Relationship Between Dose of Metformin and Vitamin B12 Deficiency
Jimena Soutelo1*, Clara FRITZ1, Sofia Moldes1, Florencia Borghi Torzillo1, Yanina Oliva1, Natalia Carbajo1, Silvina Del Duca2, María de Lujan Calcagno2 and Ruben Lutfi1
1Servicio de Endocrinologia, Hospital Churruca Visca, Argentina
2Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Argentina
Submission: January 23, 2020; Published: February 18, 2020
*Corresponding author: Jimena Soutelo, Servicio de Endocrinologia, Hospital Churruca Visca, Uspallata 3400, Buenos Aires Argentina, Argentina
How to cite this article: Soutelo J, Frizt C, Moldes S, Borghi Torzillo F, Oliva Y et al. Relationship Between Dose of Metformin and Vitamin B12 Deficiency. Curre Res Diabetes & Obes J. 2020; 12(5): 555846. DOI: 10.19080/CRDOJ.2020.12.555846
Abstract
Introduction: Metformin [MET] is a first-line pharmacological therapy in the treatment of type 2 Diabetes [T2DM]. It has been reported to cause a decrease in serum Vitamin B [VB12] concentrations.
Objectives: To describe whether there is a relationship between the use of MET and VB12 deficiency and to evaluate the metformin ratio according to the time of exposure and the dose used.
Materials and Methods: Patients with T2DM taking MET where included and a control group without T2DM. Patients and controls were excluded if history of cancer, malabsorptive diseases, bariatric surgery, alcoholism, pregnant women, chronic kidney disease or vitamin supplementation. Personal data, habits, blood pressure and anthropometric measurements where noted. Laboratory data included hemogram, hepatogram, creatinine, glycemia, HbA1c, lipids and VB12. Microvascular and macrovascular complications were searched for. Data were analyzed using chi-square, t-test and ANOVA, considering as significant level 0.05. [Info Stat].
Results: 118 patients in the control group [59,3±14,2 years] and 178 with T2DM taking MET [64,5±7,4 years]. T2DM patients presented lower VB12 levels than controls [p=0.0011]. T2DM with neuropathy had a higher percentage of VB12 deficiency than those without neuropathy [p=0.01]. There was no significant difference for BMI and HbA1c. Higher deficit was found in doses 1000 [p=0.0017]. No association was found between VB12 deficiency and time of exposure to MET regardless of the dose.
Conclusions: We found association between the use of MET, neuropathy and VB12 deficiency. Association was found with a dose over 1000 mg/day but not with time of exposure.
Keywords: Metformin; Vitamin B12; Type 2 diabetes; Diabetic treatment; Hypoglycemic treatment
Abbreviations: Hb: Hemoglobin; MCV: Mean Corpuscular Volume; MET: Metformin; T2DM: Type 2 Diabetes Mellitus; MNSI: Michigan Neuropathy Screening Instrument; VB12: Vitamin B12; DPP4: Dipeptidyl Peptidase 4; ACE inhibitor: Angiotensin-Converting-Enzyme Inhibitor; NHANES: National Health and Nutrition Examination Survey; BMI: Body Mass Index
Introduction
Type 2 diabetes mellitus [T2DM] is a chronic metabolic disorder that is increasingly becoming a pandemic in developed and developing countries. Both the European and American guidelines recommend the use of metformin as a first-line pharmacological therapy in T2DM [1]. Besides its low cost, high efficacy in lowering HbA1c and beneficial effects on body weight loss, the relatively safe side effect profile justifies the widespread use of metformin. Several studies have reported association of Vitamin B12 deficiency in adults with type 2 diabetes taking metformin [2-5]. Research on the subject revealed that, in 1969, Berchtold et al, [6] reported evidence of vitamin B12 malabsorption in patients who had been treated with metformin for as little as 3 months and Tomkin et al, [7] in 1971, based on a cross-sectional study, recommended that all patients on long term metformin therapy have annual serum B12 testing.
Many mechanisms were proposed to explain how metformin interferes with the absorption of vitamin B12. There are at least three hypotheses:
a) Intestinal bacteria overgrowth.
b) Impaired intestinal motility and
The most currently accepted mechanism suggests that metformin antagonizes the calcium cation and interferes with the calcium- dependent vitamin B12 complex binding to the ileal cubilin receptor. The reversal of metformin associated vitamin B12 malabsorption by calcium supplementation greatly supported the later hypotheses [8]. However, there is controversy over whether the reduction in vitamin B 12 level is due to the long-term use of metformin or if it is related to the dose. Regarding this, the American Diabetes Association in 2017 recommended the periodic measurement of Vitamin B12 in patients with metformin therapy without specifying the dose and the time of exposure [9]. The objective of this study is to evaluate the metformin ratio according to the time of exposure and the dose used.
Materials and Methods
Prospective transversal study, 296 patients were enrolled into the study from 1st April 2017 to 31st March 2018. Selected subjects were divided in two groups: 118 without type 2 diabetes [T2DM] and 178 patients with T2DM treated with metformin [MET]. Patients and controls were excluded from the study if they had a history of cancer, malabsorptive diseases, bariatric surgery, alcoholism, pregnant women, stage 4 chronic kidney disease [Glomerular Filtration Rate: 15-30 ml/min/1.73 m2], vitamin B12 [VB12] supplementation or drugs that could affect VB12 absorption or metabolism. The subjects were informed about the study and signed the informed consent. Family history was noted as well as personal data such as: habits, concomitant medication, weight [kg], height [m], blood pressure [mmHg]. Body mass index [BMI, kg/m2] was calculated. Underweight was defined as BMI <18.5, normal weight between 18.5 and 24.9, overweight between 25 and 29.9 and obesity ≥30 kg/m2. A complete physical examination was performed, which included neuropathy assessment by the Michigan Neuropathy Screening Instrument [MNSI] [10]. Microvascular [retinopathy, nephropathy and neuropathy] and macrovascular [Acute myocardial infarction and stroke] complications of diabetes were searched for. Laboratory data included hemogram, hepatogram, creatinine, glycemia by standardized methods [Autoanalyzer DxC 800, Beckman-Coulter, USA], glycated hemoglobin [HbA1c] [HPLC, Variant II, Bio-Rad, EEUU], thyroid function tests, VB12 and vitamin D3 [Chemiluminescence autoanalyzer DxI Beckman Coulter, EEUU]. All patients gave written informed consent. The medical Ethical Committees of the three participating hospitals approved the trial protocol. The trial has been and is conducted in accordance with the Note for Guidance on Good Clinical Practice [CPMP] and in accordance with the Declaration of Helsinki [Revised Version of Argentine].
Statistical Analysis
Serum VB12 concentration was determined in all participants as an Outcome variable. VB12 was used by its numerical value but, in most cases, it was categorized according to NHANES. We defined biochemical B12 deficiency as serum levels <200 ng/dl, borderline deficiency: 200 to 300 ng/ml and normal 300 ng/ml. To establish the association between VB12 deficiency and categorical data, arranged in contingency tables, we used the chi-square test. To compare the media of numerical variables between the groups of VB12 deficiency, variance analysis was applied in the NHANES categorized variables and t-test when VB12 deficiency was categorized as deficiency or normal. When the normality assumptions for the t-test were not met, the Mann-Whitney non parametric test was used to compare two samples and, to compare more than two groups, the Kruskal-Wallis test. In occasions, asymptotic tests were used, due to the large sample size. Data with significant association is presented as well as some results that show no significant association but are considered relevant for that matter. In all cases probability <0.05 was considered significant and statistical analysis was performed using Info Stat software [Universidad Nacional de Cordoba, version 2018] (Figure 1).
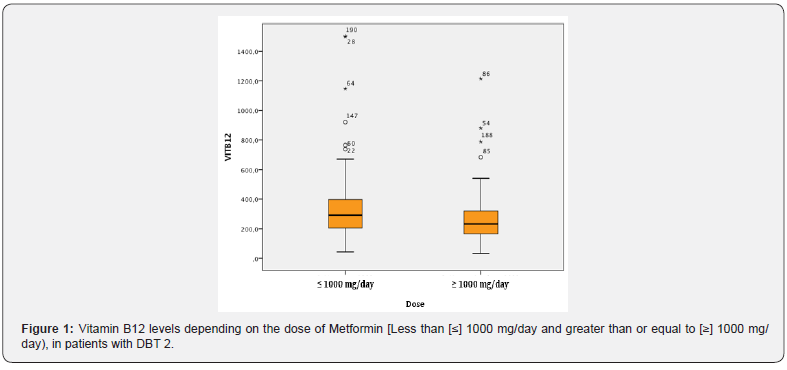
Results
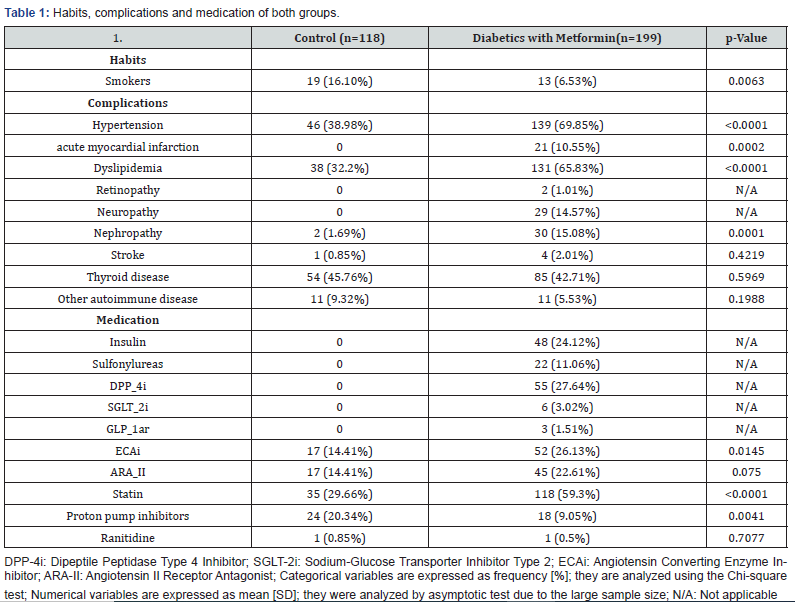
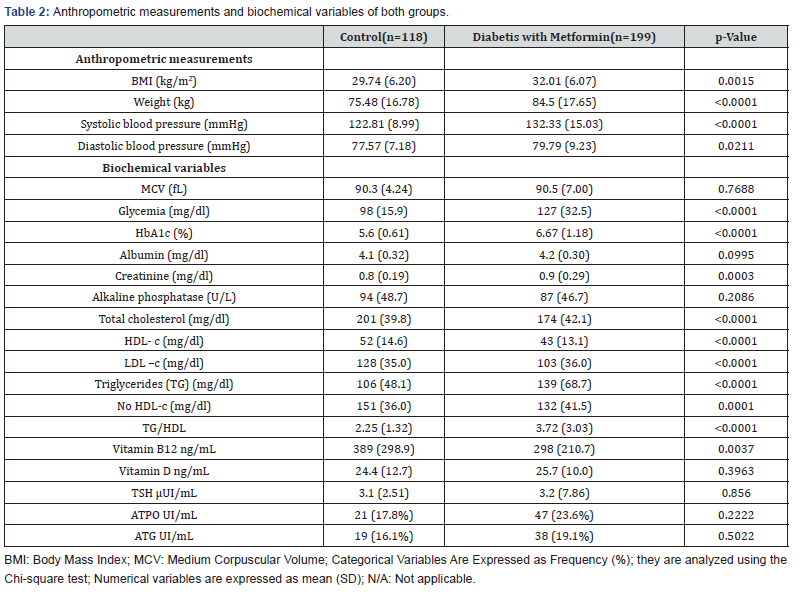
T2DM group of patients consisted of 69 men and 109 women with an average age of 65.46 ± 7.36 years old while control group enrolled 13 men and 105 women with an average age of 59.31 ± 14.20 years old. No significant difference between group’s average age was observed [p=0.052]. Table 1 shows habits, concomitant medication and complications in both groups. Anthropometric measurements and biochemical variables are shown in Table 2.
Patients with T2DM presented with higher weight and blood pressure than those in the control group. As expected, patients with T2DM reported higher glycemia, HbA1c [p<0.001] and creatinine [p=0.015] as an index of renal function and significantly higher TG/HDL-c ratio [p<0.001] as a predictor of insulin resistance and atherosclerosis. Significant differences between HDL-c and LDL-c [p<0.001] were noticed with no significant difference in serum VD3. Compared with control group [389 ng/L], T2DM metformin users [298 ng/L] had significantly lower values of VB12 [p<0.001]. When VB12 values where analyzed according to NHANES criteria in: normal, borderline and deficiency; control group individuals where 49.2%, 34.7% and 16.1% respectively, while metformin treated T2DM patients where 33.7%, 31.5% and 34.8% respectively with a significant difference in normal and deficiency levels. When relationship between VB12 and age was analyzed, both groups where divided into subgroups according to age: less than [<] 65 years old, and older or equal to [≥] 65 years old. Lower levels where observed in both subgroups in the T2DM patients [p=0.007 and p=0.001 respectively]. Regarding VB12 and neuropathy, the association was analyzed only in T2DM group given that patients in the control group had no neuropathy. Patients with neuropathy showed higher proportion of VB12 deficiency [48%] than patients without it [32.68%]; [p=0.04]. No significant difference was observed among patients treated only with metformin and those with combined therapy of metformin and sulfonylureas, dipeptidyl peptidase 4 [DPP4] inhibitors and insulin. There was no significant difference between the different drugs and VB12 deficiency. No association was established between VB12 deficiency and weight categories [p=0.96].
Metformin dose was rated in < 1000mg and 1000mg/day. Association was observed between Metformin dose and VB12 deficiency: 69.5% of VB12 deficiency values in patients taking high doses of metformin in comparison to 51.6% in low dose treatment [p-value= 0.009]. Nevertheless, no association was ascertained between time of exposure to Metformin and VB12 serum concentration in any of the doses. A significant difference was observed in VB12 concentration values in patients taking angiotensin-converting- enzyme inhibitor [ACE inhibitor] [71.2% of patients with VB12 deficiency] in comparison to patients on other antihypertensive drugs [56.8% of VB12 deficiency] [p=0.035].
Discussion
Vitamin B12 [VB12] also known as cobalamin, plays a crucial role in intracellular metabolism. Its deficiency, with the presence of classical hematological and neurological manifestations, is rather infrequent presently. Nevertheless, subclinical deficiency can affect at least 26% of the general population even though its clinical relevance remains uncertain [11]. In our study we found a significant association between VB12 deficiency and daily dose of Metformin [MET] ≥1000 mg/d. Even though this conclusion was not met by some authors[12,13], others shared the same results and even confirmed that it constituted an independent risk factor that behaved as the strongest predictor of VB12 deficiency in type 2 diabetes mellitus [T2DM] subjects [14-17]. After adjusting by multiple variables, it was observed that the increase of 1 gram of MET a day doubled the risk of VB12 deficiency. Similar results were obtained in different studies [14-17]. Liu & col [18] proposed a dose of >2000 mg/d of MET as the cut-off point for significant decrease in VB12 concentration. Moreover, with doses over 2 g/d it was determined an increase of 22 times the risk of VB12 deficiency in a study by Alharbi [16]. A study by Ko designed ROC curves about daily doses of MET with an area under the curve [AUC] of 0.69 and a cut-off point of 1.125 mg with a sensibility of 64% and specificity of 65% [15].
Other authors described serum concentrations of VB12 and its biomarkers [Methylmalonic Acid and Homocysteine] [19,20]. Three meta-analysis agreed and pointed out the interaction between time of exposure to MET and VB12 serum concentration, with a more significant result after three years of treatment [18,21]. Transversal and prospective studies reached the same results regarding risk and confirmed that the daily dose and time of exposure to MET are the most important risk factors for VB12 deficiency. Even in Ko´s study the ROC curves for duration of MET use showed an AUC of 0.72 with a cut-off point of 45.5 months with a sensibility of 80% and specificity of 57% for this deficiency [14-16]. Aroda was able to calculate a significant decrease of almost 1 pMol/L in VB12 concentration for every month of use of MET and in a controlled and randomized study, Raizada obtained an OR of 1.13 per year of treatment and, 13 years later, there was an increase of 13% of VB12 deficiency for every year of exposure to MET [5,22]. Prospective studies pointed out a positive correlation between duration of treatment with MET and the development of peripheral neuropathy due to VB12 deficiency [20,23,24]. HOME study proved that a decrease in VB12 concentration associated to the use of MET is not a transitory phenomenon but progressive and persistent [25,26]. Unlike what was previously detailed, our study found no association between VB12 deficiency and time of exposure to any of the doses of MET. Additionally, some studies found no significant increase in the prevalence of VB12 in patients with T2DM in relation to time of use of MET [2,12,13,19].
No significant association was found between VB12 levels in patients treated with combined therapy of metformin and sulfonylureas, dipeptidyl peptidase 4 [DPP4] inhibitors and insulin. In recent reviews on VB 12 deficiency it is noted that its prevalence increases in subjects over 60 years old and it is exacerbated in people older than 80 years. In this last age group, associated clinical manifestations [hematological and neurological] are generally not present or can easily be mistaken for other medical conditions related to age. What is more, Methylmalonic Acid and Homocysteine, the biomarkers of this deficiency, have less sensibility because of the concomitant renal disease which leads to their increase in the elderly population [11]. The decline in VB12 concentration associated to age is attributed to a diminished absorption and low dietary intake. This malabsorption is due to low gastric acid secretion, Helicobacter pylori infection and polypharmacy which is so frequent in the older population [8,9]. In a study done in USA amongst more than 6000 adults older than 50 years, using information from the data base of the National Health and Nutrition Examination Survey [NHANES 1999–2006], it was also concluded that the risk of T2DM and VB12 deficiency increases with age. Another prospective study in an eastern population proved correlation between age and VB12 deficiency associated to treatment with MET, with an odds ratio [OR] of 1.36 for every 10 years increase in age [8,14]. Nevertheless, multiple transversal, prospective and interventional studies also searched for this association with no positive results in relating age and VB12 serum concentration in T2DM patients treated with MET [2,14]. In our study, no significant difference was found with age between both groups. T2DM patients presented significantly lower VB12 values than the control group coincidentally with results found in the literature. When relationship between VB12 and age was analyzed, it was noticed that patients with diabetes presented lower VB12 values in both age subgroups in agreement with most authors. Regarding VB12 and body mass index [BMI], in a transversal study which involved patients with morbid obesity, BMI was adversely associated with serum VB12 due to a probable decrease in gastrointestinal absorption. Greater BMI resulted in a decrease in VB12 levels without other hematological alterations [Hemoglobin [Hb] and Mean Corpuscular Volume [MCV]]. When comparing obese BMI with VB12 deficiency and BMI of patients with normal level of VB12 a statistically significant difference was observed. Even in the multivariate model, a higher BMI could predict VB12 deficiency in morbid obesity with an OR 1.031 [p=0.007][27]. Nonetheless, in most studies carried out to analyze VB12 deficiency in patients with T2DM under treatment with MET, no significant difference was found between BMI in patients with VB12 deficiency compared to those with normal values [13,15,25,28]. Neither did we found association between VB12 deficiency and obesity categories. It is well known that in clinical VB12 deficiency, neurological manifestations have an earlier development in comparison to hematological symptoms. The reduction in serum levels of VB12 rarely present with neurological disorders and usually in the absence of anemia. [11,29,30]. While the increase in MCV often precedes the decrease in Hb concentrations in megaloblastic anemia, no statistically significant correlation was found between MCV values and VB12 levels in a research made to prove the diagnostic accuracy of this parameter. The sensibility and specificity of MCV to detect VB12 deficiency was calculated to be 10.14% and 92.82% respectively. MCV precision for this vitamin depletion was estimated in 45.05% [31]. As in our population, studies on T2DM patients treated with MET no significant difference was observed in MCV of patients with VB12 deficiency and subjects with normal values [8,12,16,17]. A greater prevalence of anemia is described amongst patients with T2DM in the NHANES population, but overall of the no macrocytic type. This anemia has probably a multifactorial origin [iron-deficiency, chronic diseases] [2]. Regarding Hb concentration, no significant difference was found between T2DM patients treated with MET with VB12 normal concentration and deficiency. Therapy with MET did not predict the risk of anemia after almost 5 years of treatment [2,5,8,13,22,32]. Some investigations found a higher percentage of anemia in patients with VB12 deficiency associated to the use of MET but with normal MCV. It is of common knowledge that macrocytic manifestation of meg aloblastic anemias can be masked by the coexistence of Thalassemia, iron deficiency and chronic diseases reasons why, amongst these patients, one could not rule out megaloblastic anemia because a normal MCV [15,23]. Nonetheless, our research did not find a significant difference in either Hb concentration or in MCV.
Classic neurological manifestations of VB12 deficiency are sensitive and motor disorders [mainly of the lower limbs], ataxia, cognitive decline that can lead to dementia and psychiatric disorders as depression. These conditions are due to central and peripheral nervous system demyelination [11,27,32]. In one of the most recent published meta- analysis on VB12 deficiency in T2DM patients treated with MET, controversial results were observed on the prevalence of peripheral neuropathy possibly owing to the different research designs and methods used in the studies involved [some based only in reported symptoms from the medical history, others using different scores like Michigan or Toronto and very few used electrophysiological nerve conducting studies to asses Nerve Conduction Velocity] [8]. Some authors found no relationship between this vitamin deficiency associated with MET and the prevalence of peripheral neuropathy. Even after adjusting by different variables they reached the conclusion that neurological complications were most likely related to T2DM duration. Results in studies and meta-analyses are controversial [13,17,22,33]. On the other hand, other research studies described a strong correlation between the severity of peripheral neuropathy [by the Toronto score] and increasing and cumulative doses of MET; but in Alharbi´s study after analyzing electrophysiological tests and correcting results by multiple variables, statistical significance of this association was revoked[16,20]. Additionally, Aroda in the secondary analysis of DPP/DPPOS observed a higher prevalence of peripheral neuropathy by using monofilament test in patients with VB12 deficiency but this was recognized in a small number of patients and could not be confirmed by Michigan score. Gupta described an earlier start of the peripheral neuropathy in individuals with this vitamin deficiency and a positive correlation with MET treatment duration [5,23]. In our research, we analyzed relationship between VB12 and neuropathy in the T2DM group. Association was significant, with a higher proportion of VB12 deficiency amongst patients with neuropathy than in those without it as it is also described in some of the studies mentioned above. As a finding, we observed, in concordance with Tal, an association between the use of ACE and VB12 deficiency even though the mechanism is yet to be elucidated [34]. As a conclusion to our work, we observed that T2DM patients treated with MET 1000 mg/d presented lower VB12 serum concentrations and a higher prevalence of diabetic neuropathy without other complications. Despite Tomkin being the first to describe malabsorption of VB12 associated to the use of MET in the year 1971 and that, since 2017, the American Society of Diabetes recommends its periodic determination in patients treated with MET, there are yet plenty of aspects to clarify. The discussion about the capacity of MET to cause VB12 deficiency and its clinical consequences continues. The lack of gold standard diagnostic tests to verify VB12 status generates controversies around the accuracy of results obtained which adds more complexity to the subject [7,8]. A limitation of our work is its transverse design, so we could not evaluate cause effect. Within the strengths we were able to count on a group of controls without T2DM and we also obtained information on confusing factors such as comorbidities, medications and use of supplements with VB12. Further research is needed to confirm this association, improve the accuracy of diagnostic tests, study long term clinical consequences and the effects of therapeutic intervention on this vitamin deficiency [35].
Authorship
Contribution to: The conception and design, or analysis and interpretation of data: Jimena Soutelo, Clara Fritz, Sofía Moldes, Florencia Borghi Torzillo, Yanina Oliva, Natalia Carbajo, María de Luján Calcagno, Silvina Del Duca., The drafting of the article or its critical review for important intellectual content: Jimena Soutelo, Clara Fritz, Sofía Moldes, Florencia Borghi, María de Luján Calcagno, Silvina Del Duca. The final approval of the version to be published: Jimena Soutelo, Clara Fritz, Sofía Moldes, Florencia Borghi, María de Luján Calcagno, Silvina Del Duca , Ruben Lutfi.
References
- Davies JM, D´Alessio DA, Fradkin J, Kernan WN, Mathieu C, et al. (2018) Management of hyperglycemia in type 2 diabetes, A Consensus Report by The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 41(12): 2669-2701.
- Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP Jr (2012) Association of biochemical B12 deficiency with metformin therapy and Vitamin B12 supplements: The National Health and Nutrition Examination Survey, 1999‑2006. Diabetes Care 35: 327‑333.
- Calvo Romero JM, Ramiro Lozano JM (2012) Vitamin B (12) in type 2 diabetic patients treated with metformin. Endocrinol Nutr 59; 487‑490.
- Niafar M, Hai F, Porhomayon J, Nader ND (2015) The role of metformin on Vitamin B12 deficiency: A meta‑analysis review. Intern Emerg Med 10: 93‑102.
- Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, et al. (2016) Long‑term metformin use and Vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 101: 1754-1761.
- Berchtold P, Bolli P, Arbenz U, Keiser G (1969) Disturbance of intestinal absorption following metformin therapy (observations on the mode of action of biguanides) (in German). Diabetologia 5: 405–412.
- Tomkin GH, Hadden DR, Weaver JA (1971) Montgomery DA. Vitamin- B12 status of patients on long-term metformin therapy. Br Med J 2: 685–687.
- Marwan Awad Ahmed (2016) Metformin and Vitamin B12 Deficiency: Where Do We Stand? J Pharm Pharm Sci 19(3): 382-398.
- American Diabetes Association (2017) Pharmacologic Approaches to Glycemic Treatment. Diabetes Care 40 (Suppl1): S64-S74.
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, et al. (1994) A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 17(11): 1281-1289.
- Green R, Allen L, Bjorke-Monsen AL, Brito A, Gueant JL, et al. (2017) Vitamin B12 deficiency. Nature Reviews Disease Primers 3: 17040.
- Marwan Awad Ahmed (2016) Metformin and Vitamin B12 Deficiency: Where Do We Stand? J Pharm Pharm Sci 19(3): 382-398.
- Damiao CP, Rodrigues AO, Castellar Pinheiro MF, Filho RA, Peres Cardoso G (2016) Prevalence of vitamin B12 deficiency in type 2 diabetic patients using metformin: a cross-sectional study. Sao Paulo Med J 134(6): 473-479.
- Rodriguez-Gutierrez R, Montes-Villareal J, Rodriguez-Velver KV (2017) Metformin use and vitamin B12 deficiency. Untangling the association. Am J Medical Sciences 354(2): 165-171.
- Ting RZ, Szeto CC, Ho-Ming Chan M, Kuen Ma K, Chow KM (2006) Risk Factors of Vitamin B12 Deficiency in Patients Receiving Metformin. Arch Intern Med 166: 1975-1979.
- Ko SH, Ahn YB, Song KH, Han KD, Park YM, et al. (2014) Association of Vitamin B12 deficiency and Metformin use in patients with Type 2 Diabetes. J Korean Med Sci 29: 965-972.
- Alharbi TJ, Tourkmani AM, Abdelhay O, Alkhashan HI, Al-Asmari AK (2018) The association of metformin use with vitamin B12 deficiency and peripheral neuropathy in Saudi individuals with type 2 diabetes mellitus. PLoS ONE 13(10): e0204420
- De Groot-Kamphuis DM, Van Dijk PR, Groenier KH, Houweling ST, Bilo HJ, et al. (2013) Vitamin B12 deficiency and the lack of its consequences in type 2 diabetes patients using metformin. Neth J Med 71: 386-390.
- Liu Q, Li S, Quan H, Li J (2014) Vitamin B12 Status in Metformin Treated Patients: Systematic Review. PLOS ONE 9(6): e100379.
- Beulens JW, Hart HB, Kuijs R, Kooijman-Buiting AM, Rutten GE, et al. (2015) Influence of duration and dose of metformin on cobalamin deficiency in type 2 diabetes patients using metformin. Acta Diabetol 52: 47-53.
- Wile D, Toth C (2010) Association of Metformin, Elevated Homocysteine, and Methylmalonic Acid Levels and Clinically Worsened Diabetic Peripheral Neuropathy. Diabetes Care 33: 156-161.
- Chapman LE, Darling AL, Brown JE (2016) Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes & Metabolism 42: 316-327.
- Raizada N, Jyotsna VP, Sreenivas V, Tandon N (2017) Serum vitamin B12 levels in type 2 diabetes patients on metformin compared to those never on metformin: A cross-sectional study. Indian J Endocr Metab 21: 424-428.
- Gupta K, Jain A, Rohatgi A (2018) An observational study of vitamin B12 levels and peripheral neuropathy profile in patients of diabetes mellitus on metformin therapy. Diabetes & Metabolic Syndrome: Clinical Research & Review 12: 51-58.
- Braza M, Hanley J, Bhatla A, Martinez M (2009) Prevalence of vitamin B12 deficiency in Hispanic patients with type 2 Diabetes Mellitus on long term Metformin - Is it associated with peripheral neuropathy. Diabetes 1(58): A153.
- De Jager J, Kooy A, Lehert P, Wulffelé M, Van der Kolk J, et al. (2010) Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomized placebo-controlled trial. BMJ 340: c2181.
- Wulffele MG, Kooy A, Lehert P, Bets D, Ogtero JC, et al. (2003) Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. Journal of Internal Medicine 254: 455-463.
- Arshad M, Jaberian S, Pazouki A, Riazi S, Rangraz MA, al. (2016) Iron deficiency anemia and megaloblastic anemia in obese patients. Rom J Intern Med 55(1): 1-13.
- Khan A, Shafiq I, Shah MH (2017) Prevalence of vitamin B12 deficiency in patients with type II Diabetes Mellitus on Metformin: a study from Khyber Pakhtunkhwa. Cureus 9(8): 1577.
- Stabler SP (2013) Clinical practice. Vitamin B12 deficiency. N Engl J Med 368: 149-160.
- Ralapanawa DM, Jayawickreme KP, Ekanayake EM, Jayalath WA (2015) B12 deficiency with neurological manifestations in the absence of anaemia. BMC Res Notes 8: 458.
- Patel S, Dhupar P, Bhattar A (2017) Accuracy of Mean Corpuscular Volume in delineating Vitamin B12 deficiency. Ann Clin Lab Res 5(3): 195.
- Kaur SNL, Nair GV, Sharma CS, Dudeja P, Puri BP (2018) A descriptive study of clinico-hematological profile of megaloblastic anemia in a tertiary care hospital. Med J Armed Forces India 74(4): 365-370.
- Dunstan DF, Rees JA, Chen S, Lansdown JA, Moast SJ (2012) An observational study of the effect of metformin on B12 status and peripheral neuropathy. Br J Diabetes Vasc Dis 12: 189-93.
- Tal S, Shavit Y, Stern F, Malnick S (2010) Association Between Vitamin B12 Levels and Mortality in Hospitalized Older Adults. JAGS 58(3): 523-526.






























