Clinical Trial on Glimepiride 2mg Single Oral Dose in Healthy Volunteers: Need for Preventive Management of Hypoglycaemia
Rivas Paterna AB1,2, Alonso Murillo S1,2, Laredo Velasco L2,3, Vargas Castrillon E2,3 and Portolés Pérez A1,2,3*
1Fundación Investigación Biomédica, Hospital Clinico San Carlos, Spain
2 Universidad Complutense de Madrid, Spain
3Clinical Pharmacology Department, Hospital Clínico San Carlos, Spain
Submission: February 06, 2020; Published: February 27, 2020
*Corresponding author: Portolés Pérez A, Clinical Pharmacology Department, Fundación Investigación Biomédica, Hospital Clínico San Carlos, Universidad Complutense de Madrid, c/ Prof. Martin Lagos s/n. 28040, Madrid, Spain
How to cite this article: Rivas P AB, Alonso M S, Portolés Pérez A, et al. Clinical Trial on Glimepiride 2mg Single Oral Dose in Healthy Volunteers: Need for Preventive Management of Hypoglycaemia. Curre Res Diabetes & Obes J. 2020; 12(5): 555847. DOI: 10.19080/CRDOJ.2020.12.555847
Abstract
Objective: The objective of our study was to assess the bioequivalence of 2 different glimepiride formulations at a dose of 2 mg. We also describe the adverse events detected, with special emphasis on those directly related to administration of glimepiride, as well as the preventive action taken.
Research Design and Methods: We performed a randomized, open-label, crossover, 2-period bioequivalence study in which a single oral dose of each formulation was administered immediately after breakfast to 30 healthy volunteers, with a 7-day washout period between doses. Extra oral carbohydrates and intravenous glucose-saline solution were administered according to blood sugar level to prevent symptomatic hypoglycaemia. An analysis of variance was performed for the different Ln-transformed pharmacokinetic parameters.
Results:Twenty-nine subjects were evaluated. A total of 28 AEs were detected, and 9 of them had a possible or close relationship. Of these, five were symptomatic hypoglycaemia (glucose blood level of 40-60 mg/dl). The symptoms were of mild to moderate intensity, since all subjects had received an adequate supply of carbohydrates and extra glucose infusion before the events occurred. All cases resolved completely after adjustment of the glucose infusion. The remaining 19 adverse events were recorded as doubtful or not related to the drug.
Conclusions: Optimization of the intravenous glucose administration schedule can prevent severe symptomatic hypoglycaemia, which could jeopardize the health status of subjects involved in a bioequivalence study with glimepiride. Our findings justify the use of such control measures and their applicability in other studies with hypoglycaemic drugs.
Keywords:Glimepiride; Hypoglycaemia; Clinical trial; Hypoglycaemic drugs; Pro-inflammatory mediators; Oxidative stress; Transcriptional mediated molecular; Type 2 diabetes mellitus
Abbreviations: AEMPS: Spanish Agency for Medicines and Health Care Products; AUC0-t: The area under the Curve to the Last Measurable Concentration; AUC0-∞: The Area Under the Curve Extrapolated to Infinity in the Elimination Phase; CIs: Confidence Intervals; Cmax: The Maximum Concentration; EMEA: European Medicines Evaluation Agency; F: Females; Ke: Elimination Rate Constant; M: Males; Tmax: The Time to Reach the Maximum Concentration
Introduction
Type 2 diabetes mellitus has long been categorized as a complex and multifactorial metabolic syndrome that is characterized by the abnormal metabolism of carbohydrates, fats and proteins which leads to augmented levels of glucose and lipids within the blood [1]. Pro-inflammatory mediators, oxidative stress, transcriptional mediated molecular and metabolic pathways are involved in the pathogenesis of tissues-specific insulin resistance. Insulin resistance is one of the major hallmarks for the pathogenesis and aetiology of type 2 diabetes mellitus [2]. Reactive oxygen species and oxidative stress play their decisive role in the development of type 2 diabetes mellitus, in this regard, current research is focus to the use of anti-oxidant treatment for the prevention and treatment; but they are still in preclinical phase. Meanwhile, several drugs have been used to treat type 2 diabetes mellitus.
Glimepiride is an orally active hypoglycaemic drug belonging to the second-generation sulfonylurea group. Glimepiride stimulates insulin release from pancreatic ß-cells and may also act via an extrapancreatic mechanism. It is administered once daily to patients with type II diabetes mellitus in whom glycaemia is not controlled by diet and exercise alone [3]. This secondgeneration sulfonylurea has a higher selective binding capacity for ß-cells, thus allowing for greater potency at lower doses than its predecessors [4]. The usual initial dose of glimepiride is 1 mg to 2 mg once daily with breakfast or with the first main meal [3], as food does not affect absorption. Nevertheless, in studies with a single oral dose in healthy volunteers and multiple oral doses in patients with type II diabetes, mean Tmax increased slightly and mean Cmax and the AUC decreased slightly when glimepiride was given with meals [5].
Glimepiride is rapidly absorbed, reaching peak serum concentrations 2 to 3 hours after administration in both healthy volunteers and diabetic patients [6]. It is completely metabolized by the liver via the isoenzyme cytochrome P450 2C9 into its main active metabolite (M1) which is converted into the inactive metabolite (M2) by 1 or several cytosolic enzymes [5]. The elimination half-life of glimepiride after a single dose is about 5 hours [7]. Pharmacokinetic parameters in healthy volunteers and patients with type II diabetes revealed no differences with regard to the action of the drug [5].
The most serious side effect of the sulfonylurea group is hypoglycaemia, which often makes it difficult to achieve optimal blood sugar levels [8]. Glimeripide has a significantly lower incidence of hypoglycaemia [4] than other second-generation sulfonylureas (10% to 20% of patients treated for 1 year) [3]. Consequently, it is more suitable for patients with increased susceptibility to this effect [9]. The objective of our study was to assess the bioequivalence of 2 different glimepiride formulations at a dose of 2 mg. Although the results of published studies show that this drug has a good safety profile at the dose administered [10-13], we developed control measures to minimize the risk of symptomatic hypoglycaemia. We also describe the adverse events detected, with special emphasis on those directly related to administration of glimepiride, as well as the preventive action taken.
Materials and Methods
Study design
This open-label, cross-over, randomized, 2-period bioequivalence study in healthy volunteers was carried out at the Clinical Pharmacology Study Unit, Hospital Clinic San Carlos, Madrid (Spain).
Ethics, consent and permissions
All the subjects received written information and signed the informed consent document before being enrolled.
Subjects
The study population comprised healthy volunteers between 18 and 40 years old, in an equal number from both sexes. Subjects were examined to verify their health status and their eligibility to participate. Smokers, pregnant women and consumers of stimulant drinks or drugs that could affect the effect of the study drug were excluded. Before inclusion, a complete medical history including vital signs and electrocardiogram was taken and laboratory tests (hematology, biochemistry, serology, urinalysis, drug abuse test, and pregnancy test [women of childbearing potential]) were performed. Alcohol and xanthine’s were not permitted for at least 48 hours before inclusion, and subjects were instructed to abstain from taking any medication within the 2 weeks preceding the study.
Methods
The study was carried out in 2 treatment periods (A and B), separated by a washout period of 7 days (minimum). During each treatment period, each volunteer received a single dose of 2 mg (one tablet). As a crossover study, the sequence of treatment was assigned randomly. Medication was administered with 200 ml of water immediately after breakfast. Subjects were seated for the first 4 hours after administration to ensure an even grade of absorption. Assignation to treatment sequence was via a computergenerated randomised balanced number table. The subjects were recruited from a database from a database of healthy volunteers existing in the Clinical Pharmacology Study Unit, Hospital Clinico San Carlos. All the task related with randomization were implemented by the research team. Venous blood samples were drawn at baseline (pre-administration), +30minutes, +1hour, +1hour 30minutes, +2hour, +2hour 30minutes, +3hours, +3hours 30minutes, +4hours, +5hours, +6hours, +8hours, +12hours, +15hours, and +24hours to determine the concentrations of glimepiride. Clinical check-ups were made at +4hours, +15hours, +24hours, and +96hours after administration.
The complete daily diet was 2500 calories, and the distribution of carbohydrates was that used by diabetic patients (6 food intakes per day). The first meal was breakfast, which was taken before drug intake. An infusion of 10% glucose (approximately 1300 ml/ day) was provided intravenously throughout the 24-hours period following administration to supply carbohydrate requirements and to prevent symptomatic hypoglycaemia. The infusion schedule was 40 to 200 ml/h to achieve a target concentration of at least 50 mg/dl, even if no symptoms had been recorded. The blood sugar level was measured using a rapid glucose test (Glucocard Memory®, Menarini & Arkray) at +2hours, +3hours, +4hours, +6hours, +12hours, +15hours and +24hours and the rate of infusion was adjusted according to the results. The infusion schedule was also modified in the case of hypoglycaemia or hypoglycaemic symptoms in order to achieve a quick recovery and ensure safety.
Pharmacokinetic analysis
Individual pharmacokinetic parameters of glimepiride were calculated using a non-compartmental model (WinNonlin.Pro ® v.4.1) upon the plasma drug concentration measured at each sampling time. Blood glimepiride level was analysed by liquid chromatography coupled to mass spectrometry tandem (HPLCMS / MS). The maximum glimepiride concentration (Cmax) and the time to reach Cmax (Tmax) were determined directly from the plasma drug concentrations. The area under the curve to the last measurable concentration (AUC0-t) was calculated using the trapezoidal linear method. The area was extrapolated to infinity (AUC0-∞) by calculating the Ke (elimination rate constant) in the elimination phase
Statistical analysis
It was estimated that a sample of 24 subjects could detect differences with a confidence interval of 90% based on the sample sizes used in previous publications. Descriptive statistics were calculated for the demographic and pharmacokinetic parameters.
An analysis of variance (ANOVA) was performed including the factors form, period, sequence, and subject (sequence). The dependent variables were the ratios of the previously mentioned parameters (Cmax, AUC0-t, AUC0-∞) and their corresponding logarithms. The 90% confidence intervals (CIs) were calculated using the Classical method. The reference interval for bioequivalence was 0.80 to 1.25.
Ethical statement
Ethics approval and consent to participate: The study protocol was approved by the institutional ethics committee (Hospital Clinico San Carlos) and authorized by the Spanish Agency for Medicines and Health Care Products (AEMPS) in February 2004 with registration number 04/029. The study was conducted in compliance with the principles of the Declaration of Helsinki and good clinical practice guidelines. All the subjects received written information and signed the informed consent document before being enrolled.
Results
Study population
Between April and May 2004 thirty subjects were enrolled and included in the safety-set analysis; 29 subjects (M/F: 15/14) were included in the pharmacokinetic-set analysis (1 subject withdrew due to poor tolerance to blood sampling). The demographic characteristics, expressed as mean ± SD, were as follows: age (22.8 ± 2.57 years), weight (65.64 ± 8.34 kg), height (170.1 ± 7.53 cm), and body mass index (22.59 ± 1.58 kg/m2).
Pharmacokinetic analysis
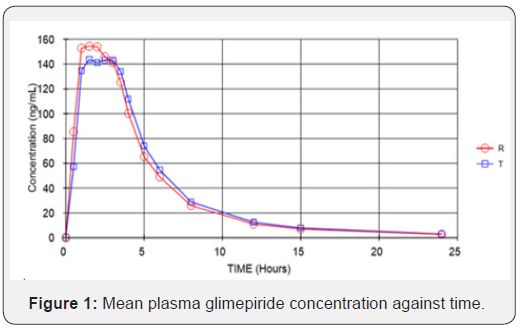
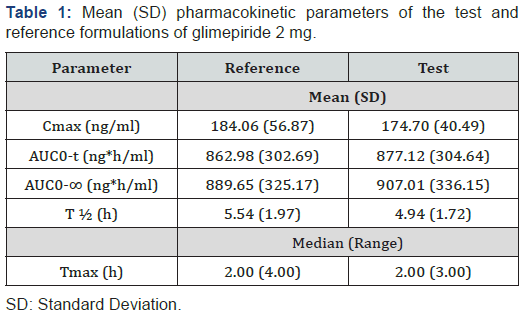
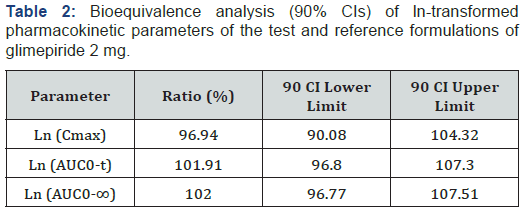
Figure 1 presents the mean plasma glimepiride concentration against time for the 2 formulations. The mean plasma concentration profiles of glimepiride for the 2 formulations were very similar. The mean (SD) of the pharmacokinetic parameters for the 2 products (reference and test) were similar, suggesting that the plasma profiles of the 2 formulations were comparable (Table 1). No significant ANOVA model effects were found. In the bioequivalence analysis, the 90% CIs for Cmax (90.08-104.32%) and AUC0-t (96.80-107.30%) were within the EMEA acceptance range (0.80 to 1.25), thus meeting the criteria for bioequivalence (Table 2).
Tolerability
Both tablet formulations of glimepiride were generally well tolerated. Twenty-eight adverse events were detected, and 9 of these had a possible or closer causal relationship. Of these, 5 (corresponding to 5 different subjects) were symptomatic hypoglycemia consisting of dizziness without loss of consciousness and/or sweating and hand tremor. In 4 subjects, the symptoms appeared 2 to 3 hours after intake (mean, 2hours 32 minutes), and in 1 subject onset was at 1hour 28 minutes after intake. The duration of symptoms was less than 10 minutes for all subjects. Blood glucose levels ranged from 40 mg/dl to 60 mg/dl. A prompt and complete recovery was achieved in all cases when the glucose infusion was adjusted to blood sugar level. Other adverse events related to glimepiride were headache (2 cases in 1 subject), dizziness (1 case), and diarrhoea (1 case). These adverse events are included in the summary of product characteristics and were therefore expected. Their duration and intensity (mild to moderate) were as expected and the subjects recovered without treatment. Finally, 19 adverse events were recorded as doubtful or not related to the drug. They were all mild or moderate, and they all resolved spontaneously or after symptomatic treatment. None of the volunteers withdrew for reasons of safety.
Discussion
The safety profile of glimepiride in diabetes patients is well known [4,12]. Although the incidence of hypo glycaemia is significantly lower than with other sulfonylurea agents, it could be present in 10% to 20% of all patients treated [3,6]. We designed a bioequivalence study with healthy volunteers, and we established control measures to prevent hypo glycaemia. Despite the preventive measures, we found a clinically significant symptomatic hypoglycaemic effect in healthy volunteers. This is interesting, since onset of hypo glycaemia was controlled by intravenous 10% glucose infusion and a specific meal regimen consisting of a rich carbohydrate breakfast before drug intake and several additional meals during the stay in our unit. Among published bioavailability studies [10-14], only Jovanovic et al. have reported hypoglycaemic events (5 in 24 subjects after an oral dose of glimepiride 6 mg). These resolved spontaneously in 30-60 minutes. No additional procedures were performed to minimize hypoglycaemic effects or to recover baseline status after onset of symptoms. By contrast, in our study, despite the 10% glucose infusion, which was modified according to blood glucose level, symptomatic hypoglycaemia (40-60 mg/dl) was reported in 5 of 28 subjects. All the subjects recovered with no sequelae, although individualized modification of the glucose infusion was required in all cases. This pharmacodynamic effect of glimepiride in healthy volunteers had been described in detail by Yun et al, [15] who found similar values of the pharmacokinetic parameters Cmax and Tmax to ours but lower glucose levels after administration. This could be explained because the authors used sugar cubes (12 g each) to supply the extra glucose after drug administration, whereas we administered a rich carbohydrate breakfast before drug intake and complementary glucose infusion during the study. Despite different control measures, our findings for detection of the lowest glycaemic serum level (2 to 3 hours after drug administration) were consistent with those of Yun et al [15].
The good tolerability of glimepiride in healthy volunteers described in other trials, even after high doses and with no preventive action against hypo glycaemia, is unlikely; therefore, relevant information may have been omitted in such publications, although it should be mandatory for bioequivalence studies. Given the potential for hypo glycaemia after intake of glimepride, the FDA’s recent draft guidance for industry “Bioequivalence recommendations for specific products” [16], recommends using the lowest glimepiride dose and subsequent administration of extra oral glucose solution for 4 hours. This recommendation is consistent with our preventive actions and highlights the need to provide more than pharmacokinetic information in the publications on bioequivalence studies.
Conclusion
It is crucial therefore to maintain safe glucose blood levels during studies with hypoglycaemic agents in healthy volunteers. Administration of long-lasting oral carbohydrates and intravenous glucose can be considered elective preventive treatment and should be available in bioequivalence glimepiride studies. Equally important is the need to report adverse events and the support strategies adopted. The lack of such information is a failing in the organization of these studies and hampers decisions affecting the safety of subjects.
Acknowledgments
All the staff from the Phase 1 Unit, Clinical Pharmacology Dept, Hospital Clinico San Carlos, Madrid.
References
- Rehman K, Akash MSH (2017) Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J Cell Biochem 118(11): 3577-3585.
- Rehman K, Akash MS (2016) Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? Journal of Biomedical Sciences 23(1): 87.
- Langtry HD, Balfour JA (1998) Glimepiride. A review of its use in the management of type 2 diabetes mellitus. Drugs 55(4): 563-584.
- Davis SN (2004) The role of glimepiride in the effective management of type 2 diabetes. J Diabetes Complications 18(6): 367-376.
- (1995) Prescribing information. Amaryl (glimepiride). In: Somerville NJ, Hoechst-Roussel Pharmaceuticals.
- Campbell RK (1998) Glimepiride: role of a new sulfonylurea in the treatment of type 2 diabetes mellitus. Ann Pharmacother 32(10): 1044-1052.
- Massi-Benedetti M (2003) Glimepiride in type 2 diabetes mellitus: a review of the worldwide therapeutic experience. Clin Ther 25(3): 799-816.
- Szoke E, Gosmanov NR, Sinkin JC Nihalani A, Fender AB, et al. (2006) Effects of glimepiride and glyburide on glucose counter regulation and recovery from hypo glycaemia. Metabolism 55(1): 78-83.
- Holstein A and Egberts EH (2003) Risk of hypoglycaemia with oral antidiabetic agents in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 111(7): 405-414.
- Malerczyk V, Badian M, Korn A, Lehr KH, Waldhäusl W (1994) Dose linearity assessment of glimepiride (Amaryl) tablets in healthy volunteers. Drug Metabol Drug Interact 11(4): 341-357.
- Pistos C, Astraka C, Kalovidouris M Vassilopoulos E, Koutsopoulou M (2005) Bioequivalence evaluation of two brands of glimepiride 4 mg tables in healthy subjects. Int J Clin Pharmacol Ther 43(4): 203-208.
- Jovanović D, Stojšić D, Zlatković M Jović-Stosić J, Jovanović M (2006) Bioequivalence assessment of the two brands of glimepiride tablets. Vojnosanit Pregl 63(12): 1015-1020.
- Borges NC, Taveira Y del A, Mazucheli JA Haddad AL, Astigarraga RE, et al. (2007) Comparison study of two glimepiride formulations bioavailability in healthy volunteers of both sexes after a single dose administration. Arq Bras Endocrinol Metab 51(6): 950-955.
- Matsuki M, Matsuda M, Kohara K Shimoda M, Kanda Y, et al. (2007) Pharmacokinetics and pharmacodynamics of glimepiride in type 2 diabetic patients: compared effects of once- versus twice-daily dosing. Endocr J 54(4): 571-576.
- Yun H-Y., Park H-C, Kang W Kwon KI (2006) Pharmacokinetic and pharmacodynamic modelling of the effects of glimepiride secretion and glucose lowering in healthy humans. J Clin Pharm Ther 31(5): 469-476.
- http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm081322






























