Clinical Application of Reduced-Carbohydrate Diets for Type 1 Diabetes Management: A Retrospective Case Series
Jessica Turton1*, Grant Brinkworth2, Helen Parker1, Amy Rush3, Rebecca Johnson3 and Kieron Rooney1
1Faculty of Medicine and Health, University of Sydney, Australia
2CSIRO -Health and Biosecurity, Australia
3Type 1 Diabetes Family Centre, Australia
Submission: April 22, 2022; Published: May 04, 2022
*Corresponding author: Jessica Turton, Faculty of Medicine and Health, University of Sydney, Australia
How to cite this article: Turton J, Brinkworth G, Parker H, Rush A, Johnson R, et al. Clinical Application of Reduced-Carbohydrate Diets for Type 1 Diabetes Management: A Retrospective Case Series. Curre Res Diabetes & Obes J 2022; 15(4): 555919. DOI: 10.19080/CRDOJ.2022.15.555919
Abstract
Background: The aim of this study was to explore the clinical application of reduced-carbohydrate (RC) diets for type 1 diabetes (T1D) management at a community-based diabetes centre.
Methods: To be included in this retrospective case series, adults with T1D must have attended at least two appointments with a Credentialled Diabetes Educator and Accredited Practising Dietitian (CDE/APD) for advice regarding: (a) advanced carbohydrate counting, (b) carbohydrate reduction, and/or (b) low-carbohydrate diet support. Data regarding specific dietary recommendations and clinical outcomes was extracted from patient records stored at the center. A semi-structured interview with the CDE/APD was conducted to collect additional information about the design and delivery of the RC diets. Thematic analysis was used to identify core components of the RC diets, and descriptive statistics were used to assess pre-post changes in clinical T1D outcomes.
Results: 26 adults with T1D were eligible and included (77% female). The RC diets represented a patient-led approach involving adjustments to energy and macronutrient intakes, glucose self-monitoring, and insulin management. 22/26 participants attended the center seeking low-carbohydrate diet support, and the average carbohydrate prescription was 63g/day (22-253g/day) which translated to a 37% reduction from baseline. HbA1c reduced from 9.0% (75mmol/mol) to 7.0% (53mmol/mol) (-5.7 to -0.1%), with an average follow-up of 55weeks (n=8). Estimated A1c reduced from 7.1% (54mmol/mol) to 6.3% (45mmol/mol) (-2.9 to+0.6%) over 21 weeks (n=19). Mean total daily insulin reduced from 44 to 31 U/day (-46 to+6 U/day), with an average follow-up of 17 weeks (n=15).
Conclusions: This study provides real-world insights into the clinical application of RC diets in the management of adults with T1D at a community-based diabetes centre. Prospective clinical trials are needed to conclusively determine the effects of RC diets on clinical T1D outcomes.
Keywords: Type 1 diabetes; Glycaemic control; Reduced carbohydrate diets; Retrospective case series.
Abbreviations: TRC: Reduced Carbohydrate; HbA1c: Hemoglobin A1c; TEI: Total Energy Intake; LC: Low-Carbohydrate; CDE: Credentialed Diabetes Educator; APD: Accredited Practicing Dietitian; EA1c: Estimated A1c; CGM: Continuous Blood Glucose Monitoring; FGM: Flash Blood Glucose Monitoring; TDI: Total Daily Insulin; BMI: Body Mass Index; ISF: Insulin Sensitivity Factor(s); VLCKD: Very Low-Carbohydrate Ketogenic; LCD: Low-Carbohydrate Diet; MCD: Moderate-Carbohydrate Diet; kcal: Kilocalories; Kilojoules
Introduction
Type 1 Diabetes (T1D) is an autoimmune condition characterized by the destruction of pancreatic beta cells and absolute insulin deficiency. Glycemic control is the strongest predictor of diabetes-related complications with a hemoglobin A1c (HbA1c) level ≤7.0% (53mmol/mol) considered the primary target in diabetes management [1-3]. Standard treatment methods include insulin therapy, blood glucose monitoring and carbohydrate counting [4].However, dietary advice for people with T1D remains consistent with national recommendations for the general population, which is a high-carbohydrate diet (45-65% total energy intake [TEI]) [4,5]. Data from T1D registries (2010-2013) across nineteen countries in Australasia, Europe, and North America (n=324,501) reported that 84% of patients’ HbA1c was >7.0% (53 mmol/mol) [6]. Further, data from the 2006 Australian National Diabetes Audit-Australian Quality Self-Management Audit (n=3,930) reported a mean HbA1c of 8.4% (68 mmol/mol) amongst T1D patients [7]. It appears that current T1D management strategies are lacking in effect and dietary approaches specifically aimed at improving glycaemic control in T1D should be explored.
It is well established that dietary carbohydrates impose the greatest impact on post-prandial blood glucose levels. Lowcarbohydrate (LC) diets providing <130 grams of carbohydrate per day (g/day) or <26% TEI have recently been acknowledged by governing health bodies as an effective approach for type 2 diabetes management and a research priority area for T1D [8-10]. Despite very LC diets being the original method to treat diabetes prior to the discovery of insulin in the early 1900’s [11] a 2018 systematic review of diet interventions containing <45% TEI from carbohydrates highlighted a sheer lack of both observational and interventional studies investigating the feasibility and effect(s) of lower-carbohydrate diets in adults with T1D [12]. Nine studies were identified of which two were randomized controlled trials with small sample sizes (n=10), four were pre-post intervention studies, two were retrospective case series, and one was a case report [12]. Nevertheless, the collective evidence demonstrated promising results, including improvements in HbA1c, total daily insulin and frequency of severe hypoglycaemia in T1D adults [12]. A recent observational study of individuals with T1D (n=316) showed that exceptional HbA1c levels of ~5.7% (39mmol/mol) can be obtained with adherence to a very LC diet (~35g/day) [13]. Until prospective clinical trials with sufficient sample sizes are conducted to conclusively determine the effect(s) of RC diets in adults with T1D, small-scale studies exploring the use and feasibility of RC diets in real-world clinical practice settings are useful to help practitioners better understand the role of RC diets for T1D management [9,12].
Therefore, a retrospective case series of adults with T1D who have been actively managed with a RC diet delivered by a Credentialed Diabetes Educator (CDE) and Accredited Practising Dietitian (APD) (hereon referred to as CDE/APD) at a communitybased diabetes centre was conducted. The objectives of this study were to 1) describe the core components of RC diets used in realworld T1D management; and 2) determine (pre-post) change(s) in clinical T1D management outcomes for patients choosing to follow a RC diet, including HbA1c, glycaemic variability, total daily insulin use, and fasting blood glucose levels. This is a hypothesisgenerating study sought to explore the feasibility and effects of prescribing a RC diet for the management of T1D.
Materials And Methods
Study design
This case series was a retrospective chart review of adults previously diagnosed with T1D who had chosen to follow a RC diet delivered by the CDE/APD between January 2017 and November 2019 at a community-based diabetes centre in Stirling, Western Australia. The study protocol was registered and included in the Australian New Zealand Clinical Trials Registry and allocated a registration number of ACTRN12619001538134 (http://www. ANZCTR.org.au/ ACTRN12619001538134.aspx). Ethical approval was obtained from the University of Sydney’s Human Research Ethics Committee (project no.: 2019/668).
Participants
An email was sent out to existing patients at the centre inviting them to complete an online survey and provide informed consent to share their data with researchers and be assessed for eligibility to participate in this retrospective analysis. Ethical approval was obtained for a single email invitation, and no further attempts were made to contact non-responders for consent to share their data. One researcher (JT) assessed the consenting patient records for study eligibility and to collect relevant data for analysis during a 1-week period in November2019. To be included, patients must have attended at least two appointments (≥2 weeks apart) with the CDE/APD for advice regarding a RC diet for the management of T1D and been between 18-60 years of age at the time of their first appointment. A RC diet was defined as any approach that involved; (a) advanced carbohydrate counting, (b) carbohydrate reduction, and/or (b) LC diet support. It was assumed that advanced carbohydrate counting led to a natural reduction in carbohydrate intake irrespective of any specific prescription due to greater participant awareness of the carbohydrate content in various foods. Participants must have also had at least one clinical T1D outcome measured on at least two time-points (≥2 weeks apart) that was recorded in their patient record by the CDE/APD. As the diabetes centre is a private billing allied health clinic, all participants were responsible for scheduling consultations with the CDE/APD on an as-needed basis. Researchers did not contact participants for any missing data, and only data in patient records stored at the diabetes centre or provided by participants in the online survey was assessed. All eligible participants were included in the analysis.
Study outcomes
Core components of the RC diet: Content analysis was performed on the core dietary and delivery components of the RC diets, including the prescribed amounts and types of dietary carbohydrates, proteins, and fats, in addition to total energy. Reported details on the dietary delivery method(s) and any adjustment(s) made to glucose self-monitoring or insulin management were also analysed.
Clinical outcomes: The primary clinical T1D management outcomes were HbA1c and estimated A1c (EA1c), calculated as (mean glucose mmol/L + 2.59)/1.59 using the data recorded by a continuous [CGM] or flash [FGM] blood glucose monitoring device across 7-14 days [14]. EA1c has been used to assess glycaemic control in previous studies [15,16] rather than actual HbA1c, likely due to reduced need for blood collections and clinic visits [14]. Secondary clinical outcomes were time spent in blood glucose target ranges and low glucose events (<3.9mmol/L) recorded by a CGM or FGM device across 7-14 days. Additional clinical outcomes were total daily insulin (TDI) and fasting glucose levels, as measured by participants using their own devices. For each outcome, the earliest and last recorded value reported in patient records was considered the ‘pre’ and ‘post’ value, respectively.
Data collection
Patient records: Data were collected from patient records stored at the centre. Patient records included clinical notes and medical correspondence written by the CDE/APD. Specialist letters, pathology reports, and blood glucose monitoring reports were also assessed, if available. Patient records were assessed by one researcher (JT) to extract the following data for each participant: Body Mass Index (BMI) as assessed by the CDE/APD; use of CGM/FGM device; use of Insulin Sensitivity Factors (ISF) or insulin pump; use of carbohydrate counting; dietary intake at baseline as assessed by the CDE/APD (including macronutrient and energy intakes); details on the prescribed LC dietary approach; details on any specific delivery techniques used; details on any insulin and blood glucose monitoring adjustments; length of follow-up (i.e., time from first appointment to last appointment); and, all recorded clinical T1D outcome values. Due to the retrospective nature of this study, it was anticipated that not all participants would have complete data available in their patient records. Data analysis was completed on available data with no imputations made for missing data.
Participant survey: Participants completed a short online questionnaire at the time of consent to provide demographic data and general health information that may not have been available from their patient records. This data included: date of birth (for age calculation); ethnicity; religion; gender; highest level of education completed; height; body weight; year of type 1 diabetes diagnosis; and co-morbidities.
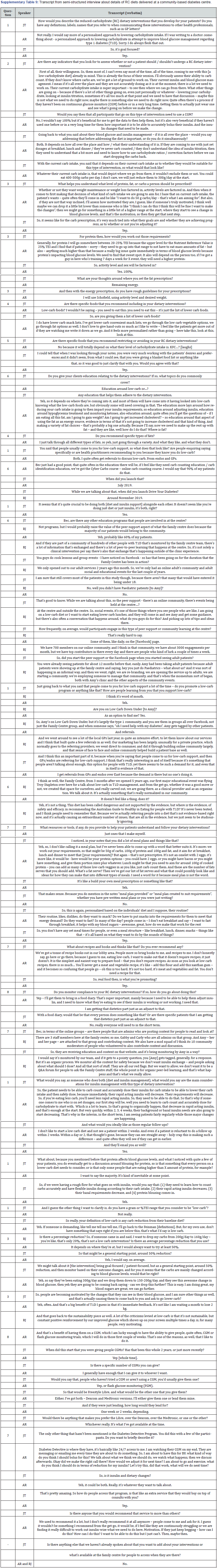
Practitioner interview: One researcher (JT) interviewed the centre’s CDE/APD and Chief Executive Officer (C.E.O.) to collect and clarify information on the RC diets delivered, in addition to information about the non-clinical social, community and educational services offered at the centre. The interview was semi-structured, and questions are included in the supplementary files (Supplementary Table 8). Answers were recorded verbatim.
Data synthesis & analysis: Thematic analysis was used to assess the dietary prescription details extracted from patient records and the semi-structured interview to identify core components of the RC diets. All investigators reviewed the initial themes identified in the collaborative data until consensus was reached on the core components and general methodologies used amongst included participants. Clinical T1D management outcome values were presented in tabular format for each participant (case-by-case) and pre-post means and mean differences were calculated using descriptive statistics. Mean changes in HbA1c, EA1c, time in target range, low glucose events, TDI and fasting glucose levels were compared using the first recorded value (pre) and the last recorded value (post) available for each participant.
Results
Participants and characteristics
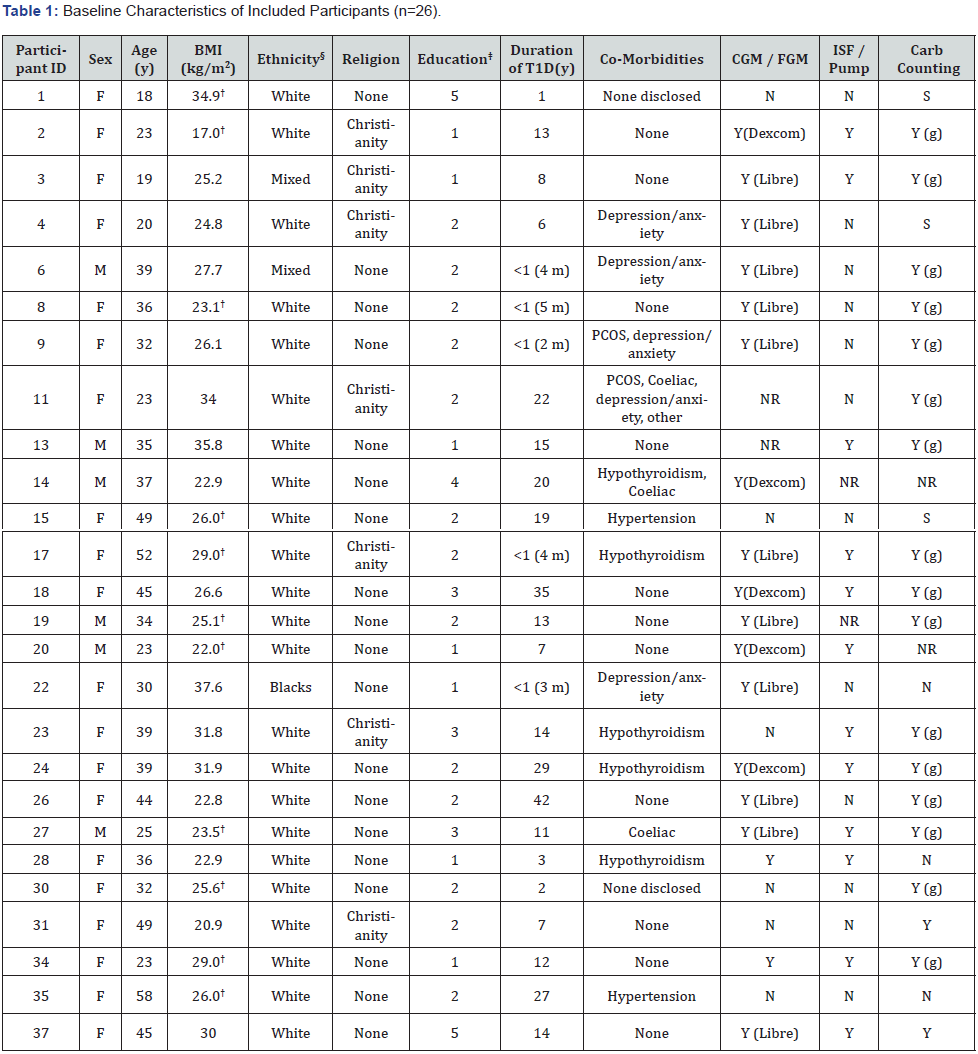
† Survey data was used for these participants and may not reflect true baseline BMI at time of initial appointment.
§ Refers to category: Black / African / Caribbean / Black British.
‡ 1, University or college degree (related to health, medical or nutritional sciences); 2, University or college degree (unrelated to health, medical or nutritional sciences); 3, University or college credit (no degree); 4, Trade/technical or vocational training; 5, High school (or equivalent secondary level).
NR: Not Reported; y: Years; m: Months; Coeliac: Coeliac disease; CGM: Continuous Blood Glucose Monitoring Device; FGM: Flash Blood Glucose Monitoring Device; ISF: Insulin Sensitivity Factor(s); Pump: Insulin Pump; Carb: Carbohydrate; S: Sometimes; G: Grams
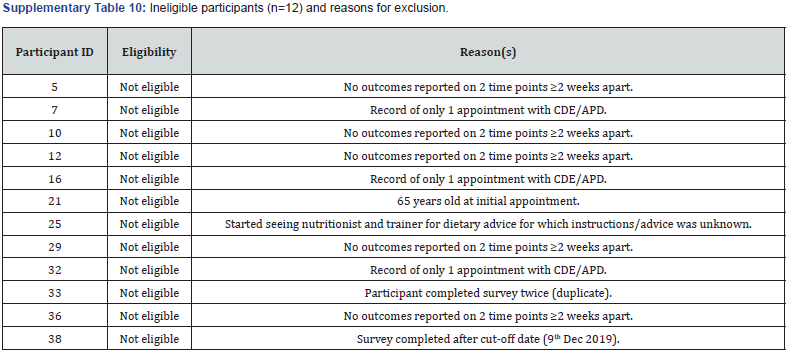
Email invitations were sent out to all existing patients at the centre (n=240). Of these, n=38 completed the online survey and provided informed consent to share their patient records with the researchers for assessment. Twenty-six participants were eligible and included in the study, while n=12 were not eligible with reasons provided (Supplementary Table 10). Baseline participant characteristics of included participants (n=26) are presented in Table 1. 77% (20/26) of participants were female with a mean age of 35 years (18-58years). The average duration of T1D was 12.4 years (2 months to 42 years), with 54% (14/26) of participants having lived with T1D for 10 years or longer. 65% (17/26) of participants were overweight or obese and 31% (8/26) were of normal weight according to BMI [17].
Baseline dietary intake
Baseline energy and macronutrient intake data is presented in Supplementary Table 1. Of the 16 participants (16/26) who reported to be following a particular diet at baseline; 75% reported to be following a LC diet, of which one was a vegetarian LC diet; and 19% were following a vegan diet. Nineteen participants (19/26) had baseline carbohydrate intake data in g/day available, with a mean of 100g/day, ranging from 20-283 g/day. According to the suggested definitions by Feinman et al. [18], 37% of participants were following a very LC ketogenic diet (VLCKD) (20-50g/day),26% were following a LC diet (LCD) (<130g/day), and 32% were following a moderate-carbohydrate diet (MCD) (130-225g/day). Thirteen participants (13/26) had baseline energy intake data, with a mean TEI of 1,662 kcal/day 250 (6,848kJ/day), ranging from 728-2,546kcal/day (3,000-10,489kJ/day).
Core components of RC diets
Participants received personalised advice and support regarding a RC diet by the centre’s CDE/APD at various timepoints between January 2017 and November 2019, and the average follow-up duration was 41 weeks (2-127 weeks) (Table 2). The RC diet represented a patient-led approach that included advice and education on the topics of dietary carbohydrates, proteins, and fats; glucose self-monitoring; and insulin management (Table 2 & Supplementary Table 9). The CDE/APD reported that the majority of patients who attend the diabetes centre are actively seeking LC diet support. Actual data from patient records indicated that 85% of participants (22/26) were seeking LC diet support. Prior to implementing any carbohydrate reduction, participants were first educated on how to dose insulin for their absolute carbohydrate intake, including proper use of insulin to carbohydrate ratios and correction factors. If participants were not properly counting carbohydrates in all foods and fluids, then advanced carbohydrate counting education was provided to ensure all digestible carbohydrates were accounted for including carbohydrates in non-starchy vegetables, low-sugar fruits, nuts, and seeds. The CDE/APD typically referred patients to an online nutrient database (Calorie King (19)), and the centre offered a carbohydrate counting course. Data extracted from patient records indicated that 65% of participants (17/26) required advanced carbohydrate counting education (Table 2).
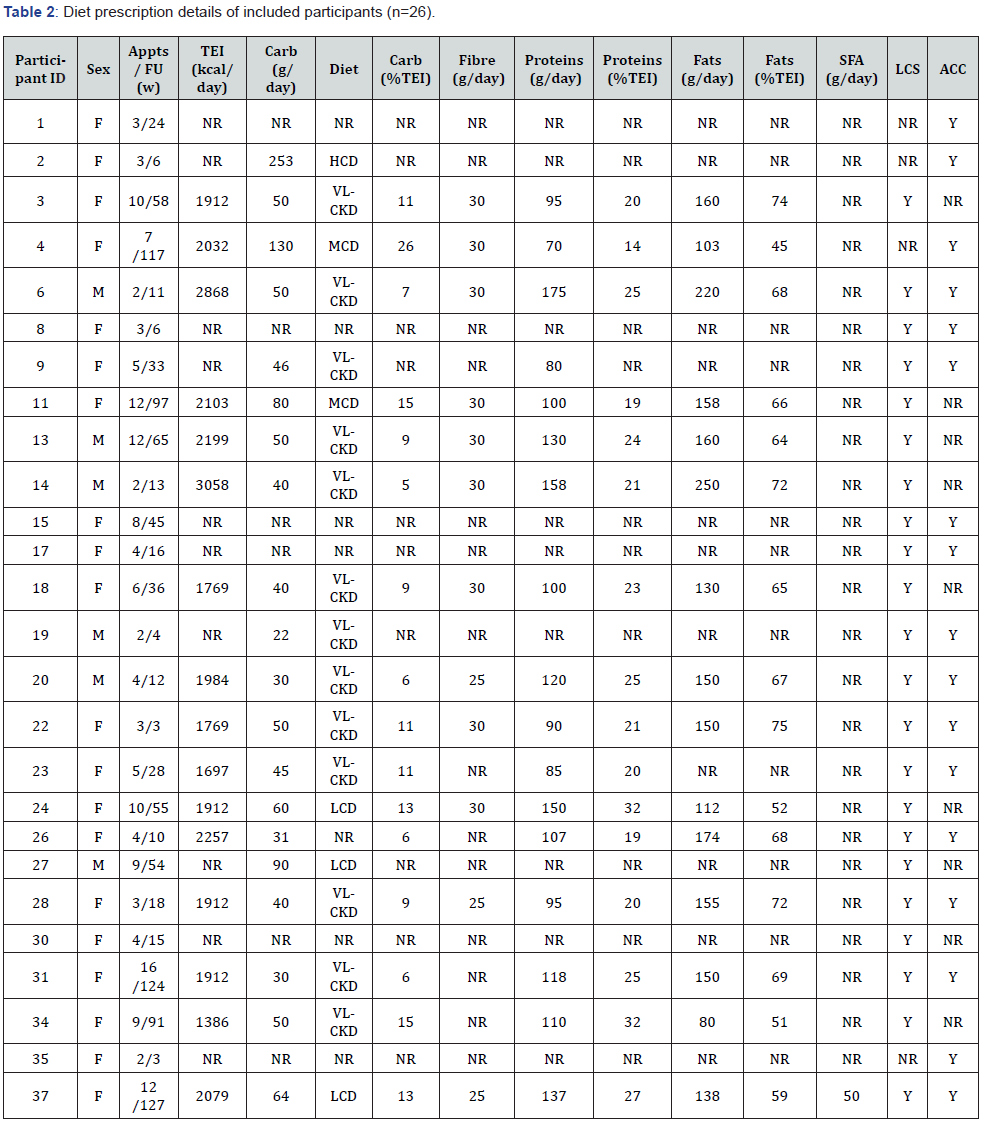
F: Female; M: Male; Appts: Appointments; FU: Follow Up Duration; w: Weeks; TEI: Total Energy Intake; kcal: Kilocalories; NR: Not Reported; Carb; Total Digestible Carbohydrates; g: Grams; VLCKD: Very Low-Carbohydrate Ketogenic Diet; LCD: Low-Carbohydrate Diet; MCD: Moderate- Carbohydrate Diet; HCD: High-Carbohydrate Diet; SFA: Saturated Fatty Acids; LCS: Low-Carbohydrate Diet Support; ACC: Advanced Carbohydrate Counting; Y: Yes
The CDE/APD reported that the initial recommended change in carbohydrates was generally a reduction of ~50% from baseline intake, except for those already following a VLCKD. Actual prescriptions were reported in g/day and/or TEI for 77% of participants (20/26) (Table 2). The average carbohydrate prescription of the RC diets was 63g/day (or 11% TEI) ranging from 22-253g/day (or 5-26% TEI), which translated to a 37% reduction from baseline (Table 2 & Supplementary Table 1). 68% of prescriptions were VLCKD (20-50g/day), 16% were LCD (<130g/day), and 11% were MCD (130-225g/day) [18] (Table 2). Participants were routinely provided with a CGM or FGM (if they were not already using one) to use over the initial 1-2 weeks. This was to assist them in identifying the required insulin adjustments to prevent hypoglycaemia in expectation that rapid acting insulin requirements would immediately reduce with carbohydrate reduction. Participants were advised to return for a follow-up within 2-4weeks of the initial appointment to receive further support on titrating insulin dosages in expectation that background/basal insulin requirements would also reduce with continued adherence to the RC diet. The carbohydrate prescription may have continued to be adjusted depending on participants’ goals, preferences, motivations, and outcome progress. Total energy prescriptions were calculated using the Schofield equation to help participants meet their energy demands on a RC diet [20]. Sixteen participants (16/26) had total energy prescriptions recorded in their patient records (Table 2). Of these, the average prescription was 2,085 kcal/day (8,590 kJ/ day), ranging from 1,408-3,106kcal (5,800-12,795kJ/day), which reflected a 25% increase from baseline (Table 2 & Supplementary Table 1). Dietary protein prescriptions were based on 20- 25% of participants’ total energy prescription, and protei n options and portion sizes were discussed with participants in terms of real food quantities (e.g., 100g cooked meat). Seventeen participants (17/26) had actual protein prescriptions recorded and of these, the average was 113g/day (or 23% TEI), ranging from 70-175g/day (or 14-32% TEI) (Table 2). Participants were recommended to meet their remaining energy requirements with a variety of dietary fats, and fat options and portion sizes were discussed. The types of foods recommended included minimally processed meat, fish, eggs, full-fat dairy, non-starchy vegetables, low-sugar fruits, nuts, seeds, avocado, olive oil, butter, and nut butters. Fifteen participants (15/26) had actual fat prescriptions recorded, with an average prescription of 153 g/day (or 64% TEI), ranging from 80-250g/day (or 45-75% TEI) (Table 2). Participants were given practical advice on structuring meals according to their macronutrient and energy requirements. The CDE/APD provided education to facilitate adherence to the RC diets. Topics included: effect(s) of carbohydrates on blood glucose levels and insulin requirements, role(s) of dietary fats as an energy source and in weight management/general health, effect(s) of increasing dietary fats on cholesterol levels and risk of cardiovascular disease, hypoglycaemia treatment, and ketone monitoring. The CDE/APD reported that participants following the RC diets eventually required education and advice on how to factor in protein when calculating mealtime insulin dosages (known as ‘protein bolusing’). Courses, meet-up events, and peer support/learning opportunities were also offered to participants by the community-based diabetes centre to complement the clinical services with the CDE/APD. For example, the centre has a Facebook group with 700 members and 3000 engagements per month.
Clinical outcomes
HbA1c:Eight participants had pre-post values for HbA1c that were either self-reported usual care measurements recorded by the CDE/APD (n=4), usual care measurements taken from pathology reports or specialist correspondence (n=1), a combination of both (n=2), or measured at the diabetes centre (n=1) (Supplementary Table 2). Mean HbA1c reduced from 9.0% (75mmol/mol) to 7.0% (53mmol/mol) (-2.2%; -5.7 to -0.1%), with an average follow-up duration of 55 weeks (10-114 weeks). Four (4/8) participants had a post HbA1c value within the T1D management target range of ≤7.0% (53mmol/mol), while only one (1/8) participant had a prevalue within this target. Two participants with the highest starting HbA1c values experienced the greatest reductions at follow-up (13.4 to 7.7%; -5.7% and 10.6 to 5.3%; -5.3%). No participants had an increase in HbA1c reported.
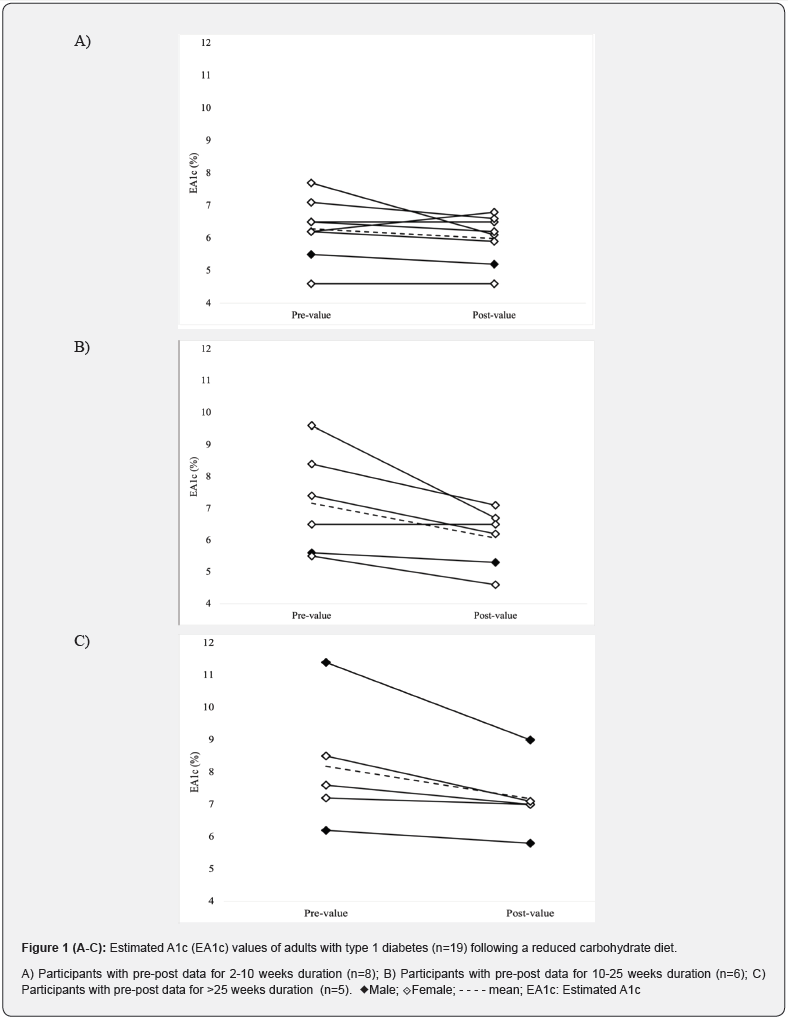
Estimated A1c:Nineteen participants had pre- and postvalues for EA1c measured using an FGM (n=14, FreeStyle Libre™), a CGM (n=1, Dexcom) or both (n=1, Libre + Dexcom; n=3, Libre + Medtronic) (Figure 1 & Supplementary Table 3). Of these participants, eight (8/19) had pre-post values over a duration of 2-10 weeks, of which five experienced a reduction, two experienced no changes, and one experienced an increase, with an average absolute change in EA1c of -0.3% (-1.6 to +0.6%). Six participants (6/19) had pre-postvalues over a duration of 10-25weeks, of which five experienced a reduction and one no change, with an average absolute change of -1.1% (-2.9 to 0.0%); and five participants (5/19) had pre-post values for durations greater than 25weeks of which all experienced a reduction and average absolute change of -1.0% (-2.4 to -0.2%). Across all 19 participants, the average follow-up for measuring changes in EA1c was 21 weeks (2-55weeks), with an overall mean pre-post change of -0.7% (-2.9 to+0.6%), with mean EA1c values reducing from 7.1% (54mmol/mol) (4.6 to 11.4%) to 6.3% (45mmol/mol) (4.6 to 9.0%).
Time in target range: Twelve participants had pre-post values for time in target range that were measured using a FGM (n=7), CGM (n=2) or both (n=3) (Supplementary Table 4). Three participants (3/12) used non-standard blood glucose target ranges within 3.9-8.5mmol/L. Of these, all participants (3/3) experienced an increase in time in target, from an average of 58% to 65% (+7%; +3 to +13%). Nine participants (9/12) used the standard target range of 3.9-10.0 mmol/L and their overall mean time in target increased from 58% to 76% (+18%; -8 to +46%), with an average follow-up duration of 19 weeks (4-52weeks).
Low glucose events: Fourteen participants had pre-post values for low glucose events (<3.9 mmol/L) measured over a 2-week period using an FGM (n=10), CGM (n=1) or both (n=3) (Supplementary Table 5). The mean frequency increased by one event following an average duration of 17 weeks, with 50% (7/14) experiencing a decrease in low glucose events ranging from -1 to -13 events over a 2-week period. Ten participants (10/14) had pre-post minute data available (Supplementary Table 5). Of these, the mean minutes spent in the low glucose range (<3.9 mmol/L) for each event reduced from 120 to 94 over a 2-week period.
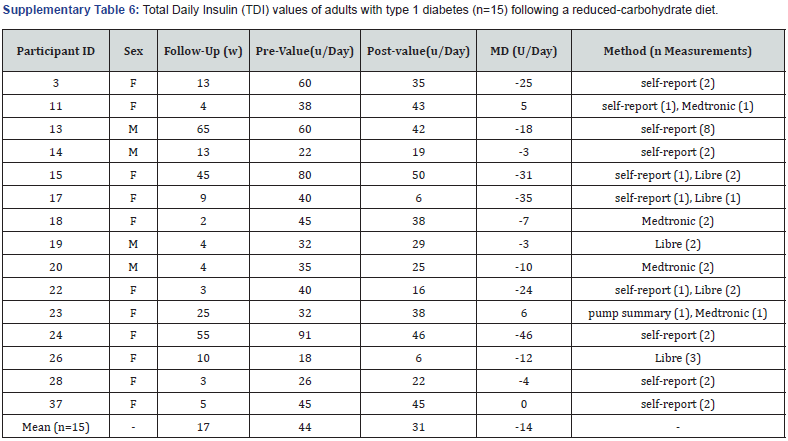
Total daily insulin: Fifteen participants had pre-post values for TDI that were either self-reported measurements recorded by the CDE/APD (n=6), taken from CGM or FGM reports (n=4), a combination of both (n=4), or taken from a combination of an insulin pump summary report and a CGM report (n=1) (Supplementary Table 6). Mean TDI reduced by 30% from 44 U/ day to 31 U/day (-13 U/day; -46 to +6 U/day), with an average follow-up duration of 17 weeks (2-65weeks). Five participants (5/15) reported reductions in TDI of >20 U/day, ranging from -24 to -46 U/day. The participant who experienced the greatest reduction in TDI (-46 U/day) had the highest reported pre-value (91 U/day), with a follow-up duration of 55weeks.
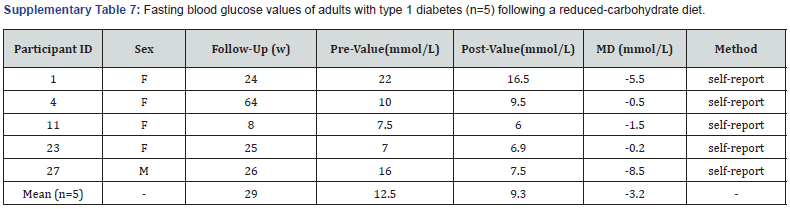
Fasting blood glucose: Five participants self-reported pre-post fasting blood glucose values at least two weeks apart (Supplementary Table 7). All five reported lower post-values with a mean reduction from 12.5mmol/L to 9.3mmol/L (-8.5 to -0.2mmol/L) following an average duration of 29weeks.
Discussion
This retrospective case series explored the practical application and effect(s) of RC diets for T1D patients at a community-based diabetes centre. Professionally supported RC diets appear to be a feasible management option for adults with T1D and may lead to maintenance or improvements in clinical outcomes in some patients. The RC diets described in this analysis were predominantly patient-led approaches involving individuals with an a priori interest in LCD who were actively seeking professional support. In addition to dietary adjustments, the RC diets involved delivery of diet-related education and changes to blood glucose self-monitoring and insulin management practices. The main clinical findings were that RC diets led to reductions in HbA1c and EA1c, increased time spent in target ranges, reduced time spent in the hypoglycaemic range, and less insulin being required overall. However, prospective clinical trials are needed to confirm these findings and conclusively determine the effects of RC diets on clinical outcomes in T1D management.
This study provides real-world insights into the use of professionally supported RC diets, including VLCKD and LCD [18] in the management of adults with T1D. It has been previously reported that individuals with T1D following RC diets experience difficulties in seeking professional support. An online survey of 316 people with T1D self-engaging in a VLCKD reported high levels of overall health and satisfaction with their diabetes management but not with their professional diabetes care team, with only 49% of respondents agreeing or strongly agreeing that their diabetes care providers were supportive of their dietary choices [13]. In the current study, 85% of participants presented to the community-based diabetes centre specifically seeking support with a LC dietary approach for T1D management. Given the sheer dearth of available evidence investigating lowercarbohydrate diets in T1D, the present analysis is a useful contribution to the evidence base on this topic and may assist healthcare professionals in better supporting T1D patients who choose to follow a RC dietary approach.
This study highlights a patient population that is particularly interested in LCD for the management of their T1D. Although the RC diet involved a reduction in total carbohydrates, the mean carbohydrate intake at baseline was already low (100g/day or 24%TEI) relative to the estimated population average. Data from a previous study which analysed the macronutrient profiles of adults with T1D from the Australian Health Survey 2011- 13 reported that the average carbohydrate intake was 213g/ day (45%E) [21]. Nevertheless, the RC diets involved additional reductions in dietary carbohydrates alongside other dietary manipulations which may have been uniquely beneficial. The CDE/ APD recommended that dietary fats make up ‘remaining energy requirements’ after carbohydrates and proteins were accounted for. The mean dietary fat prescription was 153g/day (or 64%TEI) which was a clinically significant increase of 94% from a baseline fat intake of 79g/day (or 41%TEI). This is consistent with dietary fat prescriptions identified in previous LCD intervention studies of type 2 diabetes patients which were ‘unrestricted’ in fat (n=9 studies) or ‘high fat’ diets (>35%E) (n=9 studies) [22]. Dietary fat provides an important source of energy and fat-soluble nutrients for individuals on a RC diet, and substitution of carbohydrates with fats results in a lower glycaemic load [23,24]. With that said, minimally processed fats from meat, fish, eggs, full-fat dairy, nuts, seeds, avocado, olive oil, and butter tended to be the recommended sources of fat in the collective studies [22]. These whole foods contain a natural balance of monounsaturated, polyunsaturated, and saturated fatty acids [24]. On the other hand, ultra-processed oils containing excessively high amounts of omega-6 polyunsaturated fatty acids have been identified as drivers for obesity and cardiovascular disease (CVD) [25, 26]. Although HbA1c is a primary determinant of CVD risk in diabetes, future prospective studies investigating RC diets for T1D should measure an array of CVD risk factors, including lipid changes, to help us better understand the long-term efficacy of higher-fat LCDs in T1D.
In the present analysis, more participants had reliable data for EA1c than HbA1c given that CGM and FGM devices were recommended by the CDE/APD, and reports were available to validate the results. The 0.7% absolute reduction in EA1c shown in this study (7.1% to 6.3%) is consistent with findings from previous research in T1D. Nielsen et al. 2012 [27] showed in a 4-year intervention study (n=48) that a LCD (≤75g/ day) significantly reduced HbA1c by 0.7% (7.6% to 6.9%) in adults with T1D. Additionally, a 12-week randomised controlled trial (n=10) showed that a LCD (~100g/day) decreased HbA1c by 0.7% (7.9% to 7.2%) compared to no change with a diet higher in carbohydrates (~200g/day) [28]. A 0.7% HbA1c reduction is considered clinically relevant for patients with T1D [29], particularly if HbA1c is maintained below the recommended target of 7.0%. A large Swedish study (n=10,000) showed that T1D patients with an HbA1c between 6.5% and 6.9% had a lower risk of developing retinopathy and early kidney disease compared to patients with an HbA1c ≥7.0% [30].
RC diets may be a useful option in T1D for improving or maintaining glycaemic control with less use of insulin overall. The present analysis demonstrated a clinically significant reduction in TDI of -14 U/ day from 44 U/day to 31 U/day across 2-65weeks. Similarly, a previous case-series of 10 adults with T1D reported a reduction in TDI of -17 U/day (47 to 30U/day) across 8-61 months with a VLCKD (30g/day) [31]. Excessive long-term reliance on high doses of insulin may lead to additional complications related to hyperinsulinemia, such as obesity, type 2 diabetes [32] and hypertension [32-34]. In addition, requiring less insulin is also expected to result in significant cost-savings for people affected by T1D and the health system [35]. On the other hand, previous dietary modelling showed that comparedto a traditional highcarbohydrate, low-fat diet, a low-carbohydrate, high-fat diet may be associated with negligible, marginally higher costs ($2.06 per person per week) [36]. Hence, potential insulin cost savings, marginally higher dietary intake costs, and costs associated with changes in health outcomes should be considered collectively in determining the value of a RC diet approach for individuals with T1D.
A commonly cited concern of the use of LC diets in T1D is an increased risk of severe hypoglycaemia. However, the present data showed that the overall time spent within the target blood glucose range of 3.9-10 mmol/L increased by 18% with a RC diet, and minutes per low glucose event (<3.9mmol/L) decreased by 25%. Similarly, a 12-month study of 46 adults who self-reduced carbohydrates to ~162 g/day in combination with flexible insulin therapy reported a reduction of severe hypoglycaemia from 3.7 to 0.2 episodes per year. Nevertheless, it must be acknowledged that any diet or lifestyle change in individuals with T1D may increase the risk of hypoglycaemia in the short-term due to the consequent changes in blood glucose self-monitoring and insulin titrations that are often required. Participants in the current study were encouraged to re-appoint within two weeks of commencing dietary change to review their insulin management with the CDE/APD. Unfortunately, evidence-based guidelines for adjusting pharmaceuticals for patients with T1D wishing to follow RC diets are scant, but referring to practical guides used in type 2 diabetes may be a useful approach for practitioners [37].
This study has several limitations. The retrospective nature of this study meant there were major inconsistencies in the outcomes reported for included participants. To some extent, the available data was limited because the CDE/APD did not write clinical notes intending for them to be assessed by external researchers, and researchers did not contact participants to collect missing data. In addition, limitations in the reporting of the data meant that we were unable to assess adherence to the RC diets prescribed or determine pre-post changes in patient wellbeing, or whether any associations exist between the level of dietary carbohydrate prescribed and changes in T1D management outcomes. However, to our knowledge, the diabetes centre selected for this study is the only T1D centre in Australia actively promoting RC diets as an option for T1D management and considering the major lack of available research on this topic, the present analysis is useful and clinically relevant work. The high risk of selection bias must also be emphasised because most patients attending the centre were actively seeking LCD support, with the majority already following some form of LCD (albeit unsupported). For ethical reasons that only permitted a one-off invitation request, data from non-responders could not be examined and therefore any differences between responders and non-responders could not be determined. Finally, the lack of a control group or control period and the multi-modal nature of our exploratory study design precludes the ability to draw causality between the RC diet or other specific aspects of the approach imposed and the observed changes in clinical T1D outcomes. The authors acknowledge the need for high-quality prospective interventional studies investigating this important research area to better inform clinical practice guidelines.
Discussion
In summary, this hypothesis-generating study provides realworld insights into the practical application of RC diets in the management of adults living with T1D. Professionally supported RC diets appear to be a feasible option for motivated T1D patients and may lead to maintenance or improvements in clinical outcomes. Prospective clinical trials are needed to conclusively determine the effects of RC diets, including LCD and VLCKD, on clinical outcomes in T1D management.
Acknowledgements
Thank you to the participants of this study for agreeing to participate and share their data for this research. Thank you to the staff at the community-based diabetes centre in Stirling, Western Australia for assisting with administrative tasks.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Jessica Turton: Conceptualization, Methodology, Formal Analysis, Investigation, Writing – Original Draft, Writing – Editing & Review, Visualization, Project Administration. Grant Brinkworth: Conceptualization, Methodology, Writing – Editing & Review, Visualization. Helen Parker: Conceptualization, Methodology, Writing – Editing & Review, Visualization. Amy Rush: Conceptualization, Methodology, Writing – Editing & Review, Resources. Rebecca Johnson: Conceptualization, Methodology, Writing – Editing & Review, Resources. Kieron Rooney: Conceptualization, Methodology, Writing – Editing & Review, Visualization.
Competing Interests
Ms Rush and Ms Johnson have financial involvements with the community-based diabetes centre where the research was undertaken as an employee and as the C.E.O., respectively. The centre could obtain indirect benefit from this research by receiving information on the effectiveness of their services not normally obtained if not participating in this study. However, no direct financial benefit is expected to be obtained. No other authors have any competing interests to declare.
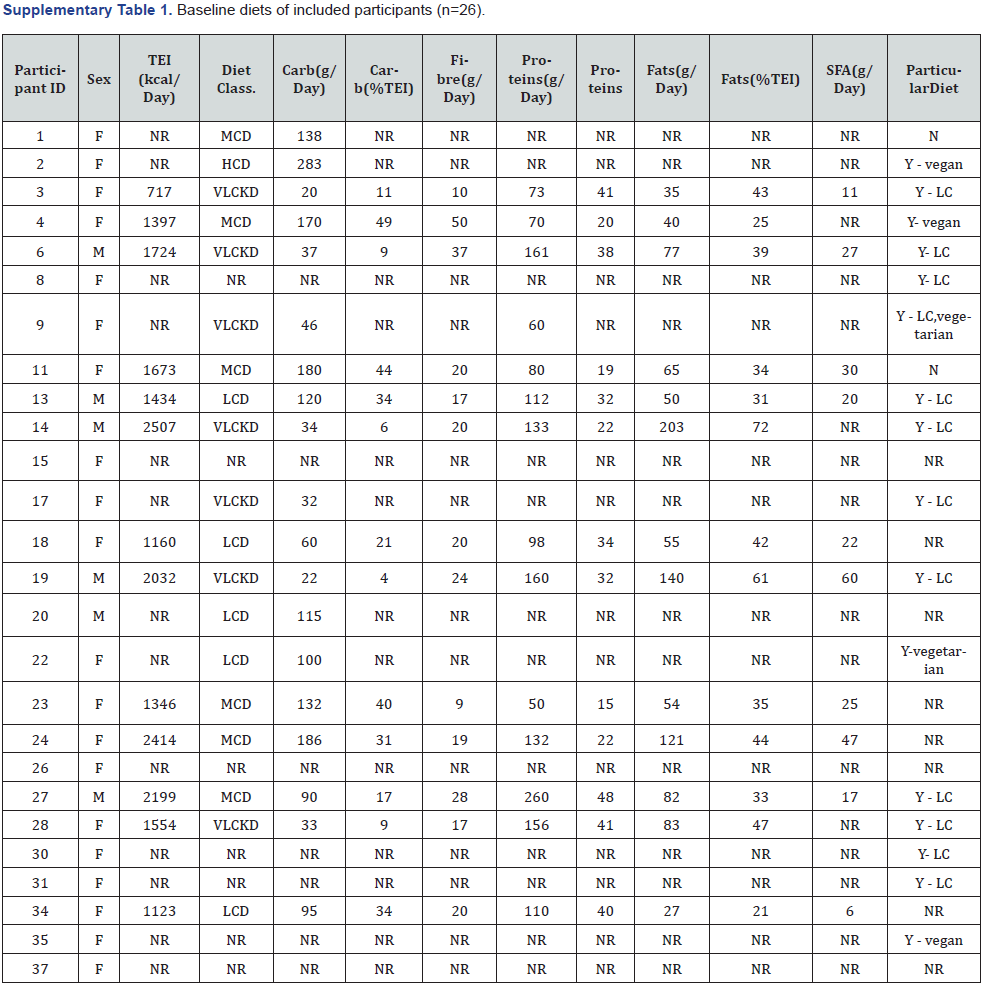
ID: Participant ID; F: Female; M: Male; TEI: Total Energy Intake; kcal: Kilocalories; NR: Not Reported; Carb: Total Digestible Carbohydrates; g: Grams; class: Classification; VLCKD: Very Low-Carbohydrate Ketogenic Diet; LCD: Low-Carbohydrate Diet; MCD: Moderate- Carbohydrate Diet; HCD: High Carbohydrate Diet; SFA: Saturated Fatty Acids.
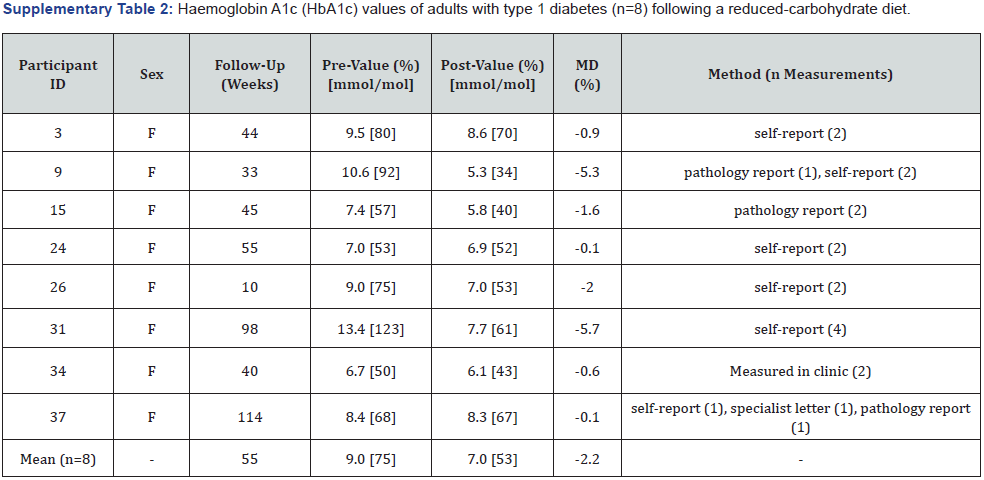
F: Female; M: Male; MD: Mean Difference.
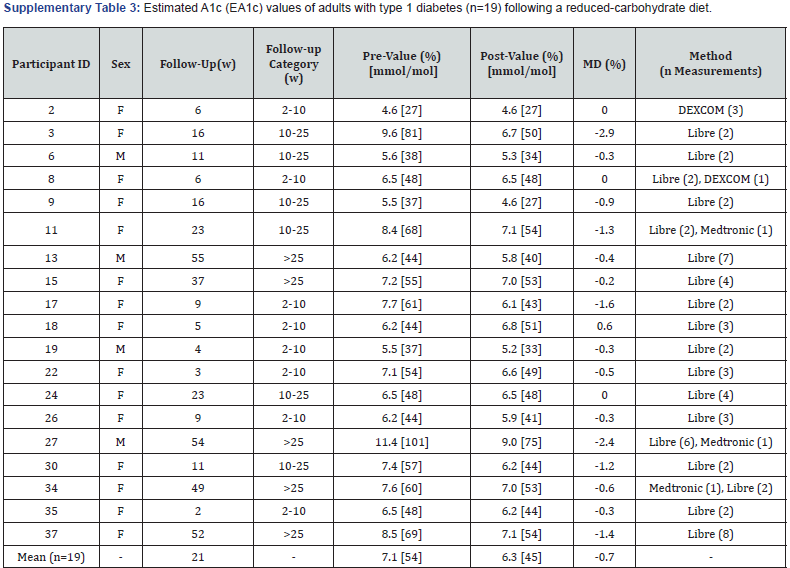
F: Female; M: Male; W: Weeks; MD: Mean Difference.
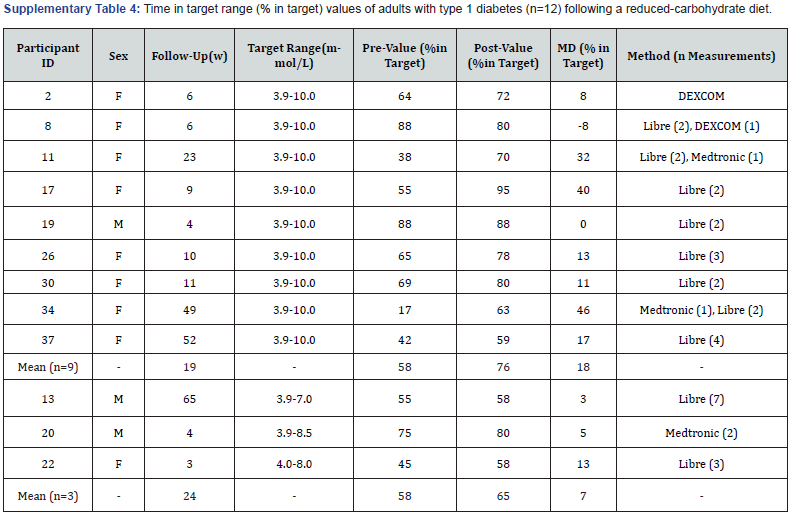
F: Female; M: Male; W: Weeks; MD: Mean Difference.
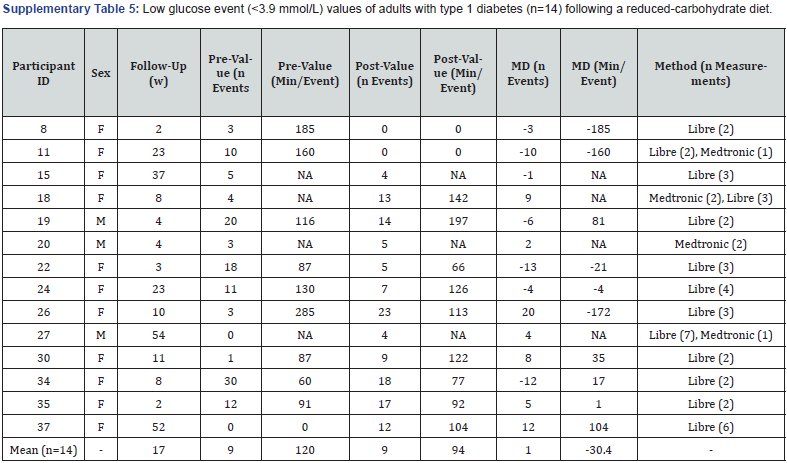
F: Female; M: Male; W: Weeks; Min: Minutes; NA: Not Available; MD: Mean Difference.
F: Female; M: Male; W: Weeks; U: Units; MD: Mean Difference.
F: Female; M: Male; W: Weeks; MD: Mean Difference.

Carbs: Carbohydrates; TEI: Total Energy Intake.
References
- Cheung NW, Conn JJ, D’Emden MC, Gunton JE, Jenkins AJ, et al. (2009) Position statement of the Australian Diabetes Society: individualisation of glycated haemoglobin targets for adults with diabetes mellitus. Med J Aust 191(6): 339-344.
- D M Nathan, S Genuth, J Lachin, P Cleary, O Crofford, et al. (1993) The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med 329(14): 977-986.
- Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M (2008) Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. DiabetesCare 31(4): 714-719.
- Craig M, Twigg S, Donaghue K, Cheung N, Cameron F, et al. (2011) National evidence-based clinical care guidelines for type 1 diabetes in children, adolescents and adults Canberra: Australian Government.
- (2013) National Health and Medical Research Council. Australian Dietary Guidelines. Commonwealth of Australia.
- McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, et al. (2015) Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med A journal of the British Diabetic Association 32(8): 1036-1050.
- (2016) National Association of Diabetes Centres (NADC). The Australian National Diabetes Audit - Australian Quality Self-Management Audit (ANDA-AQSMA) Final Report.
- (2017) Position statement: Low-carb diets for people with diabetes, Diabetes, UK.
- (2018) Position Statement: Low carbohydrate eating for people with diabetes. Diabetes, Australia.
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, et al. (2018) Management of Hyperglycaemia in Type 2 Diabetes, A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41(12): 2669-2701.
- Osler W, Mc Crae T (1921) The Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. D. Appleton.
- Turton JL, Raab R, Rooney KB (2018) Low-carbohydrate diets for type 1 diabetes mellitus: A systematic review. PLoS One 13 (3): e0194987.
- Lennerz BS, Barton A, Bernstein RK, Dikeman RD, Diulus C, et al. (2018) Management of Type 1 Diabetes with a Very Low–Carbohydrate Diet Pediatrics 141(6): e20173349.
- Hu Y, Shen Y, Yan R, Li F, Ding B, et al. (2020) Relationship Between Estimated Glycosylated Hemoglobin Using Flash Glucose Monitoring and Actual Measured Glycosylated Hemoglobin in a Chinese Population. Diabetes Ther 1 1(9): 2019-2027.
- Dunn TC, Xu Y, Hayter G, Ajjan RA (2018) Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: A European analysis of over 60 million glucose tests. Diabetes Res Clin Pract 137: 37-46.
- Al-Agha AE, Kafi SE, Zain Aldeen AM, Khadwardi RH (2017) Flash glucose monitoring system may benefit children and adolescents with type 1 diabetes during fasting at Ramadan. Saudi Med J 38(4): 366-371.
- (2019) National Centre for Chronic Disease Prevention and Health Promotion. Defining Adult Overweight and Obesity.
- Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, et al. ( 2015) Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base . Nutrition 31(1): 1-13.
- Calorie King (2021) Australia's trusted food database.
- Schofield WN (1985) Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39 (Suppl 1): 5-41.
- Turton JL, Struik NA, Riley M, Brinkworth GD (2020) Adults with and without type 1 diabetes have similar energy and macronutrient intakes: an analysis from the Australian Health Survey 2011-2013. Nutr Res 84: 25-32.
- Turton J, Brinkworth GD, Field R, Parker H, Rooney K (2019) An evidence-based approach to 604 developing low-carbohydrate diets for type 2 diabetes management: A systematic review of interventions and methods. Diabetes Obes Metab 21(11): 2513-2525.
- (2019) GI Foods Advanced Search: The University of Sydney.
- (2019) United States Department of Agriculture. USDA Food Composition Databases.
- DiNicolantonio JJ, O’Keefe JH (2018) Omega-6 vegetable oils as a driver of coronary heart disease: the oxidized linoleic acid hypothesis. Open Heart 5(2): e000898.
- Simopoulos AP, DiNicolantonio JJ (2016) The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 3(2): e000385.
- Nielsen JV, Gando C, Joensson E, Paulsson C (2012) Low carbohydrate diet in type 1 diabetes, long-term improvement and adherence: A clinical audit. Diabetol Metab Syndr 4(1): 23.
- Krebs J, Strong A, Cresswell P, Reynolds A, Hanna A, et al. (2016) A randomised trial of the feasibility of a low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account Asia Pac J Clin Nutr 25(1): 78-84.
- Lenters-Westra E, Schindhelm RK, Bilo HJG, Groenier KH, Slingerland RJ (2014) Differences in interpretation of haemoglobin A1 c values among diabetes care professionals. Neth J Med 72(9): 462-466.
- Lind M, Pivodic A, Svensson AM, Ólafs dóttir AF, Wedel H, et al. (2019) HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 366: l4894.
- O'Neill DF, Westman EC, Bernstein RK (2003) The effects of a low-carbohydrate regimen on glycaemic control and serum lipids in diabetes mellitus. Metab Syndr Relat Disord 1(4): 291-298.
- Sung KC, Jeong WS, Wild SH, Byrne CD (2012) Combined Influence of Insulin Resistance, Overweight/Obesity, and Fatty Liver as Risk Factors for Type 2 Diabetes. Diabetes Care 35(4): 717-722.
- Purnell JQ, Zinman B, Brunzell JD (2013) The effect of excess weight gain with intensive 635 diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation 127(2): 180-187.
- Kelly CT, Mansoor J, Dohm GL, Chapman WH 3rd, Pender JR, et al. (2014) Hyperinsulinemic syndrome: the metabolic syndrome is broader than you think. Surgery 156(2): 405-411.
- Palmer AJ, Weiss C, Sendi PP, Neeser K, Brandt A, et al. (2000) The cost-effectiveness of different management strategies for Type I diabetes: a Swiss perspective. Diabetologia 43(1): 13-26.
- Zinn C, North S, Donovan K, Muir C, Henderson G (2020) Low-carbohydrate, healthy-fat eating: A cost comparison with national dietary guidelines. Nutr Die 77(2): 283-291.
- Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M (2019) Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract 69(684): 360-361.






























