Molecular Basis of Neurodegeneration and Therapies in Diabetic Neuropathy
Reena Kumari and Renu Bist*
Department of Zoology, University of Rajasthan, India
Submission: November 30, 2021; Published: December 07, 2021
*Corresponding author: Renu Bist, Associate Professor, Molecular Biology Lab, Department of Zoology, University of Rajasthan, Jaipur, India
How to cite this article: Reena K, Renu B. Molecular Basis of Neurodegeneration and Therapies in Diabetic Neuropathy. Curre Res Diabetes & Obes J 2021; 15(2): 555909.DOI: 10.19080/CRDOJ.2021.15.555909
Abstract
Neuropathy is one among the known disorders corresponding diabetes. The biochemical mechanisms of this devastating neurodegenerative disorder of diabetic neuropathy (DN) have not yet clearly understood. DN is loss of function nerves beginning distally in the lower extremities that is also characterized by pain and substantial morbidity. It leads to distressing and expensive clinical complications such as foot ulceration, leg amputation, and neuropathic pain. Despite countless promising therapeutic research efforts, effective drugs are still lacking for the treatment of DN. Therefore, current review emphasizes on complications and therapeutic strategies which could be effective in DN. Various search engines like Google Scholar, PubMed, SpringerLink, Medline and Science direct were used for accessing different articles of world-wide journals to harness the information of previous work done with our relevance. Databases like PDB and NCBI were used to understand molecular information of proteins and DNA in DN. In present review, we discussed causes, mechanisms that lead to promotion of DN and possible therapies of DN. The information provided in this review provide research gap to investigators to understand molecular mechanisms underlying DN and to attempt natural substances as possible effective therapy. Current review discusses significant research gaps for making an attempt to investigate a successful natural product and its molecular target for DN. We also accentuate the use of natural product instead of a synthetic drug for treatment of DN.
Keywords: Diabetes; Diabetic Neuropathy; Natural products; Nerves; Therapy; Molecular
Abbreviations: DN: Diabetic Neuropathy; DM: Diabetes Mellitus; IDF: International Diabetes Federation; PKC: Protein Kinase C; AGEs: Advanced Glycation End Products; GSK: Glycogen Synthase Kinase; DSP: Distal Symmetric Polyneuropathy; DSPN: Distal symmetric polyneuropathy; EDIC: Epidemiology of Diabetes Interventions and Complications; DCCT: Diabetes Control and Complications Trial; AND: Autonomic Diabetic Neuropathy; CAN: Cardiac Autonomic Neuropathy; ED: Erectile Dysfunction; ROS: Reactive Oxygen Species; ER: Endoplasmic Reticulum; DPN: Diabetic Peripheral Neuropathy; SDH: Sorbitol Dehydrogenase; NO: Nitric Oxide; ARI: Aldose Reductase
Introduction
Diabetic neuropathy (DN) term is referred to a condition where symptoms of neuronal aberrations are found in a person with diabetes, in whom other factors of atrophy of brain have been excluded [1]. DN has complications of diabetes that result in damage to the nervous system. DN is a prevalent complication of type l and type 2 diabetes both and influences over 90% of the diabetic sufferers [2]. It involves decline of sensory functions that are start from distal to proximal in both extremities that is also characterized by pain and substantial morbidity. Progressively, at least 50% of individuals with diabetes develop DN [3]. Diabetes mellitus (DM) is one among the most common cause of mortality worldwide, and it accounts for about 63.5% of deaths due to its relation to chronic complications [4]. International Diabetes Federation (IDF) reported a number of cases where diabetes is expected to ascend approximately 629 million by 2045 [5].
In accordance with current survey of International Diabetes Federation (IDF), there are close to 73.12 million people in India who are suffering from Diabetes and globally it is expected to move upwards from 463 million in 2019 to 700 million by 2045 [6]. Pain is one of the chief symptoms of neuropathy that associated with diabetes but this mechanism of affecting physiology associated with disease are not yet fully known [2]. DN is actually depend on several factors that can affect the function of all organs of the body either directly or indirectly, mainly due to altered glucose metabolism, which affects nervous tissue including central as well as peripheral nerves through oxidative stress mechanisms [7]. DN may be categorized into two types in which one is symptomatic another asymptomatic. Symptomatic DN is easily diagnosed due to manifestation. It may be cured by many ways. Asymptomatic diabetic peripheral neuropathy may be present up to 50% and have more risk of injury than symptomatic [8].
DN progresses due to an imbalance between nerve fiber injury and repair. The nerve harming process preferentially affects autonomic and distal sensory fibers, leading to the progressive loss of sensation. DN affects specific cellular processes including apoptosis, neuritis outgrowth, neurodegeneration, activity, and bioenergetics [9]. The manifestations of DN worsen in night time rather than day [8]. Although brain atrophy and other neurological aberrations are prominent in DN yet there is no proper mechanism which is clearly understood. Current review ascertains the molecular cascade of onset and progression of neuropathies followed by diabetes and simultaneously it helps to understand, if any molecular dependencies are there between DM and DN. lt also highlights the complications and therapeutic strategies of DN.
Causes
There are several factors that can lead to neuropathies following DM. Uncontrolled high blood glucose levels, high level of triglycerides and elevated cholesterol level in blood is found associated with DN. High blood sugar weakens walls of capillaries that supply to nerves with oxygen and nutrients. Figure l demonstrates some factors which may contribute to nerve damage following DM including age, obesity, availability of oxygen to cells, vascular supply and biochemical levels of glucose, lipids and insulin.
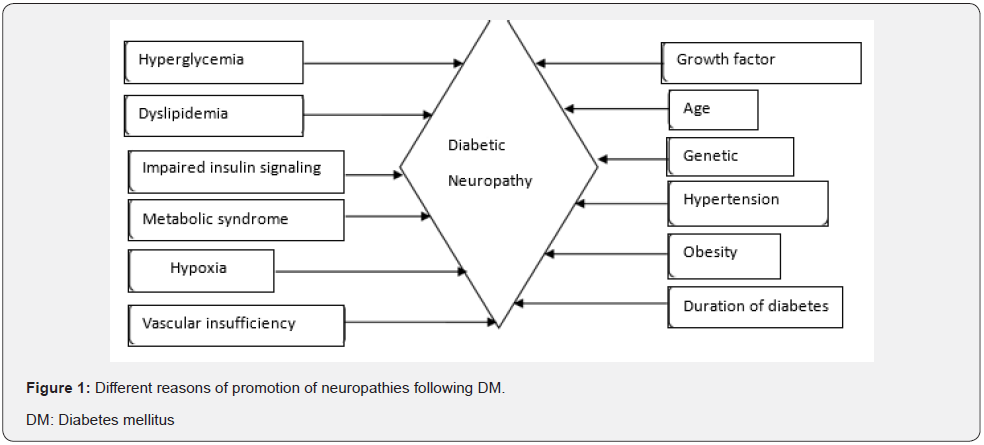
Risk Factors for DN
DN is associated with many risk factors including metabolism. Most significant and well-known initial risk factor is hyperglycemia [10]. It occurs when pancreas stop either production or secretion of insulin or both. In a second condition, insulin is present in adequate amount but cells are lacking in ability to uptake the insulin [11]. It changes several metabolic pathways like advanced glycation end products (AGEs), oxidative stress, polyol pathway influx and activation of protein kinase C (PKC) which can lead to nerve injury [12]. In another study, also reported AGEs, oxidative stress, vascular factors, growth factors and immune mechanisms to be involved in pathological processes of progression of DN [13].
It is also reported that type II diabetes insist some other pathways like TNF-alpha signaling (tumor necrosis factor), lipoxygenase pathway, glycogen synthase kinase (GSK) pathways, mitogen activated protein kinase (MAPK) pathways which are associated with onset of DN. Despite the fact that symptomatic treatment is available for diabetic neuropathy, but a few treatment options are available to eliminate the root cause [14].
A painful neuropathy has more occurrence of ache in consistently if it is defined by scoring scales and sensory loss [10,15,16]. Destruction in sensory neuron leads to hyperexcitability. During the elevated excitable period action potential generated without a stimulus is known spontaneous action linked with changes in stimulus—response activity [17- 19]. Performance of ion channels of sensory neurons can be altered by phosphorylation and trafficking that plays a critical role in excitability [20]. Nociceptors in sensory neurons are present where nerve injury is occurred and associated with voltage-gated sodium channel and identify the injurious stimuli [21]. Elevated level of triglyceride and elevated blood pressure is one more risk factors of diabetic neuropathy. Female sex is also a risk factor of DN [16].
Besides above-mentioned risk factors, smoking and alcohol consumption is found harmful in DM as it can cause nerve damage. In some cases, DN is also genetic. Obesity is one more widespread risk factor induced by weighty diet that is rich of saturated fatty acid and make possible triggering of DN that also associated with both types of DM [22]. Weight is also associated with neuropathy therefore weight loss and exercise to beneficial for neuropathy. Hypertension is considered as a peril factor for diabetic neuropathy. In DN, Na+ K“ATPase activity decreases and gets polyol disturbances which may lead to neurological disturbances and also decline the density of myelination on nerve [23]. Peripheral neuropathy is commonly caused in diabetics by a chronically high blood sugar which is shunted to polyol pathway and converts into different form like sorbitol and fructose both of which lead to reduced nerve myoinositol.
Symptoms
Individuals affected with DN who usually have clinical manifestations are called symptomatic. Those patients do not have any symptoms are known as asymptomatic type. Symptomatic patients suffer with paraesthesia as well as proprioceptive ataxia. Positive sensory symptoms occurred such as sensation of tingling, numbness, pricks and shocks. Insisted these burning feet sensation and neuropathic pain also included. Symptoms in neuropathy develop gradually. There are negative symptoms like numbness experienced by patients. Damage in sensory nerve may lead to allodynia or hyperalgesia where induced response is towards noxious stimuli. A condition that leads to increase sensitivity to tactile stimuli known as hyperesthesia while existence of pain is still later than painful stimuli is eliminated. A situation ‘areflexia’ that can lead to absent of response with stimuli and associated with large fibers [24]. Motor symptoms appeared in advanced phase of neuropathy lead to muscle atrophy.
Classification
DN is based on nerve’s types and size as well as site of nerve damage. DN also varies due to associates with various mechanisms of diabetes which involve metabolism, inflammations, vascular system and immunization. The neuropathic impediment might broadly be classified into typical and atypical DN depending upon occurrence. Distal symmetric polyneuropathy (DSP) is most prevalent form of typical length dependent diabetic neuropathy that is estimated to affect 75% of total DN cases [1]. In another way, DN is divided into acute and chronic, depending upon time duration of disorder. An acute condition is in which symptoms present suddenly and may be severe that last for weeks and month. A chronic condition is a long course developing disease. While depend on which type nerve is affected, it may be classified into three types like sensory, motor and autonomic. Further, based on which site of nerve is damaged, it may be classified into three types like generalized, focal and multifocal [8,25,26]. A simple classification is given by the American Diabetes Association for DN that is presented in figure 2.
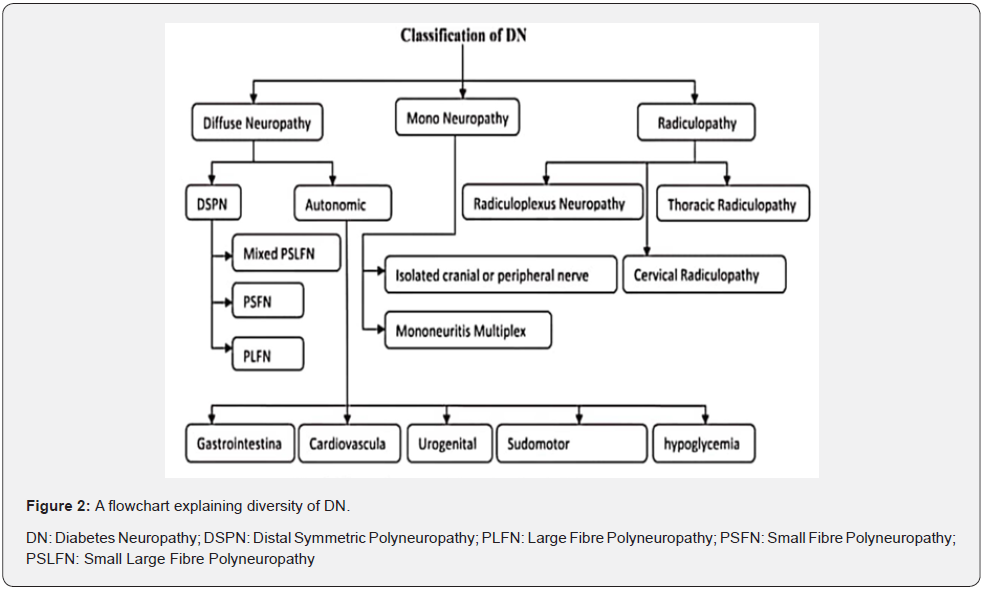
Diffuse neuropathy
This affects various parts of the body occurs in symmetrical form initially include sensation abnormality. It has earlier effect on toe and sole of lower limb feel vibration sensation. Sensory as well as motor nerve associated with diffuse neuropathy. This also included autonomic symptoms.
Distal symmetric polyneuropathy (DSPN)
It is most frequent and long-persisting disease as well as bilateral presentation that occurred in 3/4 of the patient those have neurological disorder along with DM [26,27]. In an easy way peripheral neurodegeneration disorder along with diabetes identify by the help of symptoms and marks in a conventional way. In a practical research proposed that the manner of development of DSPN is associated with several factors moreover causes still not discovered. Manner of developing DSPN related with multiple route that may lead to ultimately neurodegeneration. Survey reports of occurrence this DSPN is differ extremely [28,29]. Nevertheless, according to many confirmation reports and Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Albers & Martin et al. [26,30] mention that DSPN appeared in minimum 20% patients who suffered DM type l disorder from a large duration while DSPN occurred in minimum 10%-15% with type 2 diabetes patients who recently identify [31,32]. After first time recognition with DSPN, rate of prevalence increased up to 50% when patient have experienced already ten years [29,33]. Earliest Symptoms of DSPN is neuropathic pain that leads to patients to search cure [34]. Neurological pain related with DSPN occurred in people up to 50%. In the night pain due to nerve injury along with diabetes get more worse rather than day period. In the initial phase symptoms associated with small nerve fiber such as burning, stabbing. Due to participation of larges fibers get outcome abnormal sensation and pins and needles impaired with vibration and feel numbness in lower limb including with disturb of Achilles tendon response [1]. These symptoms get worse over time duration with disease and lead to sensory ataxia. In a severe condition, it may cause ulcers in feet, consequently amputation of limb may occur [34].
Autonomic diabetic neuropathy (ADN)
It is mostly correlated with DSPN and hardly appeared in separation [24]. It is minimum acknowledged in spite of its notable effect on quality of this disease suffering person and may lead to minor to severe [35]. This is described by automatic nerve like sympathetic nerve as well as parasympathetic nerve disorder along with diabetes. It has affected mostly on internal organs that innervated by autonomic nerves such as urinary bladder and gonads including gastro-intestine too. It may have many subtypes based on which organ is injured like cardiac autonomic neuropathy (CAN), gastro-intestinal autonomic neuropathy, erectile dysfunction (ED), urogenital autonomic neuropathy. In CAN, Patients feel discomfort and fatigue in addition loss of consciousness associated with up and down from the normal level of heartbeat. CAN along with diabetes can make a reason of cardiac vascular abnormalities such as blood pressure, heartbeat and disrupt the ability to innervate of cardiac muscle because cardiovascular system controlled by autonomic nerves. CAN is most dauce among several type of autonomic diabetic neuropathy and least elucidate too [46-38]. According to ADA, Diarrhoea and constipation is main manifest of gastrointestinal autonomic neuropathy due to injurious of that nerve which innervate the small intestine. Sometime other symptoms such as emesis, bloating, wimble, loss of hunger also occurred because of stomach nerve injury [3]. In the urogenital autonomic neuropathy erectile abnormal functioning is present in man Type 1 DM correlated with sugar regulation because damaging of that nerve which is responsible for erection. Glucose control is related with a lower incidence of erectile dysfunction (ED) in men with type l diabetes [39,40]. Due to damage to nerve which innervates urinary bladder, paralysis in bladder can occur. Abnormalities in nerve fibers that regulate the function of sweat glands may lead to complete absence of sweating, rough skin, unusual sensitivity to warm materials [41,42].
Mononeuropathy
This may be further divided into isolated cranial or peripheral nerve and mononeuritis multiplex. In the case of isolated nerve that may lead to painful neuropathy as well as motor instability that induced by scattering of single nerve regarding central as well as peripheral. One another term is mononeuropathy multiplex in which a group of nerve is damage and lead to paralysis as well as dysesthesia [43].
Radiculopathy
It may be further divided into lambosacral radiculoplexus neuropathies (RPN) and thoracic radiculopathy. RPN is correlated with ischemic injury in lumber sacral (L5-SI) nerve that supply into lower limb such as sciatic nerves [44]. Only 1% patient of diabetic neuropathy experiences with RPN [45]. Symptoms as well as any nerve disorder may not be seen in initial phase of this atypical radiculopathy [46]. In cervical radiculopathy, disabling condition refers to damage in nerve root of cervical spine and lead to pain in neck. Some inflammation factors such as cytokines secrete from injured spinal disk of cervical and progressively lead to swelling [47].
Mechanism of generation of DN (Figure 3)
DN is a unique disabling disorder of neurons especially of peripheral nervous system that preferentially affects sensory nerves, and later to damage motor nerves. Among the sensory nerves longest sensory nerve injures initially and later to proximally [48]. In progressive DN, damage starts with terminal of sensory neurons and associated Schwann cell and then it moves toward cell body. In which way sensory nerves is affected is not clear and also a topic of debate yet [3].
Excessive amount of glucose affects multiple cells of nervous system including axon and Schwann cell as well as dorsal root ganglion. lt leads to further formation of reactive oxygen species (ROS) and advanced glycation end product (AGE). Increased amounts of ROS progressively mitochondrial dysfunction and as a result of these event loss of ATP production. In following cause of activation of stress pathway and pro-inflammatory pathway that promote endoplasmic reticulum (ER) stress and DNA damage as well as apoptosis and ultimately to lead nerve injury. Diabetic peripheral neuropathy (DPN) is associated with nerve degeneration and atrophic alteration in peripheral nervous system as well as central nervous system [49,50]. Hyperglycemia leads to stress in progressive demyelination of Schwann cells that ultimately causes nerve damage due to disruption of neuronal support. Hyperglycemia included with dyslipidemia leads to neuronal death and vascular system thickening as evident in capillary basement membrane and endothelial Hyperplasia [50]. A recent study considered that hyperglycemia and dyslipidemia also include of vascular risk factors induces disrupting pathways that’s lead to downstream damaging of microvessel endothelium, axonal degeneration and Schwann cell [51].

In current advances, it is suggested that cumulative effect of these processes lead to neurodegeneration via different molecular pathways as well as mitochondrial dysfunction. Depend on mechanism and pathology of neuronal death do not distinguish painful and painless DPN [52]. A number of genes are associated with neuropathic pain in diabetes are OPRM 1, IL-6, HLA, and GCH 1, COMT TNFA [53]. Vitamin D is responsible to decrease perception of pain in diabetic nerve disorder patients [54].
Mechanism of promotion and progression of DN is associates with several molecular pathways that lead to disease Etiology and development of this disorder. Some significant pathways described here are advanced glycation end products (AGEs), protein kinase C (PKC), polyol pathway activation, hexoamine pathway, signaling pathway, oxidative stress and reactive oxygen species (ROS). Still, more focused research is needed for better understanding of mechanism of nerve injury along with diabetes.
Polyol pathway flux and aldose reductase
One of the most important pathways to understand the mechanism of DN is Polyol pathway. In this pathway intracellular elevated level of glucose is induced to convert into sorbitol (polyol) mediated by aldose reductase enzyme. These changes lead to hyper-osmolarity due to collection of large amounts of sorbitol in nerve cell following by outflow of 2-aminoethane sulfonic acid and a carbocyclic sugar such as myoinositol [51]. As a result, myoinositol and taurine level down in cell. Myo-inositol plays a significant role in usual function of NA+/K+ ATPase pump that correlated usual conduction velocity of nerve. Decline in myoinositol leads to lack in production of ATP progressively less activity of PKC. AS a result, these changes induce oxidative stress. This oxidative stress may cause of poly (ADP-ribose), polymerase (PARP) activation. This activity stimulates some death pathway and ultimately leads to apoptosis. In additional step Sorbitol further transforms into fructose mediated by sorbitol dehydrogenase (SDH) this subsequently depletion of NAD+ subsequently depletion of NADPH which is essential for antioxidant formation such as glutathione and nitric oxide (NO) which causes elevated level of ROS and lead to a major problem such as abnormal functioning by cell organs and in a severe condition may cause of cell death. Consequently, a term is used for the process ‘Metabolic Flux’ [55]. Several studies have established that inhibitor of aldose reductase (ARI) prevents most of complication of neuropathy. Polyol pathways enzymes may be manipulated that is leads to changes in expression in a result due to highly activeness of aldose reductase enzyme causes excessive metabolism of glucose to sorbitol [56-59].
Role of Hexosamine
It is well known that excessive amount of glucose is converted into fructose-6- P in glycolysis, but when fructose-6-P in changes into uridine diphosphate-N-acetyl glucosamine ultimately it promotes transformation of growth factors, and inhibition of plasminogen activator regarding neurological disorder [35].
AGEs
It has been seen that excessive amount of AGEs is deposited nearly in every component of diabetic nerve tissue as well as in stromal collagens and Schwann cells [60,61].
Dyslipidemia
During cellular studies it was revealed that a high amount of saturated fatty acid like palmitate is associated with sensory neurons lead to mitochondrial dysfunction and changes in the number of ATP formation [62]. However, palmitate induced mitochondrial abnormalities are prevented by supplementation of mono saturated fatty acid to neurons.
Potential strategies of treatment
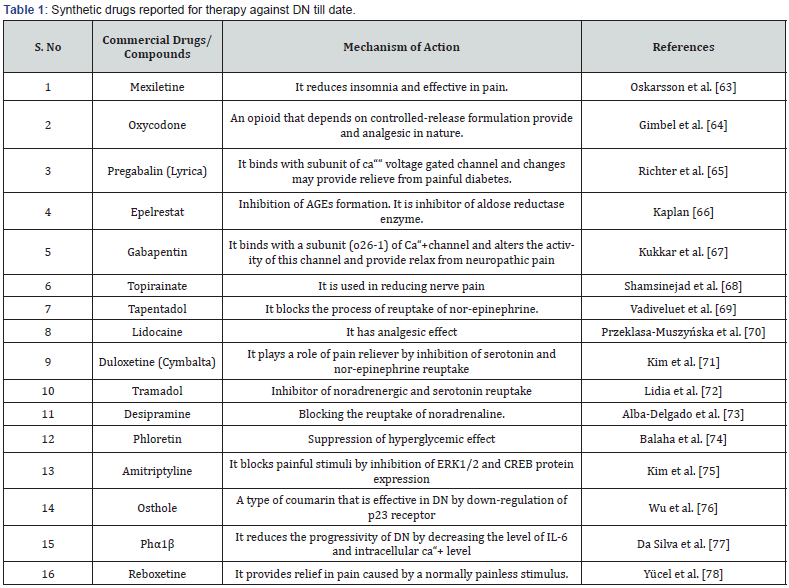
A number of medications are used for the treatment of DN yet none of them are found safe. Some of them are used for relieving nerve pain (eg mexiletine and topiramate) whereas others target specific organelles as mitochondria. Duloxetine is a pain reliever in DN which exerts its effects by impacting neurotransmitters like serotonin and epinephrine. Even after global involvement of decades in investigations, no single remedy is there which is safe for DN. Table 1 demonstrates some synthetic medications used in DN till now.
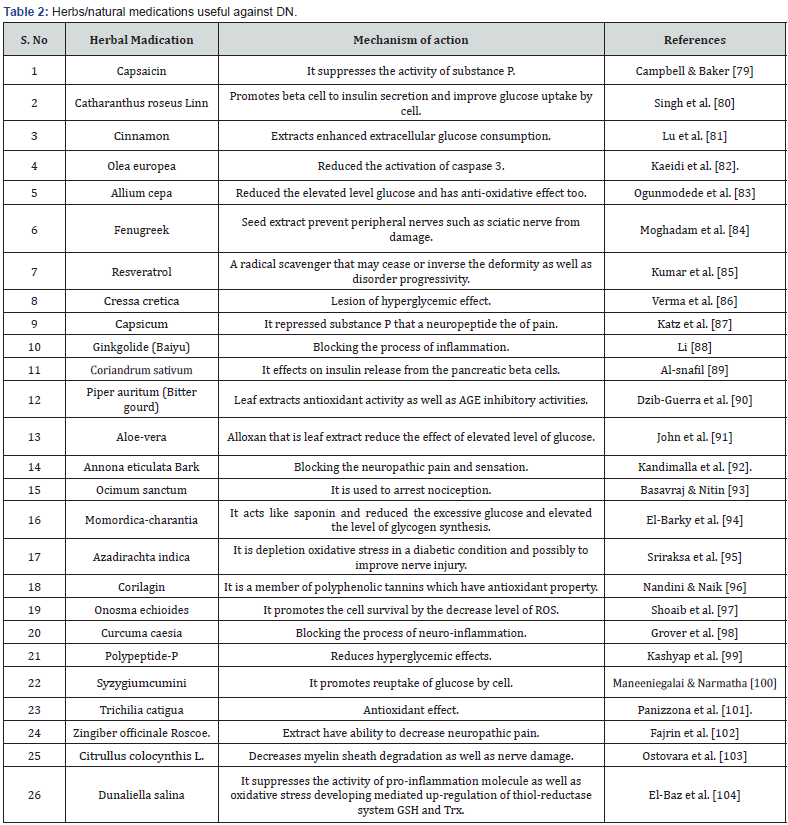
Even though, numerous drugs are attempted till date but none of them has become able to cure DN. In present time, several natural compounds and herbs have been reported that can ameliorate pathological effects of DN. Natural compounds have been reported to be effective in restoring sensation in feet, decreasing hyperglycemic effects and healing the ulcer. Herbal ingredients that are reported to prevent from DN and have no side effects are listed in table 2.
Summary and Conclusion
DN is not a single disease but rather a group of syndromic complications [105]. In DN, nervous tissue is greatly atrophied because it affects both CNS and PNS abruptly. There are various studies which exhibit severity and different molecular pathways that interplay for promotion of DN. None of the findings present an actual molecular cascade for progression of neuronal atrophy following diabetes. In current review, we studied a possible connection between various molecular and biochemical cascades such as glycolysis, dyslipidemia and polyol pathway that can trigger mitochondrial dysfunctions and microvascular damage in DN. Treatment strategies of DN, whether using synthetic or natural compounds, have their own limitations. So, in perspective of therapy, this review provides significant research gap for making an attempt to investigate a successful drug and its molecular target for DN. Currently, there is a need to develop new techniques for diagnosis of DN as early as possible. Since there is no certain cure for DN, it is cardinal to make attempts for developing drugs that trigger those pathways that are responsible to prevent the diabetic neurodegeneration together with inhibition of disease developing mechanism .
Acknowledgement
Authors are indebted to all mighty ‘God’ for giving inspiration to take a step for welfare of humans by writing this manuscript.
Ethical Statement
a) Not applicable Since, this is a review article, we did not require any animals for investigation. So, there is no need of ethical clearance by any committee.
References
- Bansal V, Kalita J, Misra UK (2006) Diabetic neuropathy. Postgrad Med J 82(964): 95-100.
- Schreiber AK, Nones CFM, Reis RC, Chichorro JC, Cunha JM (2015) Diabetic neuropathic pain: Physiopathology and treatment. World J Diabetes 6(3): 432-444.
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE (2019) Diabetic Neuropathy Nature Reviews. Disease Primers 5(41): 92-101.
- Fukrapti R, Naqiyya N (2020) Rimpang Kunyit sebagai Terapi Pencegahan Neuropati Jurnal Penelitian Perawat Profesional 2(2): 111-118.
- Cho NH, Shaw JE, Karuranga S, Huang Y, Femandes JDR, et al. (2018) IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138: 271-281.
- International Diabetes Fedearation (2019) IDF Diabetes Atlas (9th edn).
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865): 813-820.
- Pop-Busui R, Boulton AJM, Feldman EL, Beril V, Freeman R, et al. (2017) Diabetic Neuropathy: A position statement by the American Diabetes association. Diabetes Care 40(l): 136-154.
- Gardiner NJ, Freeman OJ (2016) Can Diabetic Neuropathy Be Modeled In Vitro? International Review of neuropathy 127: 53-87.
- Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, et al. (2016) The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic Pain 157(5): 1132-1145.
- Alam U, Asghar O, Azmi S, Malik RA (2014) General aspects of diabetes Handb Clin Neurol 126: 211-22.
- Duby JJ, Campbell RK, Setter SM, Rasmussen KA (2004) Diabetic neuropathy: An intensive review. Am J Health Syst Pharm 61(2): 160-173.
- Boulton AJM (2004) Diabetic somatic neuropathies. Diabetes Care 27(6):1458-1486.
- Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, et al. (2018) Molecular mechanism of diabetic neuropathy and its pharmacotheraputic targets. Euro J Pharmacol 833: 472-523.
- Keller JN, Hanni KB, Markesbery WR (1999) Oxidized low-density lipoprotein induces neuronal death: implications for calcium, reactive oxygen species, and caspases. J Neurochem 72(6): 2601-2609.
- Raputova J, Srotava V, Vlckova E, Sommer C, Üçeyler N, et al. (2017) Sensory phenotype and risk factors for painful diabetic neuropathy: a cross- sectional observational study. Pain 158(12): 2340-2353.
- Suzuki Y, Sato I, Kawanishi M, Mizumura K (2002) Lowered response threshold and increased responsiveness to mechanical stimulation of cutaneous nociceptive fibers in streptozotocin-diabetic rat skin in vitro-correlates of mechanical allodynia and hyperalgesia observed in the early stage of diabetes. Neurosci Res 43(2): 171-178.
- Orstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, et al. (2006) Abnormal function of C-fibers in patients with diabetic neuropathy. J Neurosci 26(44): l 1287-11294.
- Garcia-Perez E, Schonberger T, Sumalla M, Stierstorfer B, Sola R, et al. (2018) Behavioural, morphological and electrophysiological assessment of the effects of type 2 diabetes mellitus on large and small nerve fibres in Zucker diabetic fatty, Zucker lean and Wistar rats. Eur J Pain 22(8):1457-1472.
- Bennett DLH, Woods CG (2014) Painful and painless channelopathies. Lancet Neurol 13(6): 587-599.
- Dubin AE, Patapoutian A (2010) Nociceptors: the sensors of the pain J Clin Invest 120(11): 3760-3772.
- Callaghan BC, Gao LL, Li YF, Zhou Xi, Reynolds E, et al. (2018) Diabetes and Obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol 5(4): 397-405.
- Forrest KY, Maser RE, Pambianco G, Becker DJ, Orchard TJ (1997) Hypertension as a risk factor for diabetic neuropathy: a prospective study. Diabetes 46(4): 665-670.
- Sinnreich M, Taylor BV, Dyck PJ (2005) Diabetic Neuropathies, Classification, Clinical Features and Pathophysiological Basis. The Neurologist 11(2): 63-79.
- Thomas PK (1997) Classification, differential diagnosis, and staging of diabetic peripheral Am diabetes assoc Diabetes 46: 54-57.
- Martin CL, Albers JW, Pop-Busui R (2014) Neuropathy and Related Findings In The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 37(1): 31-38.
- Dyck PJ, Albers JW, Andersen H, Arezzo JC, Biessels GJ, et al. (2011) Diabetic polyneuropathies: Update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 27(7): 620-628.
- Boulton A, Malik R (2010) Diabetes mellitus: neuropathy. In: Jameson JL, De Groot LJ, (Eds.), Endocrinology: Adult and Pediatric, Saunders Elsevier, Philadelphia, USA, p.27.
- Ang L, Jaiswal M, Martin C, Busui PR (2014) Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep 14(9): 528.
- Albers JW, Herman WH, Busui PR, Feldman EL, Martin CL, et al. (2010) Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type l diabetes during the Epidemiology of Diabetes Intervention sand Complications (EDIC) Study. Diabetes Care 33(5):1090- 1096.
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH (1993) A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 36(2): 150-154.
- UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonyl ureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352(9131): 837-853.
- Pop-Busui R, Lu I, Brooks MM, Albert S, Althouse AD, et al. (2013) Study Group. lmpact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care 36(10): 3208-3215.
- Tesfaye S, Kempler P (2005) Painful diabetic neuropathy. Diabetologia 48: 805-807.
- Edwards JL, Vicent AM, Cheng HT, Feldman EL (2008) Diabetic neuropathy: mechanism to management. Pharmacol Therapeut 120(l): 1-34.
- DCCT Research Group (1998) the effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 41(4): 416- 423.
- Vinik AI, Maser RE, Mitchell BD, Freeman R (2003) Diabetic Autonomic Neuropathy. Diabetes Care 26(5): 1553-1579.
- Pop-Busui R (2012) What do we know and we do not know about cardiovascular autonomic neuropathy. J Cardiovasc Transl Res 5: 463-478.
- Van Den Eeden SK, Sarma AV, Rutledge BN, Cleary PA, Kusek JW, et al. (2009) Diabetes Control and Complications Trial/Epidemiology of Diabetes Research Group. Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type l diabetes. Diabetes Care 32(4): 664-670.
- Wessells H, Penson DF, Cleary P, Rutledge BN, Lachin JM, et al. (2011) Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive glycemic therapy on erectile function in men with type I diabetes. The Journal of Urology 185(5): 1828-1834.
- Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI (2013) Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther 15(11): 948-953.
- Smith AG, Lessard M, Reyna S, Doudova M, Singleton JR (2014) The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes complications 28(4): 511-516.
- Chabla PL, Wright TB (2019) Mononeuropathy, Mononeuropathy Multiplex, and Other Neuropathies. In: Abd-Elsayed A. (Eds.), Pain. Springer, Cham, USA, pp. 919-923.
- McCormack EP, Alam M, Erickson NJ, Cherrick AA, Powell E, et al. (2018) Use of MRI in diabetic lumbosacral radiculoplexus neuropathy: case report and review of the literature. Acta Neurochirurgica 160: 2225-2227.
- Tracy JA, Dyck PJB (2008) The spectrum of diabetic neuropathies. Phys Med Rehabil Clin N Am 19(1): 1-26.
- Su JL, Tan WC, Chao CC, Tsai CM, Wu CH (2010) An initial presentation of flank pain caused by thoracic disc herniaton. J emerg crit care med 21: 22l-226.
- Woods BI, Hilibrand AS (2015) Cervical J spinal Disord Tech 28(5): E251-259.
- Callaghan BC, Gallagher G, Fridman V, Feldman EL (2020) Diabetic Neuropathy: What does the future hold? Diabetologia 63(5): 89l-897.
- Tesfaye S, Selvarajah D, Gandhi R, Greig M, Shillo P, et al. (2016) Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance Pain 157: 72-80.
- Sloan G, Shillo P, Selvarajah D, Wu I, Wilkinson ID, et al. (2018) A new look at painful diabetic neuropathy. Diabetes Res Clin Pract 144: 177-191.
- Feldman EL, Nave KA, Jensen TS, Bennett DLH (2017) New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93: 1296-1313.
- Shillo P, Sloan G, Greig M, Hunt L, Selvarajah D, et al. (2019) Painful and painless diabetic neuropathies: what is the difference? Curr Diab Reports 19(6): 32-44.
- Veluchamy A, Hebert HL, Meng W, Palmer CNA, Smith BH (2018) Systematic review and meta- analysis of genetic risk factors for neuropathic pain. Pain 159(5): 825-848.
- Alam U, Nelson AJ, Cuthbertson DJ, Malik RA (2018) an update on vitamin D and B deficiency in the pathogenesis and treatment of diabetic neuropathy: a narrative review. Future Neurol 13(3):135-142.
- Oates PJ (2008) Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets 9(l): 14-36.
- Ng TF, Lee FK, Song ZT, Calcutt NA, Lee AY, et al. (1998) Effects of sorbitol dehydrogenase deficiency on nerve conduction in experimental diabetic Diabetes 47: 961 -966.
- Song Z, Fu DTW, Chan YS, Leung S, Chung SSM, et al. (2003) Transgenic mice over expressing aldose reductase in Schwann cells show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress. Mol Cell Neurosci 23(4): 638-647.
- Uehara K, Yamagishi SI, Otsuki S, Chin S, Yagihashi S (2004) Effects of polyol pathway hyperactivity on protein kinase C activity, nociceptive peptide expression, and neuronal structure in dorsal root ganglia in diabetic mice. Diabetes 53(12): 3239-3247.
- Ho CME, Lam KSl, Chen YS, Yip JCW, Arvindakshan M, et al. (2006) Aldose Reductase- Deficient Mice Are Procted from Delayed Moter Nerve Conduction velocity, Increased c-Jun NH2-Terminal Kinase Activation, Depletion of Reduced Glutathione, Increased Superoxide Accumulation, and DNA Damage. Diabetes 55(7): 1946-1953.
- Yagihashi S (1995) Pathology and pathogenetic mechanisms of diabetic neuropathy. Diabetes Metabol Res Rev 11(3): 193-225.
- Sugimoto K, Nishizawa M, Yagihashi S (2008) Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des 14(10): 953-961.
- Rumora AE, Lentz SI, Hinder LM, Jackson SW, Valesano A, et al. (2018) Dyslipidemia impairs mitochondrial trafficking and function in sensory neurons. FASEB J 32(l): 195-207.
- Oskarsson P, Ljunggren JG, Lins PE (1997) Efficacy and Safety of Mexiletine in the Treatment of Painful Diabetic Neuropathy. The Mexiletine Study. Diabetes Care 20(10): 1594-1597.
- Gimbel JS, Richards P, Russell KP (2003) Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology 60(6): 927-934.
- Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, et al. (2003) Relief of painful diabetic peripheral neuropathy with pregabalin: A randomized, placebo-controlled trial. J Pain 6(4): 253-260.
- Kaplan H (2013) Painful diabetic peripheral neuropathy. S Afr J Diabetes Vascul Dis 10(1): 23-31.
- Kukkar A, Bali A, Singh N, Jaggi AS (2013) Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res 36: 237-251.
- Shamsinejad S, Davati A, Roughani M, Ghasemlouie A, Afshinmajd S (2018) Evaluation of Topiramate Efficacy on Neuropathic Pain in Patients with Diabetic Acta Medica Iranica 56(12): 764-768.
- Vadivelu N, Kai A, Maslin B, Kodumudi G, Legler A, et al. (2015) Tapentadol extended release in the management of peripheral diabetic neuropathic pain. Ther Clin Risk Manag 11: 95-105.
- Przeklasa Muszyńska A, Kocot Kępska M, Dobrogowski J, Wiatr M, Mika J (2016) Intravenous lidocaine infusions in a multidirectional model of treatment of neuropathic pain Pharmacological Reports 68(5):1069-1075.
- Kim W, Chung Y, Choi S, Min BI, Kim SK (2017) Duloxetine Protects against Oxaliplatin-Induced Neuropathic pain and Spinal Neuron Hyperexcitability in Rodents. Int J Mol Sci 18(12): 2626.
- Lidia V, Mico JA, Berrocoso E (2017) Discovery and development of Tramadol for the treatment of pain. Expert Opin Drug Discov 12(12): 1281-1291.
- Alba DC, Llorca TM, Jaun AM, Esther B (2018) the onset of treatment with the antidepressant desipramine is critical for the emotional consequences of neuropathic pain. Pain 159(12): 2606-2619.
- Balaha M, Kandeel S, Kabel A (2018) Phloretin either alone or in combination with duloxetine alleviates the STZ-induced diabetic neuropathy in rats. Biomed Pharmacother 101: 82l-832.
- Kim Y, Kwon SY, Jung HS, Oark YJ, Kim YS, et al. (2019) Amitriptyline inhibits the MAPK/ERk and CREB pathway and proinflammtory cytokines through A3AR activation in rat neuropathic Pain models. Korean J Anesthesiol 72(1): 60-67.
- Wu B, Sheng X, Xu Z, Zhang Y, Dan Y (2020) Osthole relieves diabetics cardiac autonomic neuropathy associated with P23 receptor in rat stellate ganglia. Brain Research Bulletin 157: 90-99.
- Castro Junior CJd, Pereira EMR, Binda NS, Da silva JF, Cordeiro MN, et al. (2020) The inhibitory effect of Pholb toxin on diabetic neuropathic pain involves the CXCR4 chemokine receptor. Pharmacol Rep 72(1): 47-54.
- Yucel NT, Can OD, Ozkay UD (2020) Catecholaminergic and opioidergic system mediated effects of reboxetine on diabetic neuropathic pain. Psychopharmacology 237: 1131-1145.
- Campbell RK, Baker DE (1990) Pharmacy Update: New Drug Update: Capsaicin. The Diabetes Educ 16(4): 313-316.
- Singh SN, Vats P, Suri S, Shyam R, Kumria MML, et al. (2001) Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic J Ethnopharmaco. 76(3): 269-277.
- Lu Z, Jia Q, Wang R, Wu X, Wu Y (2011) Hypoglycemic activities of A- and B-type procyanidin oligomer-rich extracts from different Cinnamon barks. Phytomedicine 18(4): 298-302.
- Kaeidi A, Esmaeili-Mahani S, Sheibani V, Abbasnejad M, Rasoulian B, et al. (2011) Olive (Olea europaea ) Leaf extract attenuates early diabetic neuropathic pain through prevention of high glucose-induced apoptosis: in vitro and in vivo studies. J Ethnopharmacol 136(l): 188-196.
- Ogunmadede OS, Saali LC, Oguhlade B, Akunna GG, Oyewopo AO (2012) An evaluation of the hypoglycemic antioxidant and hepatoprotective potentials of Onion (Allium Cepa L.) on alloxon induced diabetes rabbits. Int J Pharmacol 8(l): 21-29.
- Moghadam FH, Vakili-Zarch B, Shafiee M, Mirjalili A (2013) Fenugreek seed extract treats peripheral neuropathy in pyridoxine induced neuropathic mice. EXCLI Journal 12: 282-290.
- Kumar A, Negi G, Sharma SS (2013) Neuroprotection by Resveratrol in Diabetic Neuropathy Concepts and Mechanism. Curr Med Chem 20(36): 4640-4645.
- Verma N, Jha KK, Chaudhary S, Garg V, Ahmad S, et al. (2014) Assessment of Antidiabetic Potential of Cressa Cretica Linn in Streptozotocin-Induced Diabetic Rats. Int Adv Res Innov 1: 181-84.
- Katz NP, Mou J, Paillard FC, Turnbull B, Trudeau J, et al. (2015) Predictors of Response in Patients with Postherpetic Neuralgia and HIV-Associated Neuropathy Treated with the 8% Capsaicin Patch (Qutenza). Clin J Pain 31(10): 859-866.
- Li JW (2015) The efficacy of ginkgolide with methyl cobalamin in treating diabetic peripheral neuropathy. Shenzhen J Int Trad Chin West Med 25: 57-58.
- Al Snafil AE (2016) the chemical constituents and therapeutic importance of Cressa cretica- A review. IOSR J Pharm 6(6): 39-46.
- Dzib Guerra WD, Escalante EF, Garcia SK, Derbré S, Blanchard P, et al. (2016) Anti-advanced glycation end product and free radical scavenging activity of plants from the Yucatecan flora. Pharmacogn Res 8(4): 276-280.
- John J (2017) Evaluation of Hypoglycemic Effect of Aloe Vera on Allaxon Induced Diabetic Int J lnf Res Review 4(3): 3865-3868.
- Kandimalla R, Dash S, Kalita S, Choudhury B, Malampati S, et al. (2017) Bioactive Fraction of Annona reticulata Bark (or) Ziziphus jujuba Root Bark along with Insulin Attenuates Painful Diabetic Neuropathy through Inhibiting NF-xB Inflammatory Cascade. Front cell neurosci 11: 73.
- Basavraj P, Nitin M (2017) Antinociceptive activity of Tulsi Amirt (A Polyherbal Formulation) in selective pain induced models in rats. Res J Pharmacol Pharmacodyn 9(4):173-177.
- El Barky AR, Hussein SA, Alm EAA, Hafez YA, Mohamed T (2017) Saponins and their potential role in diabetes mellitus. Diabetes Management 7(l): 148-158.
- Sriraksa N, Kongsui R, Thongrong S, Duangjai A, Hawiset T (2019) Effect of Azadirachta indica flower extract on functional recovery of sciatic nerve crush injury in rat models of Exp Ther Med. 17(l): 541-550.
- Nandini H, Naik P (2019) Action of corilagin on hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Chem Biol Interact 299: 186-193.
- Shoib A, Dixit RK, Badruddeen, Rahman MA, Bagga P, et al. (2019) Cure of Human Diabetic neuropathy by HPCL Validated bark extract of Onosma echioides L. root. Nat Prod Res 33(18): 2699-2703.
- Grover M, Shah K, Khullar G, Gupta J, Behl T (2019) Investigation of the utility of Curcuma caesia in the treatment of diabetic neuropathy. Royal Pharmaceutical Society. J Pharm Pharmacol 71(5): 725-732.
- Kashyap H, Gupta S, Bist R (2019) Impact of Active Antihyperglycemic Components as Herbal Therapy for Preventive Health Care Management of Diabetes. Curr Mol Med 19: 12-19.
- Maneernegalai S, Narmatha M (2019) An In-Vitro Study of Syzygiurn cumini Seed Extract on Glucose Uptake Activity in L-6 Cell Lines. J Drug Deliv Ther 9(4-A): 256-259.
- Panizzona CPNB, Miranda Netoa MH, Ramalhoa FV, Longhinib R, Mellob JCP, et al. (2009) Ethyl Acetate Fraction from Trichilia catigua Confers Partial Neuroprotection in Components of the Enteric Innervation of the Jejunum in Diabetic Rats. Cell Physiol Biochem 53: 76-86.
- Fajrin FA, Nurrochmad A, Nugroho AE, Susilowati R (2019) The improvement of pain behavior and sciatic nerves morphology in mice model of painful diabetic neuropathy upon administration of ginger (Zingiber officinale) extract and its pungent compound, 6-shogaol. J Nat Sci Bio Med 10(2): 149-156.
- Ostovar M, Akbar A, Anbardar MH, Raji A, Salmanpour M, et al. (2020) Effects of Citrullus colocynthis in a rat model of diabetic neuropathy. J Integr Med 18(1): 59-67.
- El Baz FK, Salama A, Rania A, Salama A (2020) Dunaliella salina Attenuates Diabetic Neuropathy Induced by STZ in Rats: Involvement of Thioredoxin. Hindawi Bio Med Res International 1 -
- Albers JW, Busui R (2014) Diabetic neuropathy: mechanisms, emerging treatments, and Curr Neurol Neurosci Rep 14(8): 473-475.






























