- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Retatrutide as a Novel Treatment for Obesity and Type 2 Diabetes
Nasser Mikhail1* and Soma Wali2
1Endocrinology Division, Olive View-UCLA Medical Center, David-Geffen UCLA Medical School, USA
2Department of Medicine, Olive View-UCLA Medical Center, David-Geffen UCLA Medical School, USA
Submission: July 18, 2023; Published: July 26, 2023
*Corresponding author: Nasser Mikhail, Department of Medicine, Olive View-UCLA Medical Center, David-Geffen UCLA Medical School, USA, Email: nmikhail@dhs.lacounty.gov
How to cite this article: Nasser M, Soma W. Retatrutide as a Novel Treatment for Obesity and Type 2 Diabetes. Curre Res Diabetes & Obes J 2023; 16(5): 555949.DOI: 10.19080/CRDOJ.2023.16.555949
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Abstract
Retatrutide is a triple hormone agonist of the receptors of glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide (GIP) and glucagon that can be administered subcutaneously once a week. Different doses and escalation schedules of retatrutide were evaluated in 2 phase 2 American trials of subjects with obesity (n=338) and type 2 diabetes (n=281). In the obesity trial of 48 week-duration, percentage change in body weight from baseline after 24 weeks and 48 weeks were the primary and secondary outcomes respectively. At 24 weeks, the mean percentage decrease in weight ranged from 7.2% to 17.5% across retatrutide groups versus 1.6% with placebo. At 48 weeks, the decrease was 8.7% to 24.2% versus 2.1% with placebo. In the diabetes trial, the primary endpoint was a change in glycated hemoglobin (HbA1c) values from baseline to week 24. This trial included an active treatment group receiving dulaglutide 1.5mg once weekly. Secondary endpoints included change in HbA1c and body weight at 36 weeks. At 24 weeks, mean HbA1c reductions were 0.43% to 2.02% with retatrutide, 1.4% with dulaglutide and 0.01% with placebo. At 36 weeks, mean percentage weight reductions were 3.2% to 16.9% with retatrutide, 2.0% with dulaglutide and 3.0% with placebo. While the decrease in HbA1c levels reached a plateau at 24 weeks, weight loss in the 2 studies was progressive without evidence of attenuation effect up to the end of follow-up at 36-48 weeks. The most common adverse effects of retatrutide were gastrointestinal (GI) such as nausea, diarrhea, vomiting and constipation reported by 13-50% in the retatrutide groups compared with 35% in the dulaglutide group, and 13% in the placebo group. Discontinuation rates due to adverse effects were also higher with retatrutide being 16-17% compared with 2% with dulaglutide and 0-4% with placebo. In summary, retatrutide is highly effective drug in causing substantial weight loss and HbA1c reduction. Yet, tolerance to this drug seems sub-optimal. Phase 3 trials are urgently needed to clarify efficacy and safety of retatrutide.
Keywords: Retatrutide; GLP-1; GIP; Glucagon; Obesity; Diabetes and tirzepatide
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Introduction
Retatrutide (LY3437943) is a novel triple agonist of the 3 receptors GLP-1, GIP, and glucagon [1]. Compared to the native hormones, retatrutide shows 2.9-fold less potency at the glucagon receptor (GCCR), 2.5-fold less potency at the GLP-1 receptor, and 8.9-fold greater potency at human GIP receptor [1]. The approximate half-life of retatrutide is approximately 6 days making it suitable for once weekly administration [2]. Pre-clinical studies and phase 1 trials showed that retatrutide was highly effective in promoting weight loss and lowering HbA1c levels [1,2]. Recently, 2 phase 2 studies designed to examine efficacy and safety of retatrutide for treatment of obesity and type 2 diabetes were published (Table 1) [3,4]. The main purpose of this article is to provide an appraisal of this novel agent based on available data.
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Retatrutide for Treatment of Obesity
Retatrutide, in different subcutaneous doses of 1, 4, 8 and 12mg and escalation schedules, was evaluated for treatment of obesity in a phase 2, randomized, placebo-controlled, double-blind trial of 48 week-duration in the USA (Table 1) [3]. Contrary to most preceding obesity trials that predominantly enrolled women, this study included 51.8% men [3]. Participants (n=338) had mean age of 48.2 years, and mean baseline weight of 107.7kg, and body mass index (BMI) of 37.3kg/m2 (Table 1) [3]. All subjects received lifestyle intervention including regular counseling sessions [3]. At 24 weeks, mean percentage decrease in weight (primary outcome) ranged from 7.2% to 17.5% with different retatrutide doses compared with 1.6% with placebo. At 48 weeks, there was progressive decrease in weight (secondary outcome) ranging from 8.7% to 24.2% with retatrutide versus 2.1% with placebo [3]. Weight loss was dose-related between retatrutide doses of 1 to 8mg. However, the difference in weight loss between the highest 2 doses, 8 and 12mg, was minimal [3]. Inspection of the trajectory of weight loss with time showed that weight loss with retatrutide was evident after 4-8 weeks and progressed with no evidence of plateau to the end of follow-up at 48 weeks [3]. At 48 weeks, weight reduction of ≥15% was achieved by 75-83% of subjects who received 8-12mg dose of retatrutide. Interestingly, women and subjects with BMI of ≥35 kg/m2 had more weight loss than men and subjects with BMI < 35kg/m2 [3]. Thus, among women randomized to the highest retatrutide dose, percentage mean weight loss at 48 weeks was 28.5% versus 21.9% among men. In subjects with baseline BMI ≥35 kg/m2, corresponding weight loss was 26.4% versus 21.5% in those with BMI < 35kg/ m2 [3].
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Retatrutide for Treatment of Type 2 Diabetes
In another phase 2 trial in the USA, retatrutide was evaluated for treatment of type 2 diabetes in comparison with subcutaneous dulaglutide (1.5mg/weekly) and placebo (Table 1) [4]. The study duration was 36 weeks. The primary outcome was change in HbA1c values from baseline to 24 weeks, whereas secondary endpoints were changes in HbA1c and weight after 36 weeks (Table 1) [4]. Patients (n=281, 56% women, mean age 56 years) had type 2 diabetes of approximately 8 year-duration uncontrolled on metformin with mean baseline HbA1c of 8.3% [4]. At 24 weeks, reductions in HbA1c levels were dose-related in the retatrutide groups ranging from 0.43 to 2.02%, versus 1.31% in the dulaglutide group and 0.01% in the placebo group [4]. HbA1c reductions were significantly greater with the 2 retatrutide highest doses (8mg given in slow escalation and 12mg/week) than with dulaglutide (P<0.002) [4]. At 36 weeks, a similar trend was observed but with minimal changes in HbA1c levels beyond 24 weeks in all groups [4].
There was dose-related weight loss among the retatrutide groups at 36 weeks that ranged from 3.2-16.9% compared with weight loss of 3.0% in the placebo group, and 2.0% in the dulaglutide group [4]. Contrary to the decrease in HbA1c values that attained a plateau after 24 weeks, weight reduction with retatrutide continued to progress to the end of follow-up at 36 weeks [4]. Moreover, approximately 60% of patients randomized to high dose retatrutide (8-12mg) lost ≥ 15% of weight compared with 2% with placebo [4]. It should be emphasized that exceeding the cutoff weight loss of 15% is clinically important. Indeed, sustained weight reduction of 15% or more in patients with type 2 diabetes may induce remission in a large proportion of subjects and improve metabolic status in the remaining patients [5]. Meanwhile, the magnitude of weight loss with use of retatrutide was less pronounced in patients with type 2 diabetes at 36 weeks compared with obese subjects without diabetes at 48 weeks in the obesity trial (Table 1) [3]. This observation may be due to inclusion of younger patients with more severe degree of obesity at baseline, and longer follow-up in the obesity trial compared with the diabetes trial (Table 1). In addition, for other unclear reasons, obesity drugs were generally less effective in patients with type 2 diabetes than in in people without diabetes [5].

- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Effects of Retatrutide on Cardiovascular Risk Factors
Amelioration of several cardiovascular risk factors, mainly due to weight loss, was observed with retatrutide treatment [3,4]. In the obesity study, 72% of the participants who had prediabetes at baseline in the retatrutide groups reverted to normoglycemia (HbA1C < 5.7%) as compared with 22% of individuals in the placebo group [3]. In addition, the decrease in systolic blood pressure/diastolic blood pressure (SBP/DBP) was 8.8/2.8mmHg versus 2.9/1.0 in placebo at 36 weeks [3]. In the diabetes study, SBP decreased by up to 8.8mmHg with retatrutide versus a reduction of 1.5mmHg with dulaglutide, and an increase of 1.5mmHg with placebo [4]. In the obesity study, retatrutide decreased fasting levels of plasma triglycerides by up to 40%, and low-densitylipoprotein cholesterol (LDL-C) by up to 22% at 48 weeks [3]. In the diabetes trial, retatrutide decreased plasma triglyceride by up to 35% at 36 weeks compared with 4% reduction with dulaglutide and 9% with placebo [4]. Changes in circulating levels of LDL-C, high-density lipoprotein cholesterol and free fatty acids were not statistically different between various patient groups in the diabetes study [4].
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Safety of Retatrutide
In the 2 phase 2 trials, 16-17% discontinued retatrutide compared with 0-4% with placebo due to adverse effects (Table 1) [3,4]. In general, safety profile of retatrutide mimics that of incretin-based drugs with GI adverse effects being the most common in incidence and the most frequent cause of drug discontinuation. GI adverse effects (nausea, diarrhea, vomiting and constipation) were dose-related and occurred mainly during the early dose escalation period [3,4]. In the obesity trial, proportions of subjects reporting nausea were 14-60% with retatrutide versus 11% with placebo [3]. In the diabetes study, GI adverse effects were reported by 13-50% in the retatrutide group, 35% in the dulaglutide group, and 13% in the placebo group [4]. This relatively high rate of GI adverse effect may be attributed to the glucagon receptor agonism component of retatrutide. In fact, glucagon has been shown to slow gastric emptying and inhibit GI motility [1].
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Effect of Retatrutide on Heart Rate
Increase in heart rate, of approximately 2-6 beats per minute (bpm) compared with placebo, was observed with use of all GLP-1 receptor agonists and with the dual GLP-1/GIP receptor agonist tirzepatide [6-11]. However, this adverse effect did not result in an increase in cardiovascular events in dedicated randomized trials [7,8]. With retatrutide, the increase in heart rate peaked 24 weeks after starting treatment and partially declined thereafter [3,4]. Placebo-adjusted increase in heart rate by retatrutide was 5.6bpm in the obesity trial at 48 weeks and 7.5bpm in the diabetes trial after 36 weeks [3]. In the latter study, the corresponding increase was 5bpm with dulaglutide. Furthermore, cardiac arrhythmias occurred in 4-14% and 2-3% in the retatrutide groups and placebo group, respectively in the 2 trials [3,4]. The increase in heart rate by teratrutide could be mediated in part by glucagon receptor agonism. Indeed, most, but not all, studies have shown that glucagon administration increases heart rate acutely in humans [12].
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Advantages of Retatrutide
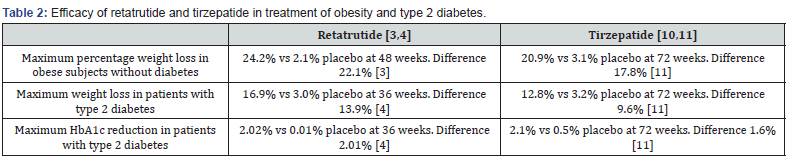
The major advantage of teratrutide is its high efficacy for weight loss that exceeds efficacy of all available anti-obesity drugs. The dual GLP-1 and GIP agonist tirzepatide is currently considered the most effective drug to lower HbA1c levels and promote weight loss [10,11]. While no direct comparison between retatrutide and tirzepatide is available and duration of use is different (36- 48 weeks with retatrutide versus 72 weeks with tirzepatide), retatrutide may be superior to tirzepatide in both parameters, particularly weight loss (Table 2) [3,4,10,11]. Furthermore, the difference in efficacy of weight reduction between the 2 agents may become even greater with more prolonged use of teratrutide beyond 48 weeks. This enhanced efficacy of retatrutide in promoting weight loss is most likely mediated through glucagon receptor agonism. In fact, Goscun et al. [1] have shown that retatrutide caused weight loss in obese mice by increasing energy expenditure through glucagon receptor engagement. Moreover, a phase 1 study has shown that retatrutide may decrease appetite and increase sensation of fullness and satiety in patients with type 2 diabetes [2]. Decreased appetite was reported by 11-31% among retatrutide-treated subjects (vs 9% placebo) in the obesity trial and by 10% (vs 2% placebo) in the diabetes trial [3,4].
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Limitations of Retatrutide
The main limitation of retatrutide is the high frequency rates of discontinuation due to adverse effects reaching 17% with the highest doses. These rates are much higher than the 7% discontinuation rate reported with the highest dose of tirzepatide (15mg/week) versus 4% with placebo [10]. Since most GI adverse effects occurred during retatrutide dose escalation, the use of smaller starting doses and slower titration might decrease the incidence of GI adverse effects. In addition, the increase in pulse rate and arrhythmias is concerning and should be inspected thoroughly in pending trials, preferably using Holter monitoring of heart rate.
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
Conclusions and Current Needs
No doubt, retatrutide is a promising novel tri-agonist of receptors of the 2 incretins GLP-1 and GIP and glucagon. This drug showed high efficacy in causing weight loss not previously recorded with any anti-obesity agent. Additionally, its efficacy for treatment of type 2 diabetes is at least comparable to tirzepatide (Table 2). Meanwhile, tolerance to retratrutide may be suboptimal with unacceptable high discontinuation rates due to adverse effects. Moreover, there are signals of increased incidence of cardiac arrhythmias associated with its use. Ongoing large-scale phase 3 trials should clarify efficacy and safety of retratrutide in subjects with obesity and type 2 diabetes. In addition, adequately powered and long-term (at least 3 years) studies designed to see the effects of retatrutide on cardiovascular events and mortality should be conducted.
- Review Article
- Abstract
- Introduction
- Retatrutide for Treatment of Obesity
- Retatrutide for Treatment of Type 2 Diabetes
- Effects of Retatrutide on Cardiovascular Risk Factors
- Safety of Retatrutide
- Effect of Retatrutide on Heart Rate
- Advantages of Retatrutide
- Limitations of Retatrutide
- Conclusions and Current Needs
- References
References
- Coskun T, Urva S, Roell WC, Qu H, Loghin C, et al. (2022) LY3437943, a novel triple glucagon GIP and GLP-1 receptor agonist for glycemic control and weight loss From discovery to clinical proof of concept. Cell Metab 34(9): 1234-1247.
- Urva S, Coskun T, Loh MT, Du Y, Thomas MK, et al. (2022) LY3437943 a novel triple GIP GLP-1, and glucagon receptor agonist in people with type 2 diabetes a phase 1b multicentre double-blind placebo-controlled randomised multiple-ascending dose trial. Lancet 400(10366): 1869-1881.
- Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, et al. (2023) Retatrutide Phase 2 Obesity Trial Investigators. Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial. N Engl J Med 387(3): 205-216.
- Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, et al. (2023) Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 140-6736(23): 1053-X.
- Hædersdal S, Andersen A, Knop FK, Vilsbøll T (2023) Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases. Nat Rev Endocrinol 19(6): 321-335.
- Lorenz M, Lawson F, Owens D, Raccah D, Roy-Duval C, et al. (2017) Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol 16(1): 6.
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, et al. (2016) Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 375(4): 311-322.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, et al. (2016) Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 375(19): 1834-1844.
- Knop FK, Aroda VR, do Vale RD, Holst-Hansen T, Laursen PN, et al. (2023) Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 140-6736(23): 01185-01186.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, et al. (2022) Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med 387(3): 205-216.
- Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, et al. (2023) Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 140-6736(23): 01200-X.
- Petersen KM, Bøgevig S, Holst JJ, Knop FK, Christensen MB, et al. (2018) Hemodynamic Effects of Glucagon: A Literature Review. J Clin Endocrinol Metab 103(5): 1804-1812.






























