Different Effects of Canagliflozin and Perindopril in the Improvement of Arterial Stiffness in Type 2 Diabetic Patients
Ramiro Sanchez1*, Maria J Sanchez2, Franco Pessana3,4 and Agustin J Ramirez1,3
1Hypertension and Metabolic Unit, University Hospital, Favaloro Foundation, Argentina
2Mater Dei Hospital, Buenos Aires, Argentina
3Institute of Translational Medicine, Transplantation and Bioengineering (IMeTTYB), CONICET-Favaloro University, Argentina
4Electronic Department, National Technological University, Argentina
Submission: November 09, 2021; Published: November 17, 2021
*Corresponding author: Sanchez, Ramiro A, Arterial Hypertension and Metabolic Unit, University Hospital, Fundación Favaloro, Belgrano 1782 P: 4 (1093), Buenos Aires, Argentina
How to cite this article: Ramiro Sanchez, Maria J Sanchez, Franco Pessana, Agustin J Ramirez. Different Effects of Canagliflozin and Perindopril in the Improvement of Arterial Stiffness in Type 2 Diabetic Patients. Curre Res Diabetes & Obes J 2021; 15(2): 555908.DOI: 10.19080/CRDOJ.2021.15.555908
Abstract
Introduction: In type 2 diabetic patients (DM), after 6 months under perindopril or Canagliflozin, we previously reported a significant cf-PWV improvement related to high serum uric acid (SUA), stronger than the observed with blood pressure.
Aims: To evaluate, in DM patients with arterial hypertension and normal renal function, the effects of canagliflozin or perindopril on SUA, 24hs ambulatory BP monitoring (ABPM) and cf -PWV.
.Methods: For 6 months, 20patients with DM and hypertension (59±4y; 10 females) received Metformin 2000mg, Amlodipine 10mg and Canagliflozin 300mg daily. Other 20 type 2 diabetic patients (62±4y, 8 female) received Metformin 2000mg, Amlodipine 10mg and Perindopril10mg daily. Parameters evaluated: HbA1C, fasting blood glucose level, glomerular filtration rate, SUA, urine uric acid excretion (urUA), 24h ABPM and cf-PWV. All evaluations were determined at baseline and after a 6-month continued treatment.
Results: After 6 months treatment, Canagliflozin induced a decrease in cf-PWV (12.0±0.67 to 8.5±0.45m/sec, p<0.001), HbA1C (8.5±0.11 to 7.0±0.09%, p<0.001), SUA (7.9±0.22 to 6.9±0.45mg%, p<0.05) and ABPM (142.0±2.04/99.0±1.34 to 134.0±2.01/85.0±1.57mmHg, p<0.001).Perindopril only reduced ABPM (140.0±2.10/95.0 to 133.0±1.57/84.0±1.79mmHg, p<0.001) and cf-PWV (11.6±0.89 to 8.0±0.45m/sec, p<0.05).Only under Canagliflozin the urUA increased (350.0±4.47 to 580.0±6.71mg/24hs, p<0.001). A strong correlation was found between variations in SUA and cf-PWV(r: 0.69, p<0.025),showing that for each 1mg% reduction in SUA there is a decrease of 0.88m/sec in PWV
Conclusions: Canagliflozin and Perindopril ameliorates arterial stiffness in patients with type 2 DM and hypertension by reducing SUA that seems related, among other mechanisms, with a vascular intrinsic effect.
Keywords: Perindopril; Canagliflozin; PWV; Arterial stiffness; Arterial hypertension; T2DBT; Diabetes mellitus; Uric acid
Abbreviations: BP: Blood Pressure; SD: Standard Deviation; ABPM: Ambulatory Blood Pressure Monitoring; SUA: Serum Uric Acid; ACEI: Angiotensin Converting Enzyme Inhibitor; SGLT2: Sodium–Glucose Cotransporter-2
Introduction
In type 2 diabetes mellitus, the blood pressure (BP) reduction has an important impact in decreasing macro vascular and micro vascular complications, as revealed by important studies such as the UK Prospective Diabetes Study Trial [1] and the Heart Outcomes Prevention Evaluation study [2]. Similarly, in patients with types 1 and 2 diabetes, benefits have been demonstrated on clinical outcomes reduction in studies using several classes of antihypertensive drugs [3]. However, in most diabetic patients, a combination of antihypertensive drugs is required to achieve the target BP goals [3].
Sodium–glucose cotransporter-2 (SGLT2) inhibitors (SGLUT2i), including dapagliflozin, canagliflozin and empagliflozin, are a newly developed class of oral anti diabetic drugs with a unique mechanism of action [4]. In the kidney, the action is in the proximal convoluted tubules, and prevents renal glucose reabsorption, that facilitates the urinary glucose excretion [5-8]. Blood pressure reduction has been also observed in recent trials such as Canvas [9], Credence [10] and EmpaREG [11], suggesting a complementary effect of these drugs along with the glucose lowering effect. Moreover, we recently found [12], in type 2 diabetic patients with arterial hypertension, a comparative antihypertensive effect between Canagliflozin and perindopril, an angiotensin converting enzyme inhibitor (ACEI). This BP reduction was observed either on 24hs ambulatory blood pressure monitoring, central systolic blood pressure or office measured systolic and diastolic blood pressure.
Arterial stiffness is an established risk factor of cardiovascular events and mortality [13-15]. In particular, the physiologic determinants, such as pulse pressure, mean arterial pressure and central arterial pressure, have been linked to cardiovascular outcomes and may provide additional information for cardiovascular risk prediction [13-15].
In a recent study, including hypertensive type 2 diabetic patients, in which we added on top of their treatment canagliflozin or perindopril, we found a similar improvement on cf-PWV, after 6 months treatment [12]. Furthermore, this data was in accordance with a recent study where empagliflozin showed an improvement on arterial stiffness in type 2 diabetic hypertensive patients [16].
Higher plasma uric acid concentration is responsible for an increase in the cardiovascular morbidity and mortality risk [17] as it was recently shown either in hypertension [18] or diabetes [19], in both middle-aged [20] and elderly individuals [21].
Different mechanisms have been suggested to explain the augmented cardiovascular risk induced by the increase in serum uric acid (SUA) levels, such as endothelial dysfunction [22] and arterial stiffness [23]. However, there is still no agreement concerning which would be the most relevant.
Aims
To evaluate, in patients with T2DM and hypertension, pretreated with amlodipine and metformin, the effects of either Canagliflozin or Perindopril, added on top, after 6 months of treatment on 24h ABPM and cf-PWV. Finally, to evaluate the effects on SUA and the possible relationships with BP and PWV changes induced by both treatments.
Methods
Forty type 2 diabetic patients aged 25-70 years old (14 women) were included in this study. All of them have hypertension and were under treatment with amlodipine 10 mg daily and metformin 2000 mg daily. Once the informed consent was signed, all subjects were clinically evaluated. Thereafter, they were randomized to receive for 6 months, either canagliflozin 300 mg daily (n= 20, 9 women, mean age: 64± 4years) or perindopril 10 mg daily (n= 20, 5 women, mean age: 59±4years), added on top of the regimen.
Exclusion criteria
Previous diagnosis of type 1 diabetes mellitus (T1DM) or diabetic ketoacidosis; recurring (i.e. 2 events over a 1-week period), fasting self-monitored plasma glucose measurements at least 13.3 mmol/l (240 mg/ dl), during the pretreatment phase, uncontrolled hypertension (i.e. the average of three seated BP readings ≥160/110 mmHg); secondary hypertension; treatment with an SGLT2 inhibitor, insulin or a glucagon-like peptide-1 receptor agonist within the 4 weeks prior to screening and/ or treatment with the following antihypertensive drugs: ACEIs, Angiotensin II Receptor blockers, loop diuretics, calcium channel blockers, other than amlodipine, or b-blockers.
All studies were conducted in accordance with ethical principles that comply with the Declaration of Helsinki and were consistent with Good Clinical Practices and applicable regulatory requirements. All patients signed a written informed consent, prior to the inclusion in the study.
Ambulatory blood pressure monitoring (ABPM) was assessed at baseline, and after 6 months of treatment. Measurements were performed with an oscillometric Mobil-O-Graph 24-h PWA Monitor (I.E.M. GmbH, Stolberg, Germany; 24,25) to obtain readings with intervals of 20 min from 0700 until 2200 h (daytime period) and every 30 min from 22:00h until 07:00h (night-time period). All the recordings took place during working days. The ARCSOlver algorithm, is included in the Mobil-O-Graph 24-h [24,25].
Measurement of cf-PWV
In all individuals, before and after 6 months of treatment, the cf-PWV was measured by tonometry, as previously described [26,27]. Briefly, all measurements were performed in a quiet room with stable temperature and the patient in a supine position, after 10 min of rest. Carotid–femoral pulse wave velocity(cf-PWV) was determined by recording the carotid and femoral waveforms using a validated technology (Arteriometer, Model V100; Oxitech, Buenos Aires, Argentina), as previously reported [14,28-30]. The hardware used two high-fidelity silicon piezo-resistive pressure sensors (Motorola MPX 2050; Motorola Inc., Schaumburg, Illinois, USA) connected to an amplifier. Briefly, the signal was acquired in a PC and during data acquisition, the pressure sensors were simultaneously positioned in the left sided carotid and femoral arteries. Records were performed continuously whereas pressure waves were monitored on the computer screen, using specific software, as previously described [31]. The software works in a Windows; Micro Redmond, Washington, USA environment, performing an online digitized pressure wave acquisition that allows several cf-PWV measurements along a single continuous record, which includes at least 10 cardiac cycles. Online cf-PWV calculation included the distance between sensors values. The mean value and the standard deviation (SD) of these measurements were always calculated and considered as the cf-PWV value for each patient. To ensure a reliable measurement, special care was taken in monitoring that the SDs of measurements were less than 10%. The same two physicians performed all data acquisition and cf-PWV calculation, one always obtaining the pressure waves and the other operating the computer. When technical mistakes or low signal quality were detected, the procedure was repeated. Recorded measurements were obtained by duplicate, in all patients [31]. Finally, in accordance with the European Recommendations [3,15], each cf-PWV value was corrected by multiplying by 0.8.
Blood and urine samples: In all individuals, before and after 6 months of treatment metabolic parameters, including plasma fasting glucose, HbA1c, creatinine, plasma sodium and potassium, and serum uric acid (SUA). In 24h urine sample, sodium, potassium, uric acid excretion, has been obtained. From these data, glomerular filtration rate was calculated.
Statistical analysis
Data were expressed as mean and standard deviation. A p <0.05 was considered significant. A paired t-test for comparison of the basal versus 6months values was performed. To verify statistical differences, about the effects of treatments on the different parameters measured, ANOVA Analysis was applied, and Post Hoc Analysis performed.
Since there was a no linear significance among values but only for variations in SUA and cf-PW, consequently, Pearson´s correlation between delta cf-PWV and delta uric acid was also performed. Statistical analysis was carried-out using IBM-SPSS software (IBM Corp. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp; United States).
Results
All individuals completed the study without significant side effects.
BP Effects
As it is shown in Figure 1, after 6 months of treatment, both drugs decreased significantly ambulatory blood pressure. The decrease in SBP or DBP was similar, either with canagliflozin (-5.63/-14.14%) or perindopril (-5.0/-11.58%). Heart rate failed to be significantly altered.
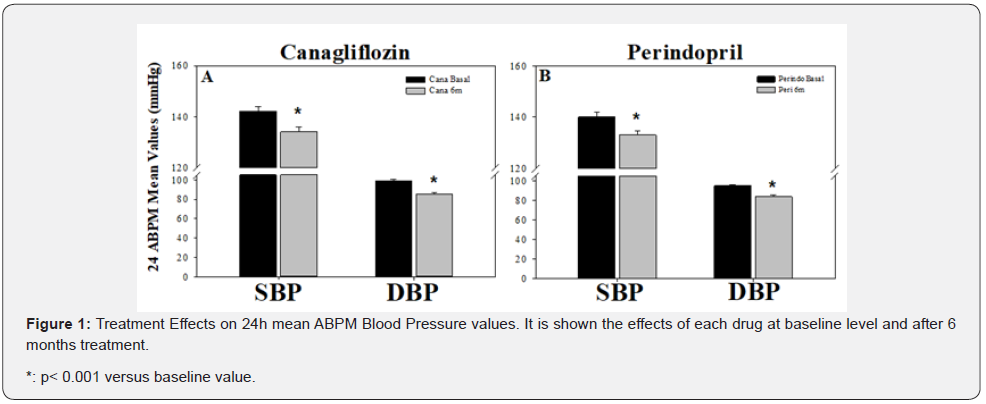
Glycemia and glycated hemoglobin effects
As expected, after 6 months treatment with canagliflozin, a significant reduction in glycemia, from 149±2.24mg% to 118±1.34mg/% (p<0.001) and glycated hemoglobin from 8.5±0.11% to 7.0±0.09% (p<0.001) was observed. Perindopril failed to alter glycemia (Basal: 138±2.24mg%, 6 months: 134±2.68mg%, ns) but glycated hemoglobin was slightly but significantly reduced from 7.5±0.11mg% to 7.0±0.09 (p<0.001).
Plasma and urinary sodium effects
Canagliflozin increased urinary sodium excretion from 150±4.03mEq/L; 6months: 170±1.79 (p<0.001), while under perindopril the excretion decreased significantly 180±4.03mEq/24h to 170±1.79mEq/24h (p <0.03). Instead of this, neither canagliflozin nor perindopril altered plasma sodium levels (canagliflozin: from 139±1.57 to 134±2.01mEq/L or perindopril: from 139±1.57 to 137±2.01mEq/L).
Kidney function
Glomerular filtration rate was significantly reduced by both treatments, Canagliflozin: from 147±2.24 to 130±1.57 ml/min 1.73m2 (p<0.001) and Perindopril: from 137±2.24 to 128±1.57ml/min 1.73m2 (p<0.003). Plasma Creatinine levels were not altered by either treatment Canagliflozin: 0.9±0.05 to 0.7±0.09mg% or Perindopril: 0.9±0.05 to 1.0±0.09mg%. Other security laboratory parameters, including hepatic function failed show any change after both treatments.
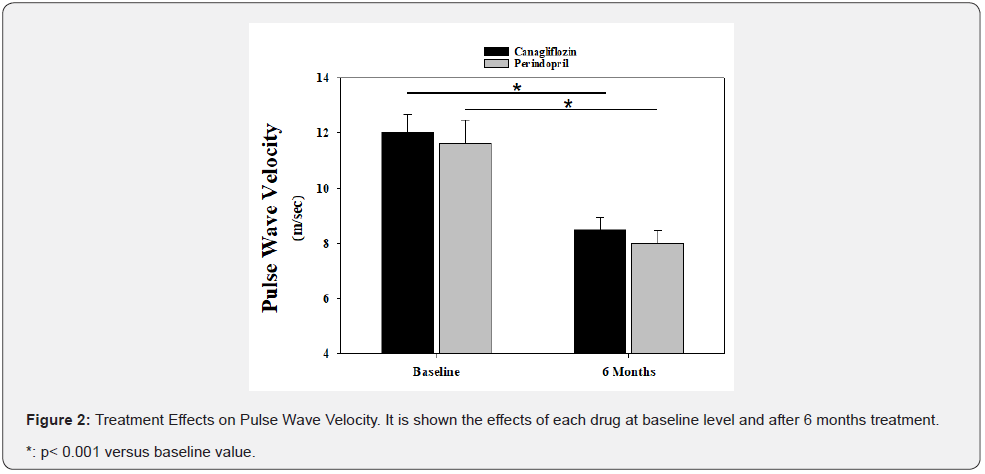
PWV effects
As shown in Figure 2, both treatments induced a similar and significant reduction in PWV.
Uric acid effects
Both treatments significantly increased urinary uric acid excretion, Canagliflozin (350±4.47 to 580±6.71mg/24h, p<0.001) and Perindopril (310±2.24 to 340±12mg/24h; p<0.02). Nevertheless, the increase induced by canagliflozin was significantly higher than the perindopril induced increase (p<0.01).
However, instead the basal SUA and urinary UA excretion levels in the group treated with perindopril were significantly lower than those under canagliflozin (Figure 3 left and right side, respectively), only canagliflozin induced a significant reduction in both parameters.
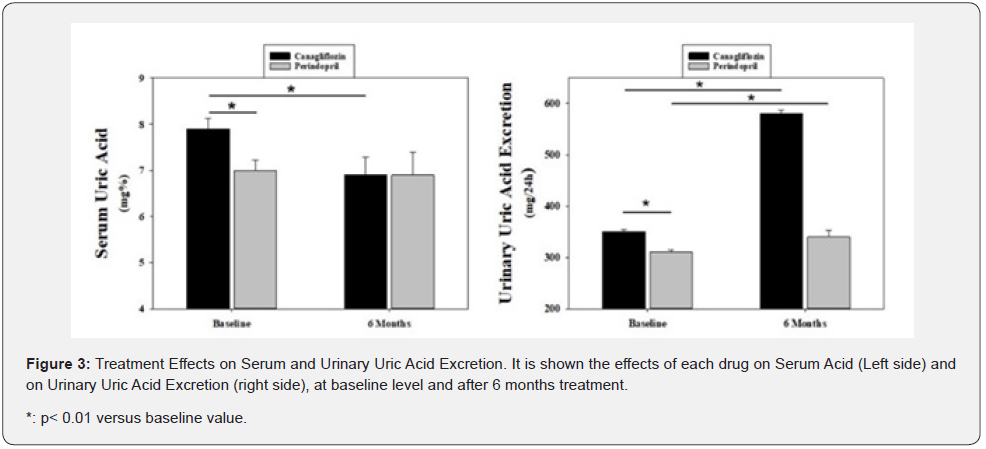
Finally, as shown in Figure 4, a significant correlation between the decrease in SUA and cf-PWV was found with canagliflozin, when corrected by blood pressure, age, weight, plasma, and urinary sodium (r=0.691, p<0025). From this correlation it was possible to calculate that for every 1mg% reduction in SUA there is a decrease in 1.18m/sec in PWV. However, no correlation was observed with perindopril.
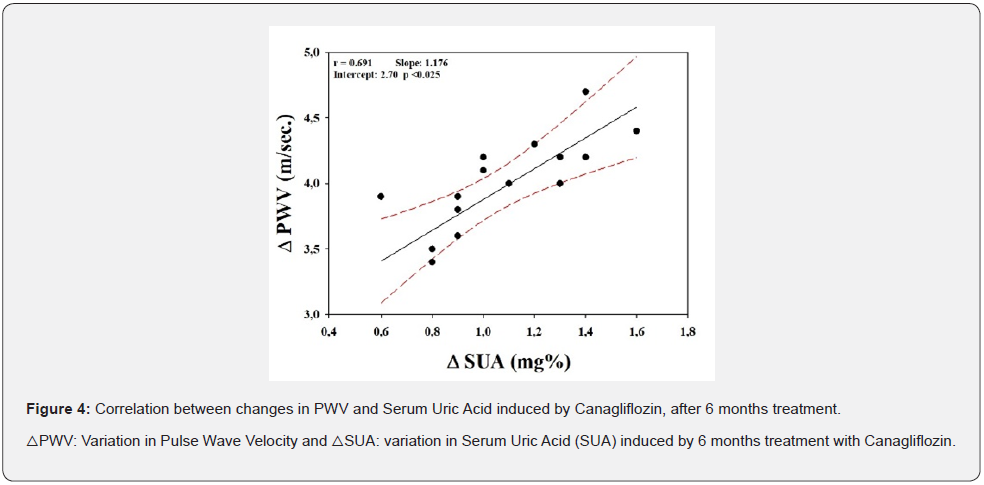
Discussion
The current study reports that, in hypertensive patients with type 2 diabetes, after 6 months of treatment with Canagliflozin, SUA and arterial stiffness were significantly improved. This is in accordance with previously reported data using other SGLT2 inhibitors, in diabetic [32] or non-diabetic patients [33]. Furthermore, 20–30% of DM patients with hyperuricemia were able to achieve normal serum uric acid levels (<6 mg/dl) with Canagliflozin [32] and the beneficial effects of SGLT2 inhibitors on SUA and urinary sodium excretion was further emphasized, in recent meta-analyses [34,35].
It is well known that around 90% of the filtered uric acid is reabsorbed in the proximal convoluted tubules through different transporters: the uric acid transporter 1 [36,37], and the organic anion transporter 4 [38,39] to the peritubular blood circulation, by a GLUT9 transporter isoform 1 (SLC2A9a; [40-42]. This is supported by experimental studies where Canagliflozin strongly increased the urinary glucose or urate-to-creatinine ratios [43] as the glycosuric and the uricosuric effects [44]. Therefore, the urinary urate excretion was negatively related to the changes in blood glucose while it was positively related to the changes in urinary glucose [43]. This suggests that the uricosuric effect of SGLT2 inhibitors is related to the increase in glucose concentrations induced in the proximal convoluted tubule. The GLUT9 isoform 2, located in the apical membrane, is stimulated by this mechanism, and increases the uric acid excretion while the uric acid reuptake is inhibited, in the distal tubule [43,45].
Perindopril, in addition to the blood pressure reduction, also showed a slight increase in serum K+ (data not shown) with a decrease in urNa+ excretion and an improvement in the glycosylated hemoglobin. This is in accordance with previous data, showing that perindopril can induce a slight serum K+ increase and, although not significantly, a slight improvement on serum glucose and insulin sensitivity [46].
The hemodynamic effect of perindopril, as we have previously shown [12], is supported by the fact that, after 6 months treatment with either canagliflozin or perindopril, 24h ABPM and office BP were significantly reduced with an improvement in c-f-PWV [12]. This effect, when the cf-PWV induced effects were corrected, either by office or 24h systolic or diastolic blood pressure values, the arterial stiffness improvement was completely blunted under perindopril, while a remaining effect was still observed under canagliflozin. This suggested that, contrarily to perindopril, the canagliflozin improvement in cf- PWV is not only related to a hemodynamic effect [12]. This agrees with the additional cardiovascular mechanisms (changes in energy metabolism, reduction in blood pressure and vessel stiffness and decrease in serum uric acid) by which SGLUT2 inhibitors improve cardiovascular risk [47]. This is further supported by the hypothesis that the additional cardiovascular protective effect induced by Canagliflozin [47] may be also related to the improvement in cf- PWV since uric acid induces an inflammatory process in the vasculature, leading to an increase in arterial stiffness [44,47]. Moreover, Albu et al. [48] have shown a direct relationship between uric acid and arterial stiffness and an 8-year longitudinal study in normotensive men [49], showed that uric acid is associated with an increase in PWV. The mechanism involved is related to pro-inflammatory and pro-atherogenic effects in the arterial wall, favoring the reduction in arterial wall distensibility [50]. However, instead the inflammatory status was not evaluated in this study, it can be suggested that the improved arterial stiffness induced by Canagliflozin could be mediated by two related mechanisms: a hemodynamic effect, resulting from the decrease in blood pressure, and the reduction in serum uric acid levels, mediated by the increase in urinary uric acid excretion, that could be associated with a reduction in the inflammatory process.
Finally, we have previously reported a linear correlation between serum uric acid and cf-PWV [51]. From this data, we suggested that PWV is related to SUA and SBP, with age being included as a forced variable. In this condition, the remaining variables analyzed: BMI, Waist Circumference, DBP and HR did not provide significant weight to predict PWV.
In accordance with this data, in the present work, we have shown that, only in the Canagliflozin treated group, a significant correlation was observed between changes in SUA to changes in cf-PWV. From this correlation it was observed that, for each 1mg% SUA reduction a decrease in 1.18m/sec in cf-PWV was induced. This is interesting because previously reported data [52] have shown that for each 1m/sec increase in PWV there is a 14% increase in cardiovascular events or a 15% in all-cause mortality, either in the general population, older subjects or individuals having a cardiovascular disease.
This further supports that the cardiovascular protective effect induced by canagliflozin, in diabetic hypertensive patients, could be related to the increased UA excretion responsible for the decrease in SUA related, a least in part, with the improvement in arterial distensibility.
Conclusion
In conclusion, our study shows the differential effect of the SGLT2 inhibitor canagliflozin, respective to perindopril, in improving arterial stiffness. This effect seems to be mediated through the reduction in blood pressure, glycemia and SUA.
The reduction in uric acid is of clinical importance given that hyperuricemia is frequently observed in type 2 diabetic patients and is related to an increased cardiovascular risk [17,21].
Limitations of the Study
a) A low number of patients included, may impair the strength of the conclusions.
b) No evaluation of the inflammatory status of the patients was performed.
c) A comparative study with a xanthine oxidoreductase drug might be important to contrast results with canagliflozin.
References
- King P, Peacock I, Donnelly R (1999) The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol 48(5): 643-648.
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, et al. (2000) Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. N Engl J Med 342(3): 145-153.
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, et al. (2006) Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. CAFE Investigators. Anglo-Scandinavian Cardiac Outcomes Trial Investigators. CAFE Steering Committee and Writing Committee. Circulation 113(9): 1213-1225.
- Kalra S (2014) Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther 5(2): 355-366.
- Lee YJ, Lee YJ, Han HJ (2007) Regulatory mechanisms of Na/glucose co- transporters in renal proximal tubule cells. Kidney Int Suppl 106: S27-S35.
- Hummel CS, Lu C, Loo DD, Hirayama BA, Andrew AV, et al. (2011) Glucose transport by human renal NAþ/d-glucose co-transporters SGLT1 and SGLT2. Am J Physiol Cell Physiol 300(1): C14-C21.
- Shyangdan DS, Uthman OA, Waugh N (2016) SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open 6(2): e009417.
- Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Masaaki M, et al. (2017) Mechanism of the blood pressure-lowering effect of sodium-glucose co-transporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol 18(1): 23.
- Mahaffey KW, Neal B, Perkovic, V, de Zeeuw D, Greg F, et al. (2018) Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events. Results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 137(4): 323-334.
- Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP (2019) Canagliflozin and Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus and Chronic Kidney Disease in Primary and Secondary Cardiovascular Prevention Groups. Results from the Randomized CREDENCE Trial. Circulation 140(9): 739-750.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. (2015) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373: 2117-2128.
- Ramirez AJ, Sanchez MJ, Sanchez RA (2019) Diabetic patients with essential hypertension treated with amlodipine: blood pressure and arterial stiffness effects of canagliflozin or perindopril. J Hypertens 37(3): 636-642.
- Blachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55(13): 1318-1327.
- Nilsson PM, Boutouyrie P, Cunha P, Kotsis V, Krzysztof N, et al. (2013) Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens 31(8): 1517-1526.
- Chow B, Rabkin SW (2015) The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta-analysis. Heart Fail Rev 20(3): 291-303.
- Bosch A, Ott C, Jung S, Striepp K, Marina VK, et al. (2019) How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc Diabetol 18(1): 44.
- Bombelli M, Ronchi I, Volpe M, Facchetti R, Stefano C, et al. (2014) Prognostic Value of Serum Uric Acid: New Onset in and Out of Office Hypertension and Long-Term mortality. J Hypertens 32(6): 1237-1244.
- Verdecchia P, Schillaci G, Reboldi GP, Santeusanio F, Porcellati C, et al. (2000) Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 36(6): 1072-1078.
- Lehto S, Niskanen L, Ronnemaa T, Laakso M (1998) Serum uric acid is a strong predictor of stroke in patients with noninsulin-dependent diabetes mellitus. Stroke 29(3): 635-639.
- Ndrepepa G, Braun S, King L, Cassese S, Tada T, et al. (2013) Prognostic value of uric acid in patients with type 2 diabetes mellitus and coronary artery disease. Clin Sci (Lond) 124(4): 259-268.
- Borghi C, Agabiti RE, Bardin T, Dawson J, Dominiczak A, et al. (2015) Serum Uric Acid and the Risk of Cardiovascular and renal disease. J Hypertens 33(9): 1729-1741.
- Elsurer R, Afsar B (2014) Serum uric acid and arterial stiffness in hypertensive chronic kidney disease patients: sex-specific variations. Blood Press Monit 19(5): 271-279.
- Doehner W, Schoene N, Rauchhaus M, Leyva LF, Darrell VP, et al. (2002) Effects of xanthinoxidase inhibition with allopurinol on endothelial function blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation 105(22): 2619-2624.
- Wassertheurer S, Kropf J, Weber T, van der Giet M, Baulmann J, et al. (2010) A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens 24(8): 498-504.
- Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, et al. (2011) Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension 58(5): 825-832.
- Diaz A, Tringler M, Galli C, Ramirez A, Cabrera FEI (2015) Arterial stiffness in a rural population of Argentina: Pilot study. High Blood Press Cardiovasc Prev 22(4): 403-409.
- Christen A, Amentano RL, Miranda A, Graf S, Santana DB, et al. (2010) Arterial wall structure and dynamics in type 2 diabetes mellitus. Methodological aspects and pathophysiological findings. Curr Diab Rev 6(6): 367-377.
- Van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, et al. (2017) SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol 12(4): 700-710.
- Mancia G, Cannon CP, Tikkanen I, Zeller C, Ley L, et al. (2016) Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension 68(6): 1355-1364.
- Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55(13): 1318-1327.
- Armentano R, Graf S, Ramirez AJ, Espinosa JD, Brandani E, et al. (2001) Mechanical vs. intrinsic components in the improvement of brachial arterial compliance. Comparison of the effects of atenolol versus ramipril in hypertensive patients. Medicina (Buenos Aires) 61(5): 535-540.
- Davies MJ, Trujillo A, Vijapurkar U, Damaraju CV, Meininger G (2015) Effects of canagliflozin on serum uric acid in patients with type 2 diabetes mellitus. Diabetes Obes Metabolism 17(4): 426-429.
- Zanchi A, Burnier M, Muller ME, Ghajarzadeh WA, Maillard M, et al. (2020) Acute and Chronic Effects of SGLT2 Inhibitor Empagliflozin on Renal Oxygenation and Blood Pressure Control in Nondiabetic Normotensive Subjects: A Randomized, Placebo‐Controlled Trial. J Am Heart Assoc 9(13).
- Zhao Y, Xu L, Tian D, Xia P, Zheng H, et al. (2018) Effects of sodium‐glucose co‐transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta‐analysis of randomized controlled trials. Diabetes Obesity Metb 20(2): 458-462.
- Xin Y, Guo Y, Li Y, Ma Y, Li L, et al. (2019) Effects of sodium glucose cotransporter-2 inhibitors on serum uric acid in type 2 diabetes mellitus: A systematic review with an indirect comparison meta-analysis. Saudi J Biol Sci 26(2): 421-426.
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, et al. (2002) Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417(6887): 447-452.
- Kolz M, Johnson T, Sanna S, Alexander T, Veronique V, et al. (2009) Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 5(6): e1000504.
- Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A, et al. (2007) Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 18(2): 430-439.
- Bahn A, Hagos Y, Reuter S, Daniela B, Brzica H, et al. (2008) Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem 283(24): 16332-16341.
- Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, et al. (2008) SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40(4): 437-442.
- Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, et al. (2008) Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 283(40): 26834-26838.
- Caulfield MJ, Munroe PB, Neill DO, Witkowska K, Charchar FJ, et al. (2008) SLC2A9 is a high-capacity urate transporter in humans. PLoS Med 5(10): e197.
- Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, et al. (2014) SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 35(7): 391-404.
- Novikov A, Fu Y, Huang W, Breemant F, Patel R, et al. (2019) SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J PhysiolRenal Physiol 316(1): F173-F185.
- Bailey CJ (2019) Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obesity and Metabolism 21(6): 1291-1298.
- Elisaf MS, Theodorou J, Pappas H, Papagalanis N, Katopodis K, et al. (1999) Effectiveness and metabolic effects of perindopril and diuretics combination in primary hypertension. J Human Hypertens 13(11): 787-791.
- Yang F, Meng R, Zhu DL (2020) Cardiovascular effects and mechanisms of sodium-glucose cotransporter-2 inhibitors. Chr Dis Translat Med 6(4): 239-245.
- Albu A, Para I, Porojan M (2020) Uric Acid and Arterial Stiffness Ther Clin Risk Management 16: 39-54.
- Tomiyama H, Shiina K, Vlachopoulos C, Iwasak Y, Matsumoto C, et al. (2018) Involvement of Arterial Stiffness and Inflammation in Hyperuricemia-Related Development of Hypertension. Hypertension 72(3): 739-745.
- Kang DH, Park SK, Lee IK, Johnson RJ (2005) Uric acid-induced C-reactive protein expression: implication on cell production of human vascular cells. J Am Soc Nephrol 16(12): 3553-3562.
- Ramirez AJ, Christen A, Sanchez RA (2018) Serum Uric Acid Elevation is Associated to Arterial Stiffness in Hypertensive Patients with Metabolic Disturbances. Current Hypertension Reviews 14(2): 154-160.
- Vlachopoulos C, Aznaouridis K, Stefanidis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55(13): 1318-1327.






























