Exploring the Mechanism of Insulin Action, Differential Insulin Sensitivity and Insulin Resistance
Vinod Nikhra*
Department of Medicine, NDMC Medical College, India
Submission: July 20, 2019; Published: October 01, 2019
*Corresponding author: Vinod Nikhra, Senior Chief Medical Officer and Consultant, Department of Medicine, Hindu Rao Hospital and NDMC Medical College, New Delhi, India
How to cite this article: Vinod Nikhra. Exploring the Mechanism of Insulin Action, Differential Insulin Sensitivity and Insulin Resistance. Curr Res Diabetes Obes J. 2019; 12(2): 555831. DOI:10.19080/CRDOJ.2019.11.555831
Abstract
Correlating Altered Insulin Sensitivity and MetS: Insulin is an anabolic hormone and regulates glucose homeostasis and other metabolic processes in various organs. With aging, there occurs an impairment of glucose-stimulated insulin pulse mass, amplitude and rhythmicity due to deterioration of β-cell function and progressive loss of β-cell mass. Further, with aging there occurs a reduction in insulin sensitivity leading to insulin resistance (IR) involving liver, striated muscle, adipose tissue and other organs. At the cellular level, the insulin triggers signaling cascades that regulate cell metabolism, cell proliferation and cell survival.
Pathophysiology of Altered Insulin Sensitivity: IR denotes an impaired signal transduction and occurs when the circulating levels of the hormone are absolutely or relatively insufficient to regulate the metabolic processes appropriately. The insulin stimulated metabolic pathways are important for mitochondrial function and the IR due to chronic relative insulin insufficiency affects the mitochondrial function. IR is a major component of metabolic syndrome and has impact on aging process, survival and longevity.
Molecular Mechanisms for Insulin Resistance: The FAs compete with glucose for substrate oxidation at the mitochondrial level. The increase in FAs causes an increase in the intramitochondrial acetyl CoA/CoA and NADH/NAD+ ratios, with subsequent inactivation of pyruvate dehydrogenase → ↑ intracellular citrate concentrations → inhibition of phosphor-fructokinase, a key rate-controlling enzyme in glycolysis. Subsequent accumulation of glucose-6-phosphate would inhibit hexokinase II activity → ↑ intracellular glucose concentrations and↓ glucose uptake. There occur FA-induced alterations in upstream insulin signaling events, resulting in decreased GLUT4 translocation to the plasma membrane and reduced glucose transport activity.
The Impact of Insulin Resistance on Homeostasis: The complex alterations in the metabolic macro- and microenvironment due to decreased insulin sensitivity lead to mitochondrial dysfunction and ER stress. The disruption of Insulin signaling impairs the entry of glucose into skeletal muscle cells and adipocytes. In addition, the cell-intrinsic mechanisms of obesity-associated IR are intensified by cell-extrinsic modulators such as endocrine and inflammatory signals. In case of MetS associated with obesity and ectopic lipid accumulation, the signaling pathways are affected by elevated FFAs, inflammatory mediators such as TNFα, IL-1 and IL−6, adipokines and hepatokines, bioactive intracellular lipid intermediates like diacylglycerols and ceramides.
IR, Aging Process, Survival and Disease: To compensate for the insulin-resistant state, β-cells initially increase basal and postprandial insulin secretion. The compensatory hyperinsulinemia is an attempt to maintain normoglycemia. Chronic hyperinsulinemia is beneficial to some extent as it overcomes the impact of subnormal insulin action in various organs. However, the metabolic derangements in tissues due to IR and differential insulin sensitivity expose the tissues to mitochondrial dysfunction and ER stress and promote the development of atherosclerosis and lead to increased risks of CVD, metabolic diseases such as T2DM and degenerative diseases such as Alzheimer’s disease, and compromised QOL and reduced longevity.
Keywords: Differential insulin sensitivity; Insulin resistance; Metabolic syndrome; PI3K/Akt pathway; MAPK; Ras pathway; Type 2 diabetes; Visceral obesity; Anabolic hormone; Triglycerides; Fat; Gluconeogenesis; Glycogenolysis; Lipid metabolism; Glucose; Amino acid; Cell proliferation; Cell survival
Abbreviations: CVD: Cardiovascular Disease; IR: Insulin Resistance; MetS: Metabolic Syndrome; FAs: fatty Acids; Om: Omental; FFAs: Free Fatty Acids; PTPases: Protein-Tyrosine Phosphatases; Sc: Subcutaneous; JNK: Jun NH2-Terminal Kinase; PKB: Protein Kinase B; PERK: Protein Kinase RNA-like Endoplasmic Reticulum Kinase; ERAD: ER-Associated Degradation
Corelating Altered Insulin Sensitivity and Mets
Insulin is an anabolic hormone and regulates glucose homeostasis and other metabolic processes in various organs. It increases rate of glucose uptake into skeletal muscle and adipose tissue, reduces hepatic glucose output (via decreased gluconeogenesis and glycogenolysis) and increases lipid synthesis in liver and fat cells, simultaneously attenuating fatty acids (FAs) release from triglycerides in fat and muscle. At the cellular level, the insulin binding to its receptors at the cell surface triggers the initiation of signaling cascades that regulate cell metabolism (including glucose, amino acid and lipid metabolism), cell proliferation and cell survival.
Insulin resistance (IR) denotes an impaired signal transduction and occurs when the circulating levels of the hormone are insufficient, absolutely or relatively, to regulate the metabolic processes appropriately leading to an impairment of insulin signaling in peripheral tissues, particularly liver, skeletal muscle, adipose tissue and heart [1]. IR is a component of metabolic syndrome (MetS) and has impact on cardiovascular aging and incidence of cardiovascular disease (CVD). Apart from IR, aging process and obesity – the adverse phenotype and metabolic triad - have impact on survival and morbidity (Figure 1).
Altered insulin signaling with adiposity
Obesity is a major contributor to the pathogenesis of IR and the increased visceral abdominal or omental (Om) fat is associated with abnormalities in insulin signaling, glucose intolerance, dyslipidemia and significantly increased CV risk. The intraabdominal adipose tissue has a relative resistance to anti-lipolytic action of insulin and enhanced sensitivity to catecholamine-induced lipolysis. The enhanced lipolysis increases the portal and systemic concentration of free fatty acids (FFAs), which reduce the insulin sensitivity to glucose metabolism and disposal in skeletal muscle and liver and impair pancreatic insulin secretion [2]. The expansion of the Om fat depot leads to raised FFA levels and contributes to IR in skeletal muscle and liver by reduced ability of insulin to stimulate the phosphorylation of several proteins like protein-tyrosine phosphatases (PTPases) which modulate insulin receptor kinase activation, intracellular signal transduction and signaling cascade in various tissues. In addition, certain PT Pases in insulin-sensitive cells, such as PTP1B, appears to be negatively related to regulation of insulin receptor autophosphorylation and post-receptor insulin signaling. The alterations in the intracellular enzymatic activity of PTP1B affect insulin action in adipose tissue. The mean endogenous PTPase activity is about 2-fold higher Om adipose tissue as compared with the subcutaneous (Sc) depot, denoting that Om adipose tissue is relatively resistant to the metabolic actions of insulin as compared to the Sc depot [3].
Altered insulin signaling with aging
Insulin secretion is pulsatile with low amplitude pulses every 8–15 min and ultradian pulses with larger amplitude at a periodicity of 60–140 min. The physiological pulse secretory pattern is disrupted in pathological conditions such as impaired glucose tolerance, obesity and T2DM. With aging, there occurs an impairment of glucose-stimulated insulin pulse mass, amplitude and rhythmicity due to a progressive loss of β-cell mass and deterioration of β-cell function [4]. Further, there occurs a reduction in insulin sensitivity leading to IR with aging involving liver, kidney, striated muscle and other organs.
There occur various physiological alterations with aging including altered body composition, decreased physical fitness, changes in hormones like growth hormone, insulin-like growth factors, leptin and sex steroids, and lipotoxicity and glucose toxicity due to sustained elevations of circulating FFAs and glucose. The reduced muscle mass, physical fitness and aerobic capacity (VO2 max) contribute to impaired insulin sensitivity. There are two pathophysiological components, IR-HOMA (Homeostatic model assessment determines β-cell function and IR) and inflammation, associated with incident frailty with age which is associated with decreased reserve in physiologic systems, functional limitations and adverse outcomes with chronic diseases [5].
The regulation of glucose homeostasis by insulin is altered during aging. There occurs a decline in glucose tolerance and peripheral glucose utilization [6]. The decreased insulin action on glucose metabolism is associated with a reduced insulin sensitivity for protein breakdown in older adults. Further, there is impaired skeletal muscle glucose uptake attributable to unopposed norepinephrine induced vasoconstriction due to decreased eNOS activity as result of IR. In addition, there occur autonomic alterations preceding the development of IR [7]. The reduced insulin action is associated with a compensatory increase in plasma insulin to improve glucose metabolism in insulindependent tissues such as skeletal muscle. Thus, the impaired glucose metabolism in older adults is associated with reduced inhibition of protein breakdown contributing to a progressive loss of body proteins, especially at the skeletal muscle level. This is accompanied by a nearly normal hepatic insulin sensitivity.
Insulin signaling in diabetes and hypertension
The muscle glycogen synthesis is the major pathway for glucose metabolism and under hyperglycemic-hyperinsulinemic conditions the defective muscle glycogen synthesis plays a major role in causing IR. Defects in glycogen synthase, hexokinase II and glucose transport have been implicated for the impaired muscle glycogen synthesis in T2DM. Even before the onset of diabetes, offspring of patients with T2DM have lower insulin sensitivity and reduced rate of muscle glycogen synthesis. Apart from this, the characteristics of IR have been shown to differ in salt-resistant (SR) and salt-sensitive (SS) subjects, independent of blood pressure. The enhanced proximal tubular sodium reabsorption by insulin is preserved in hypertensive subjects and the IR may precede and predict the development of essential hypertension [8].
There is evidence that a genetic predisposition may contribute to insulin resistance and the resultant hyperinsulinemia and hypertension. A. This concept is supported by the finding of altered glucose metabolism in normotensive offspring of hypertensive persons. Mechanisms for the development of hypertension in setting of IR and hyperinsulinemia include activation of the sympathetic nervous system, renal sodium retention, altered transmembrane cation transport, growth-promoting effects of vascular smooth muscle cells and vascular hyperreactivity. Hypertension may be associated with enhanced salt sensitivity and IR. Insulin has a peripheral vasodilatory effect, but this response is lost in insulin-resistant/obese persons suggesting resistance to the action of insulin to induce vascular NO production [9].
Pathophysiology of Altered Insulin Sensitivity
The insulin stimulated metabolic pathways are important for mitochondrial function and the IR due to chronic relative insulin insufficiency and FA-induced differential insulin sensitivity affects the mitochondrial function [10].
FFAs has impact on mitochondrial activity and may play a role in the development of mitochondrial dysfunction. In fact, insulinresistant individuals have reduced expression of mitochondrial gene mRNAs and lower protein expression of respiratory chain subunits, decreased mitochondrial DNA (mt-DNA), reduced oxidative enzyme activity and decreased mitochondrial size and density.
In fact, the FAs compete with glucose for substrate oxidation at the mitochondrial level. The increase in FAs causes an increase in the intramitochondrial acetyl CoA/CoA and NADH/NAD+ ratios, with subsequent leads to inactivation of pyruvate dehydrogenase → ↑ intracellular citrate concentrations → inhibition of phosphorfructokinase, a key rate-controlling enzyme in glycolysis. Subsequent accumulation of glucose-6-phosphate would inhibit hexokinase II activity → ↑ intracellular glucose concentrations and↓ glucose uptake. There occur FA-induced alterations in upstream insulin signaling events, resulting in decreased GLUT4 translocation to the plasma membrane and reduced glucose transport activity.
Insulin receptors and proximal signaling
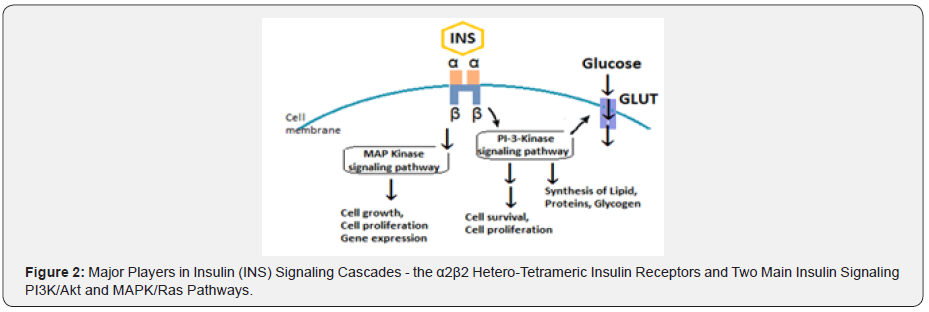
The insulin receptor is a cell-surface receptor and consists of two extracellular α-subunits linked to two intracellular β-subunits through disulfide bond to form a α2β2 hetero-tetrameric complex. Insulin binds to the α-subunits, transmits a signal across plasma membrane to activate the tyrosine kinase domain of β-subunit. Through a series of transphosphorylation reactions, the β-subunit phosphorylates its adjacent partner on specific tyrosine residues, various tyrosine residues accounting for distinct functions. In addition to tyrosine autophosphorylation, the insulin receptor is subjected to β-subunit serine/threonine phosphorylation, which attenuates receptor function. The counter-regulatory hormones and cytokines can also activate serine kinases, including protein kinase C (PKC), which leads to genesis of IR.
There are two main insulin receptor substrates, IRS-1 and IRS- 2 for propagation of insulin signal. The IRS proteins have an NH2- terminal PH domain and PTB domain having a variable-length COOH-terminal tail that contains numerous tyrosine and serine phosphorylation sites, and mediate specific interactions with the insulin and IGF-I receptor kinases. The IRS phosphorylation motifs, IRS-1/Irs-1 and IRS-2/Irs-2, which are widely expressed, whereas IRS-4/Irs-4 is limited to the thymus, brain and kidney and possibly β-cells.
On activation, the insulin receptor phosphorylates the insulin receptor substrates - IRS1/2, the Shc adapter protein isoforms, SIRP family members, Gab-1, Cbl and APS, which create recognition sites for additional effector molecules containing Src homology 2 (SH2) domains including adapter proteins Grb2 and Nck, the SHP2 protein tyrosine phosphatase and the regulatory subunit of the type 1A phosphatidylinositol 3–kinase (PI 3-kinase). IRS1 and IRS2 appear to have distinct physiological functions. In studies, the homozygous IRS1-KO mice develop a mild IR but do not become diabetic, because of β-cell compensation, whereas the disruption of the IRS2 gene results in IR, impaired insulin secretion and diabetes [11].
Downstream insulin signaling
The IRSs triggers intracellular signaling cascades by activation of two main signaling pathways: the phosphatidylinositol-3-kinase (PI3K)/Akt pathway and the mitogen-activated protein kinases/ Ras (MAPK/Ras) pathway, which regulates gene expression and insulin-associated mitogenic effects. Other Ser/Thr kinases such as protein kinase A (PKA), c-Jun amino-terminal kinase (JNK) and p38-kDa mitogen-activated protein kinase phosphorylate the insulin receptor and decrease its activity. There is variable overlap and crosstalk between the gene-regulated protein kinases having impact on the insulin signaling cascades.
The PI3K (phosphoinositide 3-kinase) pathway is the most important pathway for insulin signaling and responsible for many of the metabolic actions of insulin (Figure 2). The PI 3-kinase is activated on phosphorylation of motifs in IRS proteins and activates the Akt kinase which translocate to the mitochondria. The activated protein kinase B (PKB or Akt) phosphorylates many substrates to control various signaling reactions and plays a central role in activation and regulation of several metabolic processes including glucose transport, glycogen and protein synthesis and adipogenesis. It influences cell proliferation and cell survival through phosphorylation of the Forkhead proteins and the proapoptotic protein BAD to regulate various metabolic enzymes [12].
There occurs a relative decrease in insulin-stimulated association of IRS proteins with reduced activation of PI 3-kinase and activation of Akt in insulin-resistant skeletal muscle in T2DM [13]. The intrinsic cell-signaling pathways are also influenced by endogenous factors like oxidative stress, ER stress and mitochondrial dysfunction; whereas alterations in adipokines, increased FAs levels and the presence of inflammation in metabolic tissue are the extrinsic factors modulating the peripheral IR [14]. The TNF-α expression is high in muscle and fat in obesity and T2DM and has an inhibitory effect on insulin signaling in muscle and adipose tissue leading to IR [15].
In obesity, increased plasma FFAs concentration is associated with increased FAs exposure, leading to intracellular accumulation of FAs and FA metabolites such as diacylglycerol and ceramides, which plays an important role in the pathogenesis of IR. Various studies endorse the relationship between accumulation of intramyocellular triglyceride and IR [16]. The accumulation of intracellular fatty acid metabolites, such as diacylglycerol, fatty acyl CoA’s, or ceramides activates a serine/threonine kinase cascade, leading to phosphorylation of serine/threonine sites on insulin receptor substrates. The serine-phosphorylated forms fail to associate with or to activate PI 3-kinase, resulting in decreased activation of glucose transport and other downstream events. Evidence supporting this hypothesis comes from studies in transgenic mice that are almost totally devoid of fat because their adipocytes express the A-ZIP/F-1 protein, which blocks the function of several classes of transcription factors [17].
Insulin-stimulated glucose transport and uptake
The GLUTs are insulin-responsive molecules. The GLUT-4 is main glucose transporter and is located primarily in muscle cells and adipocytes. Following insulin stimulation, there is marked increase in the rate of GLUT4 vesicle exocytosis which recycles between the cell membrane and various intracellular organelle [18]. The GLUT4 vesicles contain the v-SNARE proteins VAMP2 and VAMP3, which interact with their t-SNARE counterparts Syntaxin-4 and SNAP23 in the plasma membrane during GLUT4 vesicle translocation. In addition, the SNARE accessory proteins, such as Munc18c, Synip and NSF, are required for the GLUT4 docking and fusion events [19].
The IR is a complex phenomenon in which certain genetic defects may combine with external stressors, as in obesity or infections, to generate the IR phenotype. In addition, several key molecules may function at lower than normal potential, resulting in poor signal transduction at multiple levels and glucose transport, or certain proteins are upregulated that inhibit the signaling pathways [20]. Skeletal muscle is the major site and to some extent adipose tissue, for insulin-stimulated glucose disposal. The resistance to the stimulatory effect of insulin on glucose utilization occurs in obesity, MetS and T2DM [21]. In addition, the impairment of insulin-stimulated glucose transport leads to decreased insulin-stimulated glycogen synthesis in skeletal muscle in T2DM [22]. Further, the chronic elevation of serum FFAs in obesity or T2DM contributes to the decreased uptake of glucose into muscles and other peripheral tissues in association with a loss of the ability of insulin to stimulate phosphoinositide-3 kinase activity [23].
The hyperglycemia itself has detrimental effects on insulin secretion and reduced insulin-stimulated glucose uptake [24]. There also occurs diversion of glucose from the glycolytic pathway to hexosamine pathway through the enzyme glutamine-fructose- 6-phosphate amidotransferase resulting in the production of glucosamine-6-phosphate and other hexosamine products [25]. The hexosamine products impair with the insulin stimulated glucose transport process and GLUT-4 translocation in skeletal muscles [26]. In addition, in T2DM, the pancreatic beta cells become insensitive to insulin. In this setting, insulin activates a different signalling pathway, through PI3K-C2α leading to proliferation and hyperplasia of pancreatic β-cells to produce extra insulin which exacerbates inflammation and has been in turn linked to IR [27].
Insulin resistance and homeostatic alterations
The insulin/IGF-signaling system plays a central role through integrating the storage and release of nutrients with the growth, development and maintenance of homeostasis throughout adult life [28]. Regulation of growth and longevity by the insulin/IGFsignaling system has been documented in Caenorhabditis elegans, as partial inhibition of insulin/IGF signaling increases nematode life span. The insulin/IGF signaling also coordinates longevity in Drosophila. There are multiple steps which are specific to the insulin- or IGF-signaling pathways and some elements are shared with other systems. There occurs dysregulation in insulin signaling cascade at certain steps leading to IR. At the physiological level, overnutrition, obesity, inactivity and aging are common causes of IR. The chronic hyperinsulinemia exacerbates IR and contributes directly to β-cell failure.
The mutations in the insulin receptor are a rare cause of lifelong IR. The elevated activity of protein or lipid phosphatases, including PTP1B, SHIP2 or PTEN can be a cause of IR. The high circulating FFAs, fatty acyl-CoAs, diacylglycerol and ceramides, and the stress-induced cytokines and metabolites promote serine phosphorylation of the IRS proteins, which impairs insulin signaling. Another TNF-α-signaling involves activation of the c-Jun NH2-terminal kinase (JNK) pathway, which is stimulated during acute or chronic inflammation [29]. JNK phosphorylates numerous cellular proteins, including IRS-1 and IRS-2, Shc and Gab1 and promotes phosphorylation of a serine residue located on the COOH-terminal side of the PTB domain inhibiting the function of the PTB domain and disrupting the IRS-1 tyrosine phosphorylation, leading to IR.
In addition, the partial failure of the insulin/IGF-signaling system is associated with various metabolic disorders including dyslipidemia, hypertension, female infertility and glucose intolerance in the prediabetic phase. The degradation of IRS proteins due to enhanced activity of the ubiquitin/ proteasome system in diabetes also exacerbate IR. The chronic hyperinsulinemia to compensate for glucose intolerance appears to differentially stimulate IRS-1 and the IRS-2 signals in tissues and cells leading to generation of free radicals and accelerated aging process.
IR: Molecular Mechanism and Pathways
There are endocrine, inflammatory and neuronal pathways link obesity to IR [30]. In case of MetS associated with overweight and obesity, the insulin sensitivity of signaling pathways is blunted by factors such as FFAs, the bioactive intracellular lipid intermediates such as diacylglycerols and ceramides, inflammatory mediators such as TNFα and Interleukins-1 and IL−6, adipokines and hepatokines which have been linked to ectopic lipid accumulation and lead to nutrient stress in the ER and mitochondria [31]. The increased proinflammatory cytokines such as TNF-α inhibit lipogenesis, promote lipolysis, disrupt insulin signaling and reduce the expression of GLUT4 in adipose tissue and myocytes, acting through the IKK-beta/NF-kappa-B pathway. IR is often present in in setting of visceral adiposity, hypertension, hyperglycemia and dyslipidemia involving elevated triglycerides and decreased HDL cholesterol levels. Further, the visceral adiposity is related to NAFLD, leading to an excessive circulating FFAs due to increased lipolysis and increased hepatic glycogenolysis and gluconeogenesis, which exacerbates peripheral IR [32]. The dyslipidemia and IR are also associated with a hypercoagulable state due to impaired fibrinolysis and increased inflammatory cytokine levels.
There is close interrelationship between obesity, inflammation, oxidative stress and IR. The obesity-associated increase in FAs triggers IR through intracellular metabolites that activate PKC, leading to the activation of serine/threonine kinases that impair insulin signaling. In addition, FAs also trigger IR by direct activation of TLR4 and the innate immune response. The decreased production of GLUT4 on the cell membrane may also contribute to IR. There are obesity-associated changes in secretion of adipokines that alter insulin signaling. There occurs an obesity-related alteration in the central response to hormonal and nutrient signals. An associated factor is leptin deficiency and resistance. The endocrine and inflammatory mediators converge on serine/threonine kinases that inhibit insulin signaling. The studies in mice with obesity and diabetes, leptin replacement in has been found to reduce glucose and insulin levels and improve insulin sensitivity [33]. Cortisol is another endocrine factor produced by adipose tissue and elevated glucocorticoid levels cause IR.
An important kinase likely to mediate the crosstalk between inflammatory and metabolic signaling is JUN N-terminal kinase1 (JNK1), a serine/threonine protein kinase that is activated by many inflammatory stimuli including TNFα. Activation of JNK1 leads to serine phosphorylation of IRS-1 impairing insulin action. Another class of inflammatory mediators contributing to obesity-induced IR are SOCS proteins, which constitute a negative feedback pathway in cytokine signaling. The SOCS family proteins, induced by adipokines, induce IR either by interfering with IRS- 1 and IRS-2 tyrosine phosphorylation or by targeting IRS-1 and IRS-2 for proteosomal degradation. At least three members of the SOCS family (SOCS-1, SOCS-3, and SOCS-6) have been implicated in cytokine-mediated inhibition of insulin signaling. The overexpression of SOCS-1 and SOCS-3 in the liver causes systemic IR [34].
There are neuronal mechanisms linking food intake, obesity and IR. The brain regulates feeding behavior and substrate metabolism to promote homeostasis of energy stores and fuel metabolism. It processes information from adiposity signals such as insulin and leptin, which is related to body fat mass and signals from nutrients such as FAs. In addition, there is impact on insulin sensitivity through the CNS regulation of the circadian rhythm. Impaired food intake with altered expression of neuroactive peptides, such as leptin, causes metabolic imbalances affecting insulin sensitivity. The mice lacking a key component of the molecular circadian clock in the hypothalamus develop a metabolic syndrome of hyperlipidemia, hepatic steatosis, and hyperglycemia [35]. The human study in workers with alternating shift work had a higher risk for developing diabetes compared with their day shift counterparts [36].
There is increased nitric oxide (NO) production in setting of obesity as a consequence of inducible nitric oxide synthase (iNOS), which is markedly increased in macrophages and other inflammatory cells. In the presence of O2, NO covalently attaches to cysteine residues of target proteins forming S-nitrosothiol adducts through protein S-nitrosation. The obesogenic diet increases S-nitrosation of the insulin receptor and AKT/PKB in adipocytes and in skeletal muscle and induces IR. The studies in aging mice have shown increased iNOS expression with S-nitrosation of the insulin receptor IRS-1 and AKT/PKB in skeletal muscle [37].
Concepts in IR and Altered Homeostasis
The inter-relationship and alterations in the metabolic macroand microenvironment leading to decreased insulin sensitivity are complex. The disruption of Insulin signaling impairs the entry of glucose into skeletal muscle cells and adipocytes. In addition, the cell-intrinsic mechanisms of obesity-associated IR are intensified by cell-extrinsic modulators such as endocrine and inflammatory signals [38].
The complex insulin signaling system
The insulin signaling is an integrated multisystemic network involving the insulin target tissues such as pancreas, liver, muscle and adipose. The peripheral IR is modulated by increased insulin secretion, as the insulin signaling cascade modulates β‐cell function [39]. The increased circulating FAs and other lipids in MetS and obesity lead to ectopic intracellular lipid accumulation in muscle and liver, which impairs insulin signaling through activation of PKC. It also triggers an increase in ROS leading to mitochondrial dysfunction and cellular ER stress which impair insulin signaling (Figure 3).
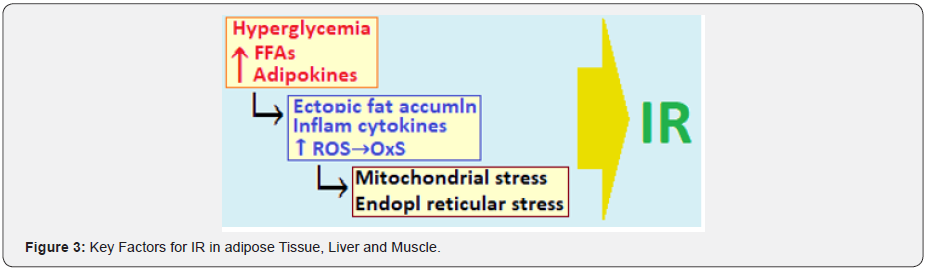
There are various molecules involved in the intracellular processing of the insulin signaling and include IRS1/2, the protein kinase B (PKB) isoforms and the Forkhead transcription (FoxO) factors. The FoxO family consists of FoxO1, FoxO3, FoxO4 and FoxO6 proteins in mammals and mediate the inhibitory action of insulin or insulin-like growth factor on key functions involved in cell metabolism, growth, differentiation, oxidative stress, senescence, autophagy and aging. The FoxO factors also control upstream signaling elements governing insulin sensitivity and glucose metabolism and the altered FoxO expression, especially FoxO6 in liver, adipose tissue and brain is associated with the pathogenesis of IR, dietary obesity and T2DM and risk of neurodegeneration disease [40].
Insulin signaling via PI3K leads to elevated AKT activity. AKT-mediated phosphorylation of FoxO promotes cytoplasmic localization of FoxO, thereby lowering its activity in the nucleus. The ER stress is a potential contributor to IR. During ER stress JNK mediated signaling phosphorylates IRS proteins and impairs PI3K/AKT signaling in response to insulin, lowering AKT activity which leads to elevated nuclear FoxO localization. Thus, the ER stress pathway involves the protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway to modulate cellular insulin responsiveness. The newly synthesized proteins normally are folded, processed and assembled in the ER and the misfolded proteins are eliminated via the ER-associated degradation (ERAD) pathway. Under conditions of cellular nutrient overload, the ER stress leads to impaired regulatory capacity of the ERAD pathway causing misfolded proteins to accumulate. Elevated ER stress is known to cause IR due to reduced activity of FoxO factors through PERK-mediated FoxO phosphorylation [41].
Metabolic and clinical fallouts of IR
IR and differential insulin sensitivity: IR in both the periphery (primarily muscle and fat) and in the liver aggravates the altered carbohydrate and lipid metabolism. To compensate for the insulin-resistant state, β-cells initially increase basal and postprandial insulin secretion. The compensatory hyperinsulinemia is an attempt to maintain normoglycemia. Chronic hyperinsulinemia is beneficial to some extent to the resistant tissues as it overcomes the impact of subnormal insulin action in liver, muscle and adipose tissues; however, it brings about metabolic derangements in tissues bearing normal sensitivity to insulin [42]. Again, within the same tissue, some of the insulin-regulated pathways, such as the glucose metabolic pathway, may be more resistant to insulin than others like the mitogenic pathway, which has impact on the cellular functions and survival [43]. The compensatory hyperinsulinemia result in activation of the mitogen-activated protein kinase pathways, which stimulates cellular migration, vascular smooth muscle cell proliferation and a prothrombotic state, and shifts the balance of insulin signaling toward a mitogenic state leading to accelerated atherosclerosis and CV aging [44]. Simultaneously, the IR leads subnormal response to insulin and associated with dyslipidemia and hypertension. Eventually, β-cells can no longer compensate, and IR and loss of β-cell function eventually lead to the deterioration of glucose homeostasis and to the development of hyperglycemia [45].
Insulin sensitivity and longevity: Metabolic syndrome consists of metabolic defects associated with IR, prothrombotic and inflammatory states. In older adults, there is rise of visceral adiposity and accumulation of senescent cells with inflammatory phenotype resulting in increased levels of proinflammatory cytokines that are likely to interfere with insulin signaling [46]. The dysfunctional IRS–PI3–kinase/Akt pathway causes impaired glucose uptake in muscle and fat cells, reduced glycogen synthesis/ storage in the liver and failure to suppress hepatic glucose production, along with impaired lipid uptake by adipose tissue. The increased plasma levels of lipids and proatherogenic apoBcontaining/ triglyceride-rich lipoproteins have a causal role in adverse aging phenotypes and age-related conditions [47]. Thus, IR is accompanied by increased risks of CVD, metabolic diseases such as T2DM and degenerative diseases such as Alzheimer’s disease, and reduced longevity [48].
However, there is evidence that insulin sensitivity and longevity may involve different causal pathways that are not necessarily interconnected. The enhanced insulin sensitivity is neither a necessary nor a sufficient step toward increased longevity. In fact, the improvement of insulin sensitivity may not reverse certain features of MetS. Further, it is held that IR is an evolutionary conserved protective mechanism against certain threats to life and the decreased insulin signaling has been linked to regulation of stress response [49]. Further, in the face of increased nutrient availability, IR may be necessary to limit glucose uptake in muscle cells where glycogen and lipid stores are already saturated [50]. Thus, although insulin sensitivity is essential for normal functioning of organs, IR in aging humans may play an adaptive role and may contribute to increased longevity with reduced insulin signaling [51].
IR and beneficial ‘spill over’ effect
With the excess caloric intake, there occurs deposition of lipids in non-adipose tissue, a deregulation of adipokines and other products of adipocytes, which set in an inflammatory process in adipose tissue and other organs, involving the recruitment of macrophages and other immune cells. This results in mitochondrial dysfunction, ER stress and IR at the level of various key organs, such as the liver, muscle and the heart and vasculature. Mitochondrial β-oxidation of fatty acids generates ROS and leads to increased NFκB activity, which triggers systemic IR. In addition, adiposity generated conditions increase the demand on the secretory pathways and lead to ER stress response.
The ‘spill-over’ effect from adipose tissue appears to prevent the tissue damage due to the ectopic lipid accumulation if adipose tissue is able to expand beyond the limits present under normal physiological conditions. Allowing adipose tissue to expand further results in improvements in metabolic parameters related to glucose and lipid metabolism. There occur reduced triglyceride levels in the liver and muscle with improved hepatic and systemic insulin sensitivity, preservation of β cell mass and a positive impact on the inflammatory profile. The underlying mechanism appears to be related to increased PPARγ activity in adipocytes and adiponectin which results in a redistribution of lipids from ectopic deposits in liver and muscle to the subcutaneous adipose depots. Experimentally, in diabetic ob/ob mice, modestly increasing the levels of circulating adiponectin increased expression of PPARγ target genes and led to reduction in macrophage infiltration in adipose tissue and systemic inflammation. These mice thus, represent a novel model of morbid obesity associated with an improved metabolic profile [52].
Conclusion: Dealing with T2DM, Obesity and Aging
The strongest relationship between insulin resistance and disease risk is observed in middle-aged persons rather than in older individuals. Though, as concerned the cardiovascular morbidity and mortality which increase with age [53]. IR promotes the development of atherosclerosis through hyperinsulinemia and hyperglycemia [54]. IR also reduces the ability of adipose tissue to store proatherogenic lipids and produces a variety of proinflammatory mediators from the adipose tissue, which contribute to atherosclerosis [55].
Better glucose control in the elderly has been associated with improvement in cognitive functioning and lower mortality following adverse CV events [56]. CR or restricting food intake can reduce ectopic lipid accumulation and improve hepatic and muscle insulin action. Indeed, euglycemic clamp studies in caloricrestricted animals revealed enhanced insulin sensitivity compared with normally fed controls [57]. Surgical removal of visceral fat in aging rodents restored insulin sensitivity and prolonged life span [58]. Administration of resveratrol, a compound that activates sirtuin in aging mice on a high-calorie diet improves insulin sensitivity and normalizes their life span [59]. In a review study which included trials involving healthy human subjects, the resveratrol supplementation along with T2DM treatment resulted in significant and clinically important changes in the levels of fasting plasma glucose and insulin [60].
References
- Zhao X, Han Q, Liu Y, Sun C, Gang X, et al. (2016) The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. J Diabetes Res 2016: 2794591.
- Su X, Magkos F, Zhou D, Eagon JC, Fabbrini E, et al. (2015) Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: Effects of obesity and weight loss. Obesity (Silver Spring) 23(2): 329-334.
- Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, et al. (2018) Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol 596(4): 623-645.
- Yamada C, Kondo M, Kishimoto N, Shibata T, Nagai Y, et al. (2015) Association between insulin resistance and plasma amino acid profile in non-diabetic Japanese subjects. J Diabetes Investig 6(4): 408-415.
- Lynch CJ, Adams SH (2014) Branched-chain amino acids in metabolic signaling and insulin resistance. Nat Rev Endocrinol 10(12): 723-736.
- Yoon MS (2016) The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 8(7).
- Holecek M (2018) Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond) 15: 33.
- Okekunle AP, Zhang M, Wang Z, Onwuka JU, Wu X, et al. (2018) Dietary branched-chain amino acids intake exhibited a different relationship with type 2 diabetes and obesity risk: a meta-analysis. Acta Diabetol 56(2): 187-195.
- Zhang S, Zeng X, Ren M, Mao X, Qiao S (2017) Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol 8: 10.
- Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, et al. (2016) Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol 12(1): 15-21.
- Jang C, Oh SF, Wada S, Rowe GC, Liu L, et al. (2016) A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 22(4): 421-426.
- Pallares-Mendez R, Aguilar-Salinas CA, Cruz-Bautista I, Del Bosque-Plata L (2016) Metabolomics in diabetes, a review. Ann Med 48(1-2): 89-102.
- Irving BA, Carter RE, Soop M, Weymiller A, Syed H, et al. (2015) Effect of insulin sensitizer therapy on amino acids and their metabolites. Metabolism 64(6): 720-728.
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, et al. (2016) Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep 6(2): 520-530.
- Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, et al. (2013) Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 304(11): E1175-1187.
- Lu J, Xie G, Jia W, Jia WJFoM (2013) Insulin resistance and the metabolism of branched-chain amino acids. Front Med 7(1): 53-59.
- Adeva-Andany MM, López-Maside L, Donapetry-García C, Fernández-Fernández C, Sixto-Leal CJAA (2017) Enzymes involved in branched-chain amino acid metabolism in humans Amino Acids 49(6): 1005-1028.
- Barcelo A, Morell-Garcia D, Salord N, Esquinas C, Perez G, et al. (2017) A randomized controlled trial: branched-chain amino acid levels and glucose metabolism in patients with obesity and sleep apnea. J Sleep Res 26(6): 773-781.
- Yadao DR, MacKenzie S, Bergdahl A (2018) Reducing branched-chain amino acid intake to reverse metabolic complications in obesity and type 2 diabetes. J Physiol 596(16): 3455-3456.
- Labonte CC, Farsijani S, Marliss EB, Gougeon R, Morais JA, et al. (2017) Plasma Amino Acids vs Conventional Predictors of Insulin Resistance Measured by the Hyperinsulinemic Clamp. J Endocr Soc 1(7): 861-873.
- Giesbertz P, Daniel H (2016) Branched-chain amino acids as biomarkers in diabetes. Curr Opin Clin Nutr Metab Care 19(1): 48-54.
- Arany Z, Neinast MJCDR (2018) Branched Chain Amino Acids in Metabolic Disease Curr Diab Rep 18(10): 76.
- Bloomgarden Z (2018) Diabetes and branched-chain amino acids: What is the link? J Diabetes 10(5): 350-352.
- Brunetta HS, de Camargo CQ, Nunes EAJAA (2018) Does l-leucine supplementation cause any effect on glucose homeostasis in rodent models of glucose intolerance? A systematic review. Amino Acids 50(12): 1663-1678.
- Rietman A, Stanley TL, Clish C, Mootha V, Mensink M, et al. (2016) Associations between plasma branched-chain amino acids, beta-aminoisobutyric acid and body composition. J Nutr Sci 5: e6.






























