Tirzepatide Reduced Significantly the Body Weight in Obese Patients
László Gerő*
Semmelweis University, Clinic of Internal Medicine and Oncology, Hungary
Submission: October 10, 2022; Published: November 02, 2022
*Corresponding author: László Gerő, Semmelweis University, Clinic of Internal Medicine and Oncology, Budapest, Hungary
How to cite this article: László G. Tirzepatide Reduced Significantly the Body Weight in Obese Patients. Curre Res Diabetes & Obes J 2022; 16(1): 555930.DOI: 10.19080/CRDOJ.2022.16.555930
Abstract
Background: Tirzepatide, a new combined GIP+GLP-1 receptor agonist underwent an extensive trial among a group of non-diabetic obese patients.
Methods: 5, 10 and 15 mg Tirzepatide or placebo were given once a week, as a subcutaneous injection to 2539 patients for a period of 72 weeks.
Results: Tirzepatide significantly reduced the body weight and other obesity parameters, as compared to the placebo.
Conclusion: Tirzepatide, the dual GIP+GLP-1 RA combination surpassed the placebo, and any other weight-reducing drugs, and its effect can be compared only to bariatric surgery.
Keywords: Tirzepatide; Obesity; Non-diabetic patients; Bariatric surgery; Non-diabetic obese patients
Introduction
Obesity has become a world-wide pandemic during the last 2-3 decades. As of today, the number of obese patients reached 700 million worldwide and obesity results in numerous complications including cardiovascular illnesses, type 2 diabetes, hypertension, joint problems, etc.
Tirzepatide, a new combined chimeric peptide was capable of reducing significantly the body weight, much more, than semaglutide, or any other previous GLP-1 RA-s or other drugs or slimming cures. The degree of weight-loss of obese patients in the SURMOUNT-1 trial could only be compared to the results of using bariatric surgery. Indeed, the loss of more than 20% of body weight during a 72-week long trial surpasses the results of all other earlier drugs and investigations.
The author demonstrates the results of the SURMOUNT-1 study.
Patients
The 2539 patients were divided into four groups, receiving 5, 10 and 15 mg Tirzepatide or placebo as subcutaneous injection once in a week, respectively. Patients were also educated for appropriate diet by a dietitian and exercised for at least 150 minutes per week.
Results
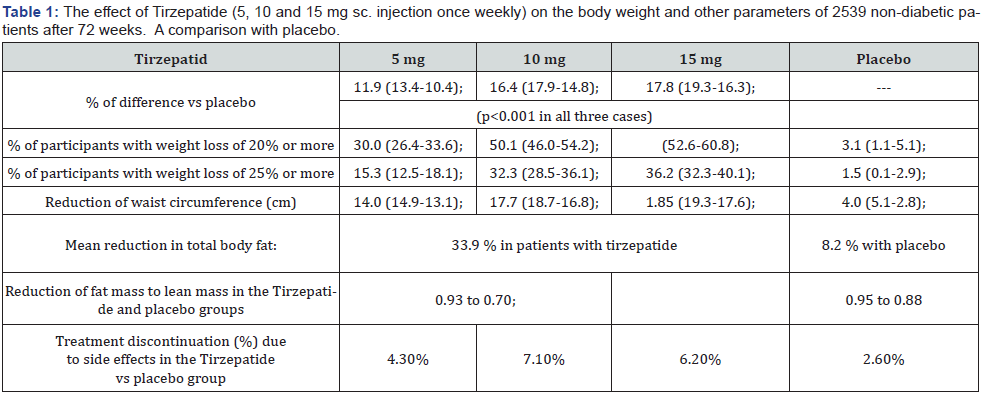
The results of the 72-week long trial are shown in Table 1. The mean body weight at the beginning of the trial was 104.4 kg. The percentage weight loss of patients treated with 5-10-15 mg Tirzepatide, respectively, were 15 %, 19.5 % and 20.9 %, while 3.1 % in the placebo group. The deviation to the placebo group were 11 % (13.4 % -10.4 %), 16.4 % (17.9 % -14.8 %) and 17.8 % (19.3-% -16.3 %) at p<0,001 for patients given 5-10-15 mg Tirzepatide, respectively.
At the end of 72 weeks, the percentage of patients losing 20% body weight, or more were significantly higher in the Tirzepatide group (p<0,001). Patients with reduction of body weight of 25 % or more were approximately 85 % in the Tirzepatide group, and 1.5 % in the placebo-treated group.
The percentage change in body-fat-mass was assessed in a subgroup of 255 patients who underwent a dual-energy x-ray absorptiometry. The body fat-loss was 33.9 % in the Tirzepatidetreated group, as compared to the 8.2 % in the placebo group.
The ratio of total fat mass to total lean mass decreased more with Tirzepatide therapy: 0.93 to 0.70, versus 0.95 to 0.88 with the placebo treatment. Discontinuation of the study due to side effects were 4.3 %, 7.1 % and 6.2 % in the Tirzepatide and 2.6 % in the placebo-treated groups, respectively. Reduction of waist circumference was also significantly higher in the Tirzepatide groups [1].
Discussion
The substantial degree of weight loss with Tirzepatide is higher than one could expect from a usual GLP-1 RA and can be compared only to the weight-loss achieved by bariatric surgery. The study shows that such a weight reduction can be achieved with a weekly-given subcutaneous drug, without the change of normal body anatomy as it is in bariatric.
The precise explanation for this „enormous” weight loss is not easy to determine. The process of the combination of GIP with GLP-1 RA is an exceptionally lucky and well-performed peptide unification.
As it is known, the effects of GLP-1 RA-s can be antagonized or at least modified with different peptides, and the original effect can be streghtened or ameliorated or even reversed [2]. In such cases, the influence of gastrointestinal hormones will change. For example, GIP was known as a weight gaining hormone, increasing appetite and body weight [3]. This effect has substantially changed with the development of Tirzepatide, which hormone, being a combination of GIP and GLP-1 RA, had strong weightlosing effects.
The individual contribution to postprandial insulin secretion is 26 % by glucose, 45 % by GIP and 29 % by GLP-1. According to calculations, the slimming effect is approximately the same as above. Thus, the main weight-reducing factor in the combination should be GIP, as it has a 4-5-times stronger binding to the receptors, than GLP-1. The precise mechanism of the combined drug is still under investigation [4,5]. Differential internalization of GIP and GLP-1 receptors might be an explanation.
The use of GLP-1 is accompanied with side effects like nausea, vomiting, and loss of appetite. Such side effects are lacking with the use of GIP. The new combination will be available in Europe in the first part of 2023. This will allow us to test Tirzepatide on our own obese patients and determine, whether the results and tolerability of the drug is in line with the results found in previous studies.
References
- Jastreboff AM, Aronne LJ, Ahmad NN, Sean Wharton, Lisa Connery, et al. (2022) Tirzepatide once weekly for the treatment of obesity. N Engl J Med 387(3): 205-216.
- Holst JJ, Rosenkilde MM (2020) GIP as a therapeutic target in diabetes and obesity: insight from incretin co-agonists. J Clin Endocrinol Metab 105(8): 2710-2716.
- Marks W (1988) GIP: the obesity hormone. In: James WPT, Parker SW, (Eds.), Current Approaches: Obesity. Southampton, England. Duphar Medical Relations, p. 13-19.
- Willard FS, Douros DJ, Gabe MBN, Showalter AD, Wainscott DB, et al. (2020) Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. J Clin Invest 5(17): e140532.
- Holst JJ, Gasbjerg LS, Rosenkilde MM (2021) The role of incretins on insulin function and glucose homeostasis. Endocrinology 162(7): 1-10.






























