To Study the Prevalence and the Risk Factors of Diabetes Mellitus in Patients Attending a Tertiary Center of Nepal
Anil Shankhadev*, Gopal Prasad Shrestha, Dipendra Khadka, Ayushi Shrestha and Ayush Shrestha
Internal Medicine, Nepalgunj Medical College and Teaching Hospital, Kathmandu University, Banke, Nepal
Submission: August 15, 2024; Published: October 28, 2024
*Corresponding author: Anil Shankhadev, Internal Medicine, Nepalgunj Medical College and Teaching Hospital, Kathmandu University, Banke, Nepal
How to cite this article: Anil Shankhadev*, Gopal Prasad Shrestha, Dipendra Khadka, Ayushi Shrestha and Ayush Shrestha. To Study the Prevalence and the Risk Factors of Diabetes Mellitus in Patients Attending a Tertiary Center of Nepal. Curre Res Diabetes & Obes J 2024; 17(4): 555970.DOI: 10.19080/CRDOJ.2024.17.555970
Abstract
Introduction: Diabetes mellitus is one of the most common non communicable chronic debilitating disorders. Its burden has increased globally and, so has the prevalence. The contributing risk factors include age, sex, ethnicity, obesity and sedentary lifestyle.
Objective: To study the prevalence and the risk factors of diabetes mellitus in patients attending a tertiary center of Nepal.
Materials and methods: A hospital based observational study conducted in the department of Internal Medicine of Nepalgunj Medical College and Teaching Hospital, Nepal in 2019. The data were collected through interviews and questionnaires. Physical examination, anthropometric measurements, and laboratory measurement for random blood sugar, fasting blood sugar and HbA1c were conducted. American Diabetic Association diagnostic criteria were applied to establish the diagnosis of diabetes mellitus.
Results: The prevalence of diabetes was 7.12%. Diabetes was more prevalent in the age group of 65 years and above. There was male predominance with male to female ratio of 7.6:6.6. Out of total 200 patients enrolled in the study, 44.5 % of diabetic patients were obese, 19% had family history of diabetes, 62.5% were smoker, 40.5% consumed moderate alcohol, 54% had dyslipidemia and 39.5% had hypertension. Diabetes was higher in urban areas (71.5%) than in rural areas (28.5%). Chhetri caste had highest diabetes prevalence (30.5%) followed by Brahmin (24%) and Chaudhary (16.5%).
Conclusion: Diabetes mellitus is a common disease in Nepali population and the prevalence rate is growing rapidly. The common risk factors for diabetes mellitus were advancing age, obesity, physical inactivity, smoking, urbanization and dyslipidemia.
Keywords: Alcohol, Diabetes Mellitus, Dyslipidemia, Epidemiology, Hypertension, Obesity, Prevalence, Risk Factors, Smoking
Introduction
Diabetes mellitus is a common metabolic disorder characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both [1]. According to International Diabetic Federation (IDF), the worldwide prevalence of diabetes has risen dramatically over the past two decades. IDF reported that 463 million individuals had diabetes in 2019 and the number is estimated to increase to 578.4 million in 2030. The global prevalence of diabetes in 2019 was 9.3% and is expected to increase to 10.2% in 2030 if similar growth is continued [2]. World Health Organisation (WHO) reported a diabetes prevalence of 9.1% in Nepal with higher male prevalence 10.5% compared to 7.9% in females in 2016.
Various factors contribute to diabetes mellitus. Advancing age, often after 45 years, is commonly associated with diabetes due to decreasing glucose tolerance with the growing age [4]. Family history of diabetes in any first degree relative has a two-to-three-fold increased risk of diabetes. The risk of diabetes was found to be higher (five- to- six -fold) in those with both a maternal and paternal history of diabetes [5]. Obesity, predominantly abdominal obesity causes insulin resistance of peripheral glucose to insulin dependent tissues, which is the primary mechanism of diabetes [6].
Physical inactivity and unhealthy diet change the body composition and proportions of myocyte and adipocyte insulin receptors [7] Moderate alcohol intake is associated with a lower risk of diabetes. Excessive alcohol impairs insulin sensitivity and hence increase risk of diabetes [8]. Both active and passive smoking increase the risk of chronic illness including diabetes. Smoking increases triglyceride and decreases high density lipoprotein which contributes to decreased insulin sensitivity [9]. Dyslipidemia, hypertension and diabetes are the components of metabolic syndrome [10]. Hypertension is presented in twothirds of diabetic patients and its development coincides with development of hyperglycemia [11]. Thus, the aim was to find the prevalence and risk factors of diabetes mellitus in the patients attending Nepalgunj Medical College and Teaching Hospital in Nepal. To our knowledge, this study is the first to assess hospitalbased prevalence of diabetes in western Nepal.
Materials and Methods
This is a hospital based prospective observational study conducted from 15th February, 2018 to 15th February, 2019 in the department of Internal Medicine, Nepalgunj Medical College and Teaching Hospital, located in the center of western region of Nepal.
Data collection
During this period, every patient aged 24 or higher, clinically presenting with diabetes as defined American Diabetes Association or taking oral hypoglycemic agents or insulin was enrolled after getting their informed consent. Pregnant women, patients taking drugs that raise blood glucose level and those refusing to give consent were excluded from the study. Information about the interviews and questionnaire were filled up as per Performa. For calculation of the prevalence of diabetes, the total number of patients visiting the medical outpatient and inpatient department were recorded from the hospital database. Similarly, all the patients who were newly diagnosed or previously diagnosed with diabetes mellitus were also recorded. The prevalence was calculated using following formula. Prevalence = (Total number of diabetes/ total population attending department of internal medicine) *100. During the process of population registry, it was ensured that patients who were seen in the medicine outpatient department and inpatient department were not counted twice.
Risk factor stratification
Out of all the patients with diabetes, 200 were randomly selected for further evaluation of various risk factors. A detailed history, clinical examination, anthropometric measurements and various blood investigations were performed. Height was measured with a standard stadiometer to the nearest cm; weight was measured in light clothing and without shoes, to the nearest 100gm using a standard analogue weighing machine (model SAM 50) that was checked regularly for accuracy. Body Mass Index (BMI) was calculated (BMI= w/h2; w=weight in kg, h=height in m).
Overweight and obesity were defined using WHO criteria. Waist circumference and hip circumference was measured according to the WHO Data Gathering Protocol. Waist circumference was measured at the midpoint between the lower margin of the last palpable ribs and the top of the iliac crest, using a stretch‐resistant tape that provided a constant 100 g tension. Hip circumference was measured around the widest portion of the buttocks, with the tape parallel to the floor. The waist-to-hip ratio (WHR), a dimensionless ratio of the circumference of the waist to that of the hips, was calculated with waist measurement divided by hip measurement (W ÷ H).
Data analysis
Data was collected using a structured Performa covering relevant details. Incomplete data was discarded. Statistical analysis was conducted using Microsoft Office Excel and SPSS Statistical Package for the Social Sciences Software version 20.0.0. Data is presented in the form of tables and figures.
Results
The total number of populations was 31338. 48.9% were male and 51.1% were female. The prevalence of diabetes was 7.12 %. It was most prevalent in age group above 65 years of 7.12%, 1.39 % were newly diagnosed cases of diabetes whereas 5.73 % were previously diagnosed cases. The prevalence was higher in males than in female (7.6 % vs 6.6 %) and it was higher after 65 years (11.25%) and 45 years (10.24%) as shown in Table 1.

Demographic profile of diabetic patients
2234 patients were diagnosed to have diabetes mellitus. The mean age of diabetic patients was 54.92±13.15 years. Majority of the patients were in the age group 45-65 (47.90%) and more than half were male (52.6%). Table 2 shows the demographic profile of these patients. For the risk factor stratification, 200 out of 2234 patients were enrolled in the study. 44.5 % of the diabetic patients were obese, 19% had positive family history of diabetes, 62.5% smoked, 40.5% were alcoholic who consumed at least moderate alcohol, 54% had dyslipidemia and 39.5% had hypertension. When considering waist circumference and waist to hip ratio, there was increased risk of developing diabetes in males compared to females (27.5% and, 32% respectively). Table 3 gives the description of the risk factors.


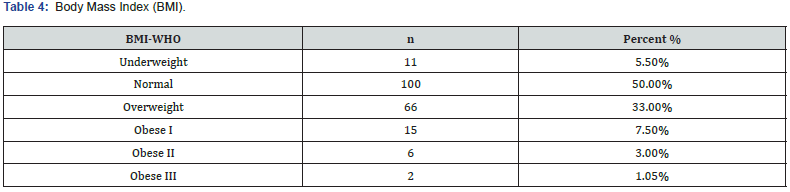
Body Mass Index (BMI)
According to the BMI category of WHO, of the 200 participants, 50% had normal BMI, 5.5% were underweight, 33% were overweight and rest were obese as shown in Table 4.
Rural or urban region as risk for Diabetes
A higher number of patients were from urban areas (71.5%) compared to the rural areas (28.5%) as shown in Figure 1. In our study, patients belonging to the Chhetri caste had higher prevalence of diabetes (30.5%) followed by Brahmin (24%) and Chaudhary (16.5%).as shown in Figure 2.


Discussion
Diabetes mellitus has always been the topic of global concern. Its incidence is rising day by day. This study found 7.12 % prevalence of diabetes in Nepalgunj Medical College and Teaching Hospital. The prevalence of newly diagnosed diabetes and preexisting diabetes were 1.39 %, and 5.73 % respectively. These observed epidemiological variables were similar to the findings reported by other studies. Karki et al. [12] from Dharan, reported 6.3 % prevalence of diabetes (among them 1.63% previously diagnosed and 4.67 % were newly diagnosed cases) in their a hospital based study of 1840 patients in 2000. This is a slightly lower prevalence compared to our study.
However, the prevalence of newly diagnosed diabetes was higher in their study. Similarly, a hospital based study by Fossen T et al. [13] reported 4.9% prevalence of diabetes in females in the Kavre region, which was lower than in our study (6.6%). Due to limited hospital-based studies available in the literature for comparison of data, the prevalence was also compared with community-based studies. As community-based studies usually include a larger population, the findings on prevalence were also expected to be different as compared to the hospital-based studies. However, their data could give more representative of diabetes prevalence as many diabetic patients visit a hospital late in the course of the disease. This also reflected higher prevalence of diabetes in community-based studies as compared to hospital-based studies. In a community-based study, conducted in the urban populations of Nepal by Shrestha et al. [14] in 2006, reported 19% prevalence. Similarly, Ono K et al. [15] in 2007 in a semi urban population, reported 9.5% prevalence.
Age played an important determinant in diabetes mellitus. A study conducted in a semi urban population of Nepal by Ono k et al. [15] in 2007, reported that risk of diabetes increased with advancing age. Similarly, Karki P et al12 in 2000 reported that the prevalence of diabetes mellitus was relatively lower in females (5.75%) than in males (6.73%) in their hospital-based study. In our study, the prevalence of diabetes was more common in individuals above 45years in both men and women. As per our observation, central obesity was more prevalent in diabetic patients. In contrast, we also established that patients with normal BMI had an equal risk of diabetes as nearly half of the diabetic patients had normal BMI. Additionally, a population based studies conducted by Xu l et al [16] in 2017 in China and a hospital based study conducted by Jung SH et al [17] in 2016 in Korea, studies showed that visceral/central obesity was associated with increased prevalence of diabetes.
Family history of diabetes was thought to be a strong predictor of diabetes. In contrary to it, we found that only 19% individuals had positive family history of diabetes. Many patients were unaware about their positive family history of diabetes. Lack of knowledge might be due to illiteracy, unawareness and lack of health care facilities, especially in rural part of the country [18]. However, multiple studies suggested that the positive family history of diabetes increased the risk of developing it in their offsprings [19-21]. Our study showed higher incidence of physical inactivity in diabetic patients. Many studies reported similar findings. A population based study by Hu G et al [22], Sullivan PW et al [23] showed that both physical inactivity and obesity were strongly and independently associated with diabetes and it’s comorbidities.
Both active and passive smoking was equally associated with risk of developing diabetes in this study. Heavy alcohol intake and central obesity were considered strong determinants in developing diabetes. Many studies [24-26] suggested that moderate alcohol consumption was protective for diabetes and high alcohol consumption was associated with development of diabetes. A population based study by Carlsson et al24 reported that there was no protective effect of high alcohol consumption and large intakes of alcohol might increase the risk of diabetes. Additionally, 54% of the individuals with diabetes had dyslipidemia in this study. Several studies found that dyslipidemia increased the risk of developing diabetes and the risk increased if the patient had obesity. A study by Krauss et al [27] and Mooradian et al [28] reported that diabetes was associated with abnormalities of plasma lipid and lipoprotein, which include reduced HDL cholesterol, elevated LDL, and elevated triglyceride levels. A study by Yoshino et al [29] reported hypertriglyceridemia as an independent risk for developing diabetes.
Similarly, 39.5% of patients had both diabetes and hypertension. Despite the lack of direct association between hypertension and diabetes, these factors caused chronic illness like acute coronary syndrome and cerebrovascular accidents when occurring together. This study also categorised diabetes in epidemiological variables. The study showed that 71% of the respondents lived in urban region and 28.5% lived in rural areas. The prevalence of diabetes living in urban region was 5.09%, whereas rural region was 2.03%. Sasaki H et al. [30] in 2005 reported that the prevalence was 14.6% in urban areas, and 2.5% in rural areas.
Another study by Gyawali et al. [31] in 2015 reported the 8.1% and 1.0% prevalence in urban and rural populations, respectively. Both reported higher prevalence in urban population than rural. Higher prevalence (25.9%) was reported by Chhetri et al. [32] in 2009 in a community-based study of 1633 randomly selected participants in urban and rural areas in the Kathmandu Valley of Nepal, which reflected the prevalence of diabetes in an urban population. Different ethnic groups showed varying frequency of diabetes prevalence. Higher prevalence was noted in Chhetri (30.5%) followed by Brahmin (21%), Chaudhary (16.5%). The difference in prevalence among different ethnic groups may be due to genetic predisposition, insulin sensitivity and environmental factors [33]. In this study Chhetri and Brahmin showed higher prevalence than other ethnic groups suggesting the prevalence of diabetes in certain ethnic groups than others.
Conclusion
Hospital based prevalence of diabetes was 7.12% with higher prevalence among males. Moreover, it was more prevalent in individuals above 45 years. Risk factors associated with diabetes were central obesity, high BMI, physical inactivity, smoking, dyslipidemia, moderate or high alcohol intake and hypertension. Family history of diabetes was not a common risk factor in our study. Urbanisation had direct association with diabetes however screening of diabetes in rural population is required to accurately document its prevalence.
References
- Powers AC (2015) Diabetes mellitus: Diagnosis, Classification, and Pathophysiology. Harrison’s Principles of Internal Medicine 19th ed United States of America: McGraw-Hill Education 2399- 407.
- International Diabetes Federation: IDF (2019) Diabetes Atlas. 9th edition.
- World Health Organisation (2016) diabtetes, country profiles.
- Biggs ML, Mukamal KJ, Luchsinger JA, Ix JH, Carnethon MR, et al. (2010) Association between adiposity in midlife and older age and risk of diabetes in older adults. Jama 303(24): 2504-2512.
- InterAct Consortium (2013) The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia 56(1): 60-69.
- World Health Organization (2008) Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva 8(11): 5-27.
- Eaton SB, Eaton SB (2017) Physical inactivity, obesity, and type 2 diabetes: An evolutionary perspective. Research quarterly for exercise and sport 88(1): 1-8.
- Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC (1995) Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. Bmj 310(6979): 555-559.
- Facchini FS, Hollenbeck CB, Jeppesen J, Chen Y-DI, Reaven G (1992) Insulin resistance and cigarette smoking. The Lancet 339(8802): 1128-1130.
- Reaven GM (1987) Abnormal lipoprotein metabolism in non-insulin-dependent diabetes mellitus: pathogenesis and treatment. The American journal of medicine 83(3A): 31-40.
- Ferrannini E, Cushman WC (2012) Diabetes and hypertension: the bad companions. The Lancet 380(9841): 601-610.
- Karki P, Baral N, Lamsal M, Rijal S, Koner B, Dhungel S, et al. (2000) Prevalence of non-insulin dependent diabetes mellitus in urban areas of eastern Nepal: a hospital based study. Southeast Asian journal of tropical medicine and public health 31(1): 163-166.
- Fossen T, Nossen J (2013) The prevalence of diabetes mellitus and associated risk factors in the female population of Kavre in rural Nepal: Norges teknisk-naturvitenskapelige universitet, Det medisinske fakultet.
- Shrestha U, Singh D, Bhattarai M (2006) The prevalence of hypertension and diabetes defined by fasting and 2‐h plasma glucose criteria in urban Nepal. Diabetic Medicine 23(10): 1130-1135.
- Ono K, Limbu YR, Rai SK, Kurokawa M, Yanagida J, Rai G, et al. (2007) The prevalence of type 2 diabetes mellitus and impaired fasting glucose in semi-urban population of Nepal. Nepal Medical College journal: NMCJ 9(3): 154-156.
- Xu L, Lam T, Jiang C, Zhang W, Jin Y, et al. (2017) Adiposity and incident diabetes within 4 years of follow‐up: the Guangzhou Biobank Cohort Study. Diabetic Medicine 34(10): 1400-1406.
- Jung SH, Ha KH, Kim DJ (2016) Visceral fat mass has stronger associations with diabetes and prediabetes than other anthropometric obesity indicators among Korean adults. Yonsei medical journal 57(3): 674-680.
- Marasini S, Sharma S, Joshi A, Kunwar S, Mahato RK, et al. (2024) Exploring knowledge, perceptions, and practices of antimicrobials, and their resistance among medicine dispensers and community members in Kavrepalanchok District of Nepal. Plos one 19(1): e0297282.
- Van't Riet E, Dekker JM, Sun Q, Nijpels G, Hu FB, et al. (2010) Role of adiposity and lifestyle in the relationship between family history of diabetes and 20-year incidence of type 2 diabetes in US women. Diabetes care 33(4): 763-767.
- Meigs JB, Cupples LA, Wilson P (2000) Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 49(12): 2201-2207.
- Cederberg H, Stančáková A, Kuusisto J, Laakso M, Smith U (2015) Family history of type 2 diabetes increases the risk of both obesity and its complications: is type 2 diabetes a disease of inappropriate lipid storage. Journal of internal medicine 277(5): 540-551.
- Hu G, Lindström J, Valle TT, Eriksson JG, Jousilahti P, et al. (2004) Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Archives of internal medicine 164(8): 892-896.
- Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO (2005) Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the US, 2000–2002. Diabetes care 28(7): 1599-1603.
- Carlsson S, Hammar N, Grill V (2005) Alcohol consumption and type 2 diabetes. Diabetologia 48(6): 1051-1054.
- Howard AA, Arnsten JH, Gourevitch MN (2004) Effect of alcohol consumption on diabetes mellitus. Annals of internal medicine 140(3): 211-219.
- Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ (2005) Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes care 28(3): 719-725.
- Krauss RM (2004) Lipids and lipoproteins in patients with type 2 diabetes. Diabetes care 27(6): 1496-1504.
- Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nature Reviews Endocrinology 5(3): 150.
- Yoshino G, Hirano T, Kazumi T (1996) Dyslipidemia in diabetes mellitus. Diabetes research and clinical practice 33(1): 1-14.
- Sasaki H, Kawasaki T, Ogaki T, Kobayashi S, Itoh K, et al. (2005) The prevalence of diabetes mellitus and impaired fasting glucose/glycaemia (IFG) in suburban and rural Nepal—the communities-based cross-sectional study during the democratic movements in 1990. Diabetes research and clinical practice 67(2): 167-174.
- Gyawali B, Sharma R, Neupane D, Mishra SR, van Teijlingen E, et al. (2015) Prevalence of type 2 diabetes in Nepal: a systematic review and meta-analysis from 2000 to 2014. Global health action 8(1): 29088.
- Chhetri M, Chapman R (2009) Prevalence and determinants of diabetes among the elderly population in the Kathmandu Valley of Nepal. Nepal Med Coll J 11(1): 34-38.
- Carulli L, Rondinella S, Lombardini S, Canedi I, Loria P, et al. (2005) Diabetes, genetics and ethnicity. Alimentary pharmacology & therapeutics 22(2): 16-19.






























