-life data from the DPV registry from Germany and Austria. Diabetes Res Clin Pract 115: 31-38.
- Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, et al. (2022) Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45: 2753-2786.
- Holt RIG, DeVries JH, Hess FA, Hirsch IR, Kirkman MS, et al. (2021) The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 44: 2589-2625.
- Wadwa RP, Laffel LM, Franco DR, Dellva MA, Knights AW, et al. (2023) Efficacy and safety of ultra‐rapid lispro versus lispro in children and adolescents with type 1 diabetes: The PRONTO‐Peds trial. Diabetes Obes Metab 25: 89-97.
- Linnebjerg H, Zhang Q, LaBell E, Dellva MA, Coutant DE, et al. (2020) Pharmacokinetics and glucodynamics of ultra rapid lispro (URLi) versus Humalog® (lispro) in younger adults and elderly patients with type 1 diabetes mellitus: a randomized controlled trial. Clin Pharmacokinet 59: 1589-1599.
- Auzanneau M, Lazinger S, Bohn B, Kroschwald P, Kuhnle KU, et al. (2018) Area deprivation and regional disparities in treatment and outcome quality of 29,284 pediatric patients with type 1 diabetes in Germany: a cross-sectional multicenter DPV analysis. Diabetes Care 41(12): 2517-2525.






























