Diabetic Foot Ulcer: An Overview, Risk Factors, Pathophysiology, And Treatment
Gudisa Bereda*
Department of Pharmacy, Negelle Health Science College, Ethiopia
Submission: May 13, 2022; Published: May 23, 2022
*Corresponding author: Gudisa Bereda, Department of Pharmacy, Negelle Health Science College, Guji, Ethiopia
How to cite this article: Gudisa B. Diabetic Foot Ulcer: An Overview, Risk Factors, Pathophysiology, And Treatment. Curre Res Diabetes & Obes J 2022; 15(4): 555920.DOI: 10.19080/CRDOJ.2022.15.555920
Abstract
Diabetic foot ulcer is one of the major health challenges that can decrease the quality of life, lengthen hospitalization and entails more cost to the patient. Diabetic foot disease outcome fifteen percent of the diabetic patients and person with diabetes are fifteen times more probably to undergo lower extremity amputation than their non-diabetic counterpart. Risk factors for ulceration are specific or systemic contributions such as uncontrolled hyperglycemia, duration of diabetes, peripheral vascular disease, blindness or visual loss, chronic renal disease, advanced age and local issues such as peripheral neuropathy, structural foot deformity, trauma and incorrectly suited shoes, callus, history of prior ulcer amputation, delayed elevated pressures, limited joint mobility. The initial goals of treatment for diabetic foot ulcer are to acquire wound closure as expeditiously as possible. Debridement includes remove of dead, injured, or exposed tissue, which improves the healing potential of the remaining healthy tissues. The principle of antibiotic management is depending on evidence provided by reports on bacteriological culture and sensitivity from distinctive centers worldwide. Use of anti-infective/antibiotics must be guided by proper cultures.
Keywords: Diabetic foot ulcer; Overview, Pathogenesis; Risk factors; Treatment
Introduction
Diabetes mellitus is a severe, chronic metabolic disorders that described by elevated sugar level either when the pancreas does not secrete sufficient insulin, or when the body cannot effectively use insulin [1]. Infection is an often complication of diabetic foot ulcers (DFUs), with up to fifty eight percent of ulcers being exposed at primary presentation at a diabetic foot clinic, elevating to eight two percent in patients hospitalized for a DFU. These diabetic foot infections are correlated with poor clinical consequences for the patient and most expenses for both the patient and the health care system. Patients with DFIs have a fifty times accelerated risk of hospitalization and one hundred fifty times elevated risk of lower extremity amputation compared with patients with diabetes and no foot infection. Among patients with DFIs, five percent will undergo a major amputation and twenty thirty percent a minor amputation, with the availability of peripheral arterial disease (PAD) highly elevating amputation risk [2-5]. DFU notifies to a cover in the continuity of the skin epithelium enclosing its full thickness or beyond, distal to the ankle joints, in a people living with DM [6,7]. DFU is one of the major health challenges that can rupture the QOL, lengthen hospitalization and entails more cost to the patient. Diabetic foot disease outcome fifteen percent of the diabetic patients and person with diabetes are fifteen times further probably to undergo lower extremity amputation than their non-diabetic counterpart [8-10]. The challenge and features of diabetic foot are infection, ulceration, or gangrene. Neuropathy, poor circulation, and vulnerability to infection are the three major contributors to the development of diabetic foot; which when available, foot abnormalities or mild trauma can readily influence to ulceration and infection [11, 12].
Ulceration is the most common precursor of amputation and has been distinguished as a component in greater than 2/3 of lower-limb amputations. The availability or absence of infection and/or ischemia, footwear and pressure relief, and overall glycemic control lead the healing of ulcers. The depth of an ulcer is other significant factor that influences the consequence of DFUs [13]. Wounds on the feet, known as DFUs, are a major complication of diabetes. DFUs can become exposed, influencing to amputation of the foot or lower limb [14]. Limb amputation has a major impact on the individual, not solely in distorting body image, but also with respective to loss of productivity, elevating dependency, and costs of managing foot ulcers if patients necessitates inpatient care [15,16]. Lower limb amputation in diabetic patients is correlated with important excess mortality. Foot ulceration is also believed to be correlated with enhanced deaths due to related cardiovascular disease. Furthermore, patients with foot ulceration frequently have developed diabetes complications [17- 19]. 15% of those with diabetes will advance at least one DFU during their lifetime [20]. Foot challenges responsible for up to fifteen percent of healthcare resources in developed countries and 40% in developing countries. Among Ethiopian diabetic patients foot ulcer is a major health challenge. Foot ulcer correlated with sepsis sequences in twelve percent of death. Low follow-up and poor glycemic control are preponderance contributing factors. Understanding of the influential factors of foot ulcer in diabetics will enable great risk patients to be recognized early [21-25].
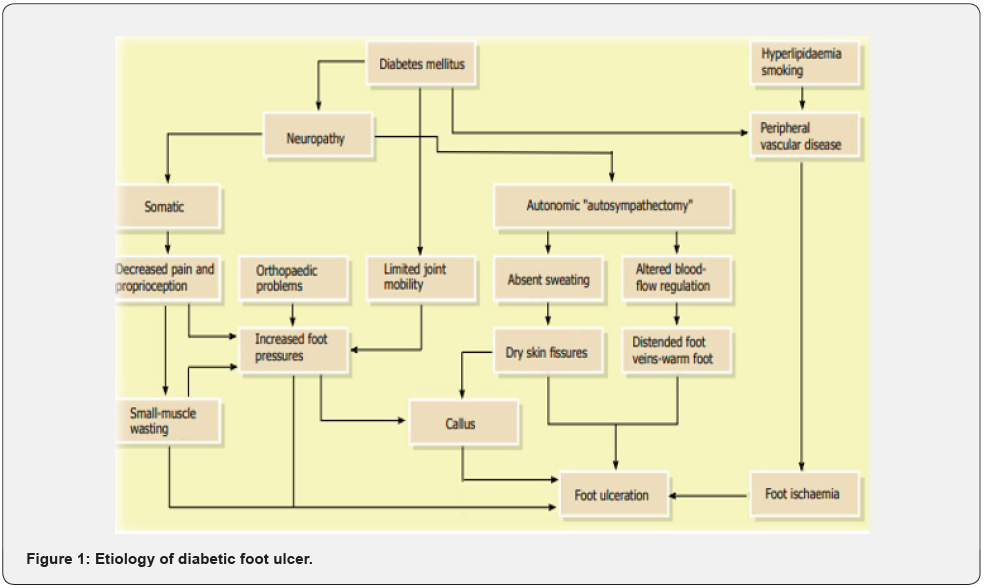
The main risk factors for DFUs involve sensory neuropathy, lower limb ischemia, and trauma [26]. Risk factors for ulceration are specific or systemic contributions such as uncontrolled hyperglycemia, duration of diabetes, peripheral vascular disease, blindness or visual loss, chronic renal disease, advanced age and local issues such as peripheral neuropathy, structural foot deformity, trauma and incorrectly suited shoes, callus, history of prior ulcer amputation, delayed elevated pressures, limited joint mobility [20,21,24]. The grades of the University of Texas (UT) system are as follows: grade zero (pre- or post-ulcerative site that has cured), grade one (superficial wound not involving tendon, capsule, or bone), grade two (wound penetrating to tendon or capsule), and grade three (wound penetrating bone or joint). Within each wound grade there are 4 stages: clean wounds (stage A), non-ischemic infected wounds (stage B), ischemic noninfected wounds (stage C), and ischemic infected wounds (stage D) [15]. DFUs can be medically categorized in several ways but all of them define the ulcer in terms of its depth and the availability of osteomyelitis or gangrene. As an example, the categorization according to Wagner’s system is depending on the following grades: grade zero (no ulcer in a foot with a high-pitfall factor of complication); grade one (partial/full thickness ulcer); grade two (deep ulcer, penetrating down to ligaments and muscle, but no bone involvement); grade three (deep ulcer with cellulitis or abscess formation); grade four (localized gangrene); and grade five (extensive whole foot gangrene). The classification of diabetic foot ulcers is significant as it perhaps facilitate the choice of suitable dressing based on the wound type and on its phase [27- 30] (Figure 1).
Pathophysiology
The most common contributing factors in creating DFU are neuropathy, peripheral artery disease (PAD), abnormality and minor trauma. Additionally contributing factors are necrosis, gangrene, infection, PAD, advancing age of the patient and other co-morbidities such as end ESRD, and heart failure. The DFU patients are ordinarily older males with a history of delayed DM combined with poor health situation. They ordinarily based on assistance of other to work their daily activities. The average age of these patients is sixty-five years and they are ordinarily presented with the disease for at least ten years. The majority of them has a history of uncontrolled diabetes additionally elevated degree of HbA1c, and in 1/3 of the cases other co morbidities are available. Neuropathy sequences in insensitivity and occasionally causes abnormalities in the foot. In these patients, even a minor trauma perhaps influences to a chronic ulcer. Additionally, the persistent walking on the influenced foot, which is insensitive to pressure sense, changes the healing procedure. In the availability of peripheral vascular disease (PVD), the wound becomes ischemic and a non-healing ulcer advance. In patients with neuro ischemic ulcer, unfortunately, the classic signs of infection such as pain, warmth and tenderness are masked. Decrease in pain and tenderness is owing to neuropathy and the warmth and redness declines indispensably because of ischemia. These alters perhaps confuse the physician and sequences in misdiagnosing for wound infection [31-33].
Diagnostic criteria
In recent clinical practices, the evaluation of DFU comprises of several important works in early diagnosis, keeping track of advancement and number of lengthy actions received in the management and treatment of DFU for each case: 1) the medical history of the patient is evaluated; 2) a wound or diabetic foot specialist examines the DFU thoroughly; 3) additional tests like CT scans, MRI, X-Ray perhaps helpful to support advance a management plan. The patients with DFU specifically have a challenge of a swollen leg, although it can be itchy and painful based on each case. Ordinarily, the DFUs have aberrant structures and unusual outer boundaries. The visual appearance of DFU and its enveloping skin based upon the several stages i.e. redness, callus formation, blisters, and significant tissues types like granulation, slough, and bleeding, scaly skin. Thereby, the ulcer evaluation with the support of computer vision algorithms would be depending on the exact assessment of these visual signs as color descriptors and texture features [34].
Treatment
The objectives of management are: (1) to create and maintain a plantigrade, stable foot; (2) to heal an ulcerated wound; (3) to heal fractures; (4) to suppress abnormalities. The initial goals of treatment for DFU are to acquire wound closure as expeditiously as possible [35]. Not all diabetic foots are preventable, but proper preventive measures can dramatically minimize their occurrences. Managing a DFI necessitates correct wound care and proper antibiotic therapy. Multiple factors involving assessment of the wound, its categorization, and the require for debridement involving sharp surgical, mechanical, chemical etc., have to be received onto consideration before proceeding with the correct selection of topical regimen [36]. The preventive measures and treatment of diabetic complications consists of the following: Lifestyle modification; BP control (the best indicator of glucose control over a period of time is HbA1C level. This test measures the average BS concentration over a ninety-d span of the average RBC in peripheral circulation. The higher the HbA1C level, the greater glycosylation of Hg in RBCs will happen. Surveys have reveal that BG levels > 11.1 mmol/L (equivalent to > 310mg/mL or an HbA1C level of > 12) is correlated with decreased neutrophil work, involving leukocyte chemotaxis [37]); lipid management; glycemic control; smoking cessation, BP control (ACE inhibitors or angiotensin receptor blockers were recommended for all patients with HTN, previous cardiovascular disease, and/or microalbuminuria, unless there was known renovascular disease. Beta-blockers were recommended for all patients with existing cardiovascular disease or in whom BP was still uncontrolled despite ACE inhibition.
Education
Patients’ training plays an indispensable function in suppression of DFU. The goal of training is to motivate the patient and create adequate skills in order to maximize the use of preventive methods. It is also crucial to make sure that the patient has understood all the instructions. Recently, a broad range and combinations of patient educational interventions have been evaluated for the prevention of DFU that different from brief education to intensive education involving observation and hands on teaching. Patients with DFU should be educated about risk factors and the necessity of foot care, involving require for self-inspection, monitoring foot temperature, proper daily foot hygiene, usage of correct footwear, and blood sugar control. Debridement: Debridement includes remove of dead, injured, or exposed tissue, which improves the healing potential of the remaining healthy tissues. Based on the wound tissue type, other debridement techniques are recommended: (1) Surgical debridement or sharp debridement-recommended for necrotic and exposed wounds. The terms surgical debridement and sharp debridement are frequently used intercalate, certain clinicians refer to surgical debridement as being settled in an operating room, whereas sharp debridement is settled in a clinic setting. Sharp surgical debridement is the most effective and quickest method of debridement; (2) Autolytic debridement-a selective procedure in which the necrotic tissue is liquefied. A wound filled in with an occlusive dressing permits concentration of tissue fluids containing macrophages, neutrophils, and enzymes, which remove bacteria and digest necrotic tissues. This is reached by a moist wound curing environment. Autolytic debridement is not advisable for the treatment of exposed pressure ulcers; (3) Mechanical debridement-includes remove of unhealthy tissue using a dressing, which is altered regularly by wound irrigation (pressure: 4-15psi), without injuring healthy/new tissues. Scrubbing the wound aids in remove of exudates and devitalized tissues, however this influences to bleeding as well as pain resulting from wound trauma. This technique is used in the treatment of surgical wounds and venous leg ulcers. The shortcomings of the method are that it is time consuming and high cost; (4) enzymatic debridement-a method of debriding devitalized tissue by topical enzymes such as collagenase, fibrinolysin, or papain. Recommended for sloughy, exposed, necrotic wounds where surgical debridement is contraindicated; and (5) Maggot debridement-a procedure in which maggots or fly larva that are accelerated in a sterile environment are used. The most frequently used fly is Lucilia sericata, which is used for human wound management when conventional managements fail. Maggots are placed on the wound pursued by wrapping with 2ndry dressing. The larvae feed on the necrotic (dead) tissue and bacteria available at the wound site and produce antimicrobial enzymes, which support in the wound healing procedure [38-40].
Wound dressings for diabetic foot ulcer treatment
Natural skin is thought-out the perfect wound dressing and thereupon an ideal wound dressing should try to duplicate its properties. Historically, wound dressings were primary thoughtout to play only a passive and protective function in the healing procedure. Although in current decades wound management has been revolutionized by the innovator that moist dressings can assist wounds heal quicker. In addition, a moist wound environment is also an indispensable factor to initiate the proliferation and migration of fibroblasts and keratinocytes as well as to accelerate collagen generation, influencing to decreased scar formation [41-43].
Antibiotic selection
The principle of antibiotic management is depending on evidence provided by reports on bacteriological culture and sensitivity from other centers worldwide. Usage of anti-infective/ antibiotics must be guided by proper cultures. Improper usage of antibiotics could influence to resistance and adverse drug reactions. Oral and parenteral antibiotics are prescribed for mild soft tissue infections and moderate to severe infections, respectively [44].
Antibacterial agents
Used only or in combination for each class except dry necrotic wounds. Topical antibiotics have wide spectrum antibacterial coverage which lasts for twelve hour and are less toxic. Metronidazole gel has excellent anaerobic coverage and supports in maintaining a moist wound healing environment. By weight, gels are highly liquid, yet they behave like solids owing to a 3-dimensional cross-linked network within the liquid. It is the crosslinking within the fluid that bestows a gel its structure (hardness) and contributes to its adhesion [45]. Off-loading: Total contact casts and therapeutic shoes are appropriate alternatives for remove of pressure from the wound. The further effective offloading technique for the treatment of neuropathic DFU is total contact casts (TCC). TCC is minimally padded and molded carefully to the shape of the foot with a heel for walking.
Surgery
Diabetic foot surgery plays a crucial function in the suppression and treatment of DFU and has been on elevate over the past two decades. Although surgical interventions for patients with DFU are not without peril, the selective correction of persistent foot ulcers can improve consequences. Vascular foot surgery such as bypass grafts from femoral to pedal arteries and peripheral angioplasty to ameliorate blood flow for an ischemic foot have been currently advanced [46-48].
Advanced dressing
A major breakthrough for DFU treatment over the last decades was the observation of novel dressings. Ideally, dressings should confer moisture balance, protease sequestration, growth factors enliven, antimicrobial activity, O2 permeability, and the capacity to accelerate autolytic debridement that facilitates the secretion of granulation tissues and the re-epithelialization procedure. Furthermore, it should have a delayed time of action, high efficiency, and improved sustained drug release in the case of medicated therapies [49].
Conclusion
Diabetic foot disease outcome fifteen percent of the diabetic patients and person with diabetes are fifteen times further probably to undergo lower extremity amputation than their non-diabetic counterpart. Risk factors for ulceration are specific or systemic contributions such as uncontrolled hyperglycemia, duration of diabetes, peripheral vascular disease, blindness or visual loss, chronic renal disease, advanced age and local issues such as peripheral neuropathy, structural foot deformity, trauma and incorrectly suited shoes, callus, history of prior ulcer amputation, delayed elevated pressures, limited joint mobility. The preventive measures and treatment of diabetic complications consists of the following: Lifestyle modification; BP control (the best indicator of glucose control over a period of time is HbA1C level. This test measures the average BS concentration over a ninety-d span of the average RBC in peripheral circulation. Historically, wound dressings were primarily considered to play solely a passive and protective function in the healing procedure.
Acknowledgments
The author would be grateful to anonymous reviewers by the comments that increase the quality of this manuscript.
Data Sources
Sources searched include Google Scholar, Research Gate, PubMed, NCBI, NDSS, PMID, PMCID, Lancet, Scopus database, Scielo and Cochrane database. Search terms included: an overview, risk factors, pathophysiology and treatment of diabetic foot ulcer.
Funding
None
Availability of Data and Materials
The datasets generated during the current study are available with correspondent author.
Competing Interests
The author has no financial or proprietary interest in any of material discussed in this article.
References
- Bereda G, Bereda G (2021) The Incidence and Predictors of Poor Glycemic Control among Adults with Type 2 Diabetes Mellitus in Ambulatory Clinic of Mettu Karl Referral Hospital, Southwestern, Ethiopia: A Prospective Cross-Sectional Study. Int Arch Endocrinol Clin Res 7: 24.
- Kristy Pickwell, Volkert Siersma, Marleen Kars, Jan Apelqvist, Karel Bakker (2015) Predictors of Lower-Extremity Amputation in Patients with an Infected Diabetic Foot Ulcer. Diabetes Care 38(5): 852-857.
- L Prompers, M Huijberts, J Apelqvist, E Jude, A Piaggesi, et al. (2007) High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 50(1): 18-25.
- Peters EJG, Lipsky BA (2013) Diagnosis and management of infection in the diabetic foot. Med Clin North Am 97(5): 911-946.
- Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, et al. (2006) Risk factors for foot infections in individuals with diabetes. Diabetes Care 29(6): 1288-1293.
- Ugwu E, Olufunmilayo Adeleye, Ibrahim Gezawa, Innocent Okpe, Marcelina Enamino, et al. (2019) Burden of diabetic foot ulcer in Nigeria: Current evidence from the multicenter evaluation of diabetic foot ulcer in Nigeria. 10(3): 200-211.
- Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ (2016) International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev 32(Suppl 1): 2-6.
- Wasim Ahmad, Ishtiaq Ali Khan, Salma Ghaffar, Farhan Khasham Al-Swailmi, Ihsanullah Khan, et al. (2013) Risk factors for diabetic foot ulcer. J Ayub Med Coll Abbottabad 25(1-2): 16-18.
- Khalid Mehmood, S Tehseen Akhtar, Abu Talib, Abu Talib, Badar Abbasi (2008) Clinical profile and management outcome of diabetic foot ulcer in a tertiary care hospital. J Coll Physicians Surg Pak 18(7): 408-412.
- Dorresteijn JAN, Kriegsman DMW, Assendelft WJJ, Valk GD (2010) Patient education for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews (5): CD001488.
- Larijani B, Hasani Ranjbar S (2008) Overview of diabetic foot; novel treatments in diabetic foot ulcer. DARU 16(Suppl 1): 1-6.
- Larijani B, Forouzandeh F (2003) Diabetic foot disorders. Iran J Diabetes and Lipid Disord 2(2): 103-193.
- S O Oyibo, E B Jude, I Tarawneh, H C Nguyen, L B Harkless, et al. (2001) A Comparison of Two Diabetic Foot Ulcer Classification Systems. Diabetes care 24(1): 84-88.
- Bill Cassidy, Neil D Reeves, Joseph M Pappachan, David Gillespie, Claire O’Shea (2021) The DFUC 2020 Dataset: Analysis Towards Diabetic Foot Ulcer Detection. Touch Reviews in Endocrinology 17(1): 5-11.
- Shahi SK (2012) Prevalence of Diabetic Foot Ulcer and Associated Risk Factors in Diabetic Patients from North India. The Journal of Diabetic Foot Complications 4(3): 83-91.
- Shobhana R, Rao PR, Lavanya A, Vijay V, Ramachandran A (2000) Cost burden to diabetic patients with foot complications: a study from Southern India. J Assoc Physicians India 48: 1147-1150.
- Young MJ, Joanne E McCardle, Luann E Randall, Janet I Barclay, et al. (2008) Improved Survival of Diabetic Foot Ulcer Patients 1995-2008. Diabetes Care 31(11): 2143-2147.
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, et al. (2007) High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe: baseline results from the Eurodiale study. Diabetologia 50(1): 18-25,
- Ghanassia E, Villon L, Thuan dit Dieudonne JF, Boegner C, Avignon A (2008) Long-term outcome and disability of diabetic patients hospitalised for diabetic foot ulcers: a 6.5 year follow-up study. Diabetes Care 31(7): 1288-1292.
- Sue E Gardner, Stephen L Hillis, Kris Heilmann, Julia A Segre, Elizabeth A Grice, et al. (2013) The Neuropathic Diabetic Foot Ulcer Microbiome Is Associated with Clinical Factors. Diabetes 62(3): 923-930.
- Clayton W, Elasy TA (2009) A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes 22: 52-58.
- Agale SV (2013) Chronic Leg Ulcers: Epidemiology, Aetiopathogenesis, and Management. Ulcers.
- Deribe B, Woldemichael K, Nemera G (2014) Prevalence and Factors Influencing Diabetic Foot Ulcer among Diabetic Patients Attending Arbaminch Hospital, South Ethiopia. J Diabetes Metab 2: 322.
- International Diabetes Federation (2012) The Global Burden. (5th), IDF Diabetes Atlas.
- Amogne W, Reja A, Amare A (2011) Diabetic foot disease in Ethiopian patients: A hospital-based study. Ethiopia J Health Dev 25(1): 17-21.
- Bakri FG (2012) Prevalence of Diabetic Foot Ulcer and its Associated Risk Factors among Diabetic Patients in Jordan. J Med J 46(2): 118-125.
- Cavanagh PR, Lipsky BA, Bradbury AW, Botek G (2005) Treatment for diabetic foot ulcers. Lancet 366(9498): 1725-1735.
- Leung PC (2007) Diabetic foot ulcers—a comprehensive review. Surgeon 5(4): 219-231.
- Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, et al. (2001) A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 24(1): 84-88.
- O´ Donnell TF, Lau J (2006) A systematic review of randomized controlled trials of wound dressings for chronic venous ulcer. Journal of Vascular Surgery 44(5): 1118-1125.
- Iraj B, Khorvash F, Ebneshahidi A, Askari G (2013) Prevention of diabetic foot ulcer. Int J Prev Med 4(3): 373-376.
- Apelqvist J, Bakker K, van Houtum WH, Schaper NC (2008) International working group on the diabetic foot (IWGDF) editorial board. Practical guidelines on the management and prevention of the diabetic foot: Based upon the international consensus on the diabetic foot (2007) prepared by the international working group on the diabetic foot. Diabetes Metab Res Rev 24(Suppl 1): S181-187.
- Prompers L, Huijberts M, ApelqvistJ, Jude E, PiaggesiA, et al. (2007) High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 50(1): 18-25.
- Goyal M (2017) DFUNet: Convolutional Neural Networks for Diabetic Foot Ulcer Classification. 4(5).
- Markowitz JS, Gutterman EM, Magee G, Margolis DJ (2006) Risk of amputation in patients with diabetic foot ulcers: a claimsbased study. Wound Repair Regen 14: 11-17.
- Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, et al. (2004) Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 39(7): 885-910.
- Laura Jean van Veen (2008) Maggot debridement therapy: a case study. J Wound Ostomy Continence Nurs 35(4): 432-436.
- Driver VR (2004) Treating the macro and micro wound environment of the diabetic patient: managing the whole patient, not the hole in the patient. Foot and Ankle Q Sem J 16: 47-56.
- Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ (2003) Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diabetes Care 26(4): 1069-1073.
- Boulton AJ (2004) Pressure and the diabetic foot: clinical science and offloading techniques. Am J Surg 187: 17S-24S.
- ] Mulder M (2011) The selection of wound care products for wound bed preparation. Prof Nurs Today 30: 15-21.
- Harding KG, Jones V, Price P (2000) Topical treatment: which dressing to choose. Diabetes Metab Res Rev 16(Suppl. 1): S47-50.
- Morton LM, Phillips TJ (2012) Wound healing update. Semin Cutan Med Surg 31: 33-37.
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, et al. (2012) 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 54: e132-e173.
- https://en.wikipedia.org/wiki/Gel.
- Capobianco CM, Stapleton JJ, Zgonis T (2010) Soft tissue reconstruction pyramid in the diabetic foot. Foot Ankle Spec 3: 241-248.
- K Game FL, Hartemann-Heurtier A, Löndahl M, Price PE, van Houtum WH, et al. (2008) A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 24(Suppl 1): S119-S144.
- Lepäntalo M, Biancari F, Tukiainen E (2000) Never amputate without consultation of a vascular surgeon. Diabetes Metab Res Rev 16(Suppl 1): S27-S32.
- Yazdanpanah L, Nasiri M, Adarvishi S (2015) Literature review on the management of diabetic foot ulcer. World J Diabetes 6(1): 37-53.






























