The Prevalence of Thyroid Disorders in Patients with Type 2 Diabetes Mellitus in Saudi Community Based Hospital
Khalid S Aljabri*
Department of Endocrinology, King Fahad Armed Forces Hospital, Kingdom of Saudi Arabia
Submission: April 17, 2019; Published: July 10, 2019
*Corresponding author: Khalid S. Aljabri, Department of Endocrinology, King Fahad Armed Forces Hospital, PO Box 9862. Jeddah 21159, Kingdom of Saudi Arabia
How to cite this article: Khalid S Aljabri. The Prevalence of Thyroid Disorders in Patients with Type 2 Diabetes Mellitus in Saudi Community Based Hospital. Curr Res Diabetes Obes J. 2019; 11(3): 555812. DOI: 10.19080/CRDOJ.2019.11.555812
Abstract
Background and objective: The association between diabetes and thyroid dysfunction were reported. Thus, the present study was conducted to find out the relationship between T2DM and thyroid dysfunction in patients with T2DM in a cohort of Saudi population.
Design: A cross-sectional study was conducted in the Diabetes centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia from January 2018 to December 2018. Thyroid stimulating hormone (TSH), free thyroxin (FT4) and HBa1c were measured.
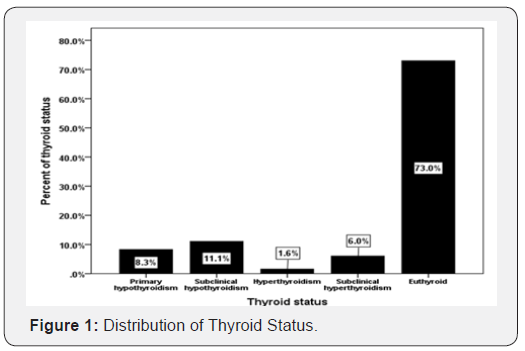
Results: A total of 2069 subjects with T2DM were included in this study. Average age of the study population was 51.3 ±16.4 years. 1511 (73%) were euthyroid, primary hypothyroid was present in 229 (11.1%) and subclinical hypothyroidism was present in 3 (3%) patients. Hyperthyroidism was present in 33 (1.6%), and subclinical hyperthyroidism was present in 125 (6%) patients. Patients with primary hypothyroid were statistically non-significant older compared to subclinical hypothyroidism, hyperthyroidism and subclinical hyperthyroidism (P=0.6), and included statistically non-significant difference between thyroid dysfunctions in younger compared to older than 50 years subjects (P=0.3).
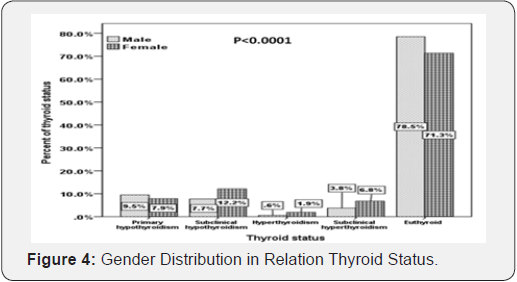
Moreover, there was a statistically non-significant difference between thyroid dysfunctions in males compared to females (P<0.0001). In addition, there was a statistically non-significant difference between thyroid dysfunctions in males compared to females (P<0.0001). Patients with primary hypothyroid were statistically significant have higher HbA1c compared to subclinical hypothyroidism, hyperthyroidism and subclinical hyperthyroidism (P=0.004). In addition, there was a statistically significant difference between HbA1c below and above 7% in correlation to thyroid dysfunctions (P=0.02).
Keywords:Hypothyroidism; Type 2 Diabetes; Thyroid dysfunctions; Endocrine diseases; Diabetes; Human body; Radioimmunoassay; Hypothalamic control; Thyroxin; Triiodothyronine
Abbreviations: T2DM: Type 2 Diabetes Mellitus; TSH: Thyroid Stimulating Hormone; T4: Thyroxin; T3: Triiodothyronine; CMIA: Chemiluminescent Immunoassay Method; FT4: Free Thyroxine; SD: Standard Deviation; TT4: Total Thyroxine; TRH: Thyrotropin Releasing Hormone
Introduction
Thyroid gland is one of the important organs in human body and the burden of thyroid diseases in the general population is enormous specially in females [1,2]. Thyroid disorders have increased recently and are considered the commonest endocrine diseases [3]. Several studies have been reported from different parts of the world showing the prevalence of hypothyroidism between 1% and 2%, and it is ten times more common in women than in men [4].
Type 2 diabetes mellitus (T2DM) is the commonest endocrine disorder [5]. The World health organization estimated diabetes prevalence was 2.8% in 2000 and 4.4% in 2030 [6]. Saudi Arabia is the seventh of the top ten countries in terms of the prevalence of diabetes among the adult population aged 20-79 [7].
The association between diabetes and thyroid dysfunction were first published in 1979 [8]. Since then a number of studies have estimated the prevalence of thyroid dysfunction among diabetes patients to be varying from 2.2 to 17 % [9,10]. However, fewer studies have estimated much higher prevalence of thyroid dysfunction in diabetes i.e. 31 % and 46.5% respectively [11,12]. The prevalence of thyroid dysfunction among Saudi diabetic patients was reported to be 16-28.5% of which 25.3% had hypothyroidism [13,14]. Thyroid dysfunction is a disorder of the thyroid gland which manifests either as hyper or hypothyroidism and is reflected in the levels of thyroid stimulating hormone (TSH) [15].
T2DM appears to influence thyroid function in two sites; at the level of hypothalamic control of TSH release and at peripheral tissue by converting thyroxin (T4) to triiodothyronine (T3). Hyperglycemia causes reduction in hepatic concentration of T4-5 deiodinase, low serum concentration of T3, raised levels of reverse T3 and low, normal, or high level of T4. Thyroid hormone regulate metabolism and diabetes can alter metabolism [16].
Epidemiological studies of thyroid dysfunction have limitations, for example the definition of overt hypothyroidism and subclinical hypothyroidism, the selection criteria of the sample used, the influence of age, sex, genetic, environmental factor, the different techniques used for the measurement of thyroid hormones and the relative paucity of incidence data [5]. Thus, the present study was conducted to find out the relationship between T2DM and thyroid dysfunction in patients with T2DM in a cohort of Saudi population.
Methods
A cross-sectional study was conducted in the Diabetes centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia from January 2018 to December 2018 for a period of 12 months which included 2069 patients who were diagnosed as T2DM on the basis of ADA criteria [17]. Patients who are pregnant were excluded. TSH was measured with a chemiluminescent immunoassay method (CMIA) (Architect i2000 system, Abbott, USA). Serum free thyroxine (FT4) was estimated by radioimmunoassay. The assays have intra- assay precision of 4.3%. TSH levels between 0.22-4.2 mIU/L and Free T4 12.0-22.0 pmol/L were regarded normal [18]. High performance liquid chromatography was used. HbA1c was expressed as percentage. Hypothyroidism was defined as a clinical syndrome of hypothyroidism associated with elevated TSH >4.2 mIU/l and decreased serum levels of FT4. Subclinical hypothyroidism was defined as elevated TSH >4.2 mIU/l and normal circulating FT4. Hyperthyroidism was defined as TSH <0.22 mIU/l with elevated FT4. Subclinical hyperthyroidism was defined as TSH <.22 mIU/l and normal circulating FT4 [19].
Statistical analysis
Data are presented as means ± standard deviation (SD) or numbers (%). Quantitative variables were compared between two groups by using the Student’s test. Differences in categorical variables were analyzed using the chi-square test. The relationship between continuous variables was assessed using coefficients of correlation. The statistical analysis was conducted with SPSS version 23.0 for Windows.
Results
A total of 2069 subjects with T2DM were included in this study. Average age of the study population was 51.3 ±16.4 years (Table 1). 24.5% were male and 75.5% were female. Mean HbA1c (%), TSH and FT4 were 7.5±2, 3.4 ±8.8 mIU/l and 14.0±5 pmol/L respectively. 1511 (73%) were euthyroid, primary hypothyroid was present in 229 (11.1%) and subclinical hypothyroidism was present in 3 (3%) patients (Figure 1). Hyperthyroidism was present in 33 (1.6%), and subclinical hyperthyroidism was present in 125 (6%) patients.
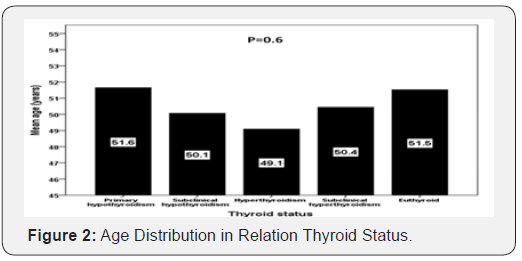

Patients with primary hypothyroid were statistically nonsignificant older compared to subclinical hypothyroidism, hyperthyroidism and subclinical hyperthyroidism (mean age:51 years versus 50, 49 and 50 years respectively; P=0.6) (Figure 2), and included statistically non-significant difference between thyroid dysfunction in younger compared to older than 50 years subjects (P=0.3) (Figure 3).
Moreover, there was a statistically non-significant difference between thyroid dysfunctions in males compared to females (P<0.0001) (Figure 4). In addition, there was a statistically nonsignificant difference between thyroid dysfunctions in males compared to females (P<0.0001) (Figure 4).
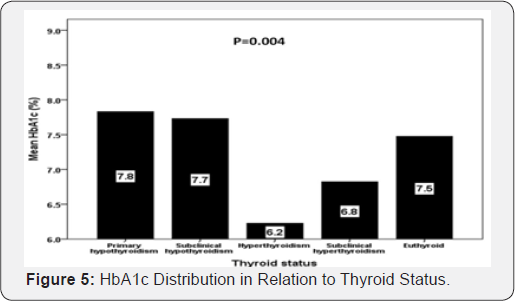
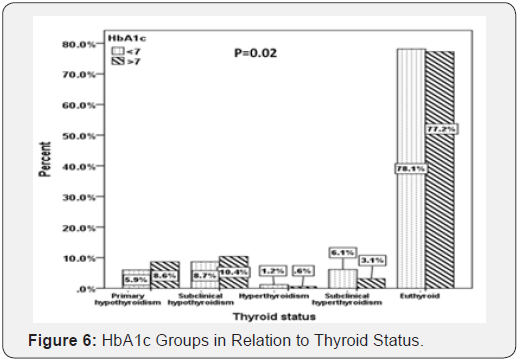
Patients with primary hypothyroid were statistically significant have higher HbA1c compared to subclinical hypothyroidism, hyperthyroidism and subclinical hyperthyroidism (mean HbA1c (%): 7.8 versus 7.7, 6.2 and 6.8% respectively; P=0.004) (Figure 5). In addition, there was a statistically significant difference between HbA1c below and above 7% in correlation to thyroid dysfunction (P=0.02) (Figure 6).
Discussion
There is a complex interaction between thyroid dysfunction and diabetes mellitus. In the present study among the 2069 subjects with T2DM, thyroid dysfunction was found in 27%. Primary hypothyroid was present in 11.1% and subclinical hypothyroidism was present in 3% patients. Moreover, hyperthyroidism was present in 1.6%, and subclinical hyperthyroidism was present in 6% patients. Brijesh et al found 26% of patients with T2DM have thyroid dysfunction [20]. Pasupathi et al in their study found that prevalence of thyroid dysfunction was 45% among type 2 diabetics.
Hypothyroidism was present in 28% and 17% had hyperthyroidism [21]. Udiong et al in their study from Nigeria found that prevalence of thyroid dysfunction was 46.5%. Hypothyroidism was present in 26.6% and 19.9% had hyperthyroidism [12]. A prevalence of 12.3% was reported among Greek diabetic patients and 16% of Saudi patients with type 2 diabetes were found to have thyroid dysfunction [13,22]. In Jordan, a study reported that thyroid dysfunction was present in 12.5% of type 2 diabetic patients [23]. Perros et al demonstrated an overall prevalence of 13.4% of thyroid diseases in diabetics [9].
It has been suggested that altered thyroid status in T2DM is linked toleration of hypothalamo-pituitary- thyroid axis causing decreased formation of total thyroxine (TT4). Also, there is less activation of AMPK (5’-Adenosine Mono Phosphate activated Protein Kinase) in T2DM that also causes decreased formation of thyroid hormones [24]. Decrease levels of thyroid hormones cause increased TSH release from anterior pituitary gland by feedback mechanism [25-27]. Most of the T2DM patients were obese who might have increased level of leptin. This increased level of leptin develops leptin resistance centrally that causes decreased formation of thyroid hormones and increase TSH secretion [24]. Moreover, the binding affinity of TT4 is increased in T2DM that causes decrease formation of FT4 in blood [26,27]. T2DM are also associated with obesity, stress, infection that caused changes in hypothalamo-pituitary thyroid axis lead to decrease level of FT4 and increased TSH level in T2DM [24].
The presence of both raised and low levels of thyroid hormones in diabetic may be due to modified thyrotropin releasing hormone (TRH) synthesis and release [21]. The hyperglycaemia seen in type- 2 diabetics is known to have negative effect on thyroid function precisely blunting the pituitary TSH response to stimulation by hypothalamic TRH. This may be due to possible alteration of post translational glycosylation of TRH hence affecting its biological activity [28]. T2DM is associated with increased insulin level and C-peptide level. Insulin is an anabolic hormone known to enhance TSH turnover, which is protein in nature. Recently, C-peptide has been shown to enhance Na+/K+- ATPase activity, an action that may also increase protein synthesis. Such an action would induce increased turnover of TSH, a protein hormone [29,30].
Our results showed a different frequency between thyroid dysfunction in both males and females. Still, detailed molecular mechanisms remain unclear, because sex hormones (such as estrogen, and testosterone) can regulate the thyroid function [31]. Boucai the difference in sex hormones may partly explain the sex-difference in the relationship between thyroid dysfunction. However, because levels of sex hormones such as testosterone and estrogen were not measured in this study, further research is needed to explore this issue. In addition, because the sample size was smaller for males (24.5%) than in females (75.5%), the precision and statistical power of the analysis may be lower for males. Further large-scale population studies are required to confirm the above findings.
In our study we found that diabetic patients with primary and subclinical hypothyroidism have higher uncontrolled diabetes HbA1c > 7 when compared to patients with HbA1c < 7 (8.6 vs. 5.9% and 10.4 vs. 8.7%) respectively whereas patients with hyperthyroidism and subclinical hyperthyroidism have lower uncontrolled diabetes HbA1c > 7 when compared to patients with HbA1c < 7 (0.6 vs. 1.2% and 3.1 vs. 6.1%) respectively. In addition, patients with primary hypothyroid were statistically significant have higher HbA1c compared to subclinical hypothyroidism, hyperthyroidism and subclinical hyperthyroidism (mean HbA1c (%): 7.8, 7.7, 6.2 and 6.8% respectively; P=0.004). These findings are supported by various studies [32-34]. Hypothyroidism falsely raises HbA1c due to decreased erythropoiesis. Thyroid hormone replacement is associated with decrease in HbA1c level, which is influenced by increased erythropoiesis rather than by changes in glucose level [35].
We aimed to identify the frequency of thyroid dysfunction in patients with T2DM in hospital-based health care setting. Furthermore, due to the cross-sectional nature of this study, the observed population reflects a selected yet comprehensive group of patients rather than the general population. In addition, the current study population may appear limited in size and therefore may underestimate the true frequency of thyroid dysfunction in patients with T2DM [36].
We conclude that despite the limitations of this hospitalbased retrospective study, hypothyroidism is highly prevalent in cohort of Saudis with T2DM. The majority of our patients with primary hypothyroidism were predominantly males. These two observations remain to be validated by population-based studies. In the absence of registry data, larger cooperative studies involving diverse population samples from multiple centers could help to provide further information on the true frequency nationally. Based on a high prevalence of hypothyroidism among Saudi T2DM patients, routine screening for hypothyroidism is highly recommended in Saudi diabetic population.
Acknowledgement
The author would like to thank all colleagues from the Department of primary care for helping in data collection.
References
- Hanna CE, LaFranchi SH (2002) Adolescent thyroid disorders. Adolesc Med 5(1): 65-86.
- Memonab A, Berrington De González A, Luqmani Y, Suresh A (1994) Family history of benign thyroid disease and cancer and risk of thyroid cancer. Eur J Cancer 40(5): 754-760.
- Okosieme OE, Marx H, Lazarus JH (2008) Medical management of thyroid dysfunction in pregnancy and the postpartum. Expert Opin Pharmacother 9(13): 2281-2293.
- Madariaga AG, Palacios SS, Guillén-Grima F, Galofré J (2014) The incidence and prevalence of thyroid dysfunction in Europe: a metaanalysis. J Clin Endocrinol Metab 99(3): 923-931.
- Vanderpump MPJ (2005) The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, (Eds.), Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. JB Lippincott-Raven, Philadelphia 9: 398-406.
- Faghilimnai S, Hashemipour M, Kelishadi B (2006) Lipid profile of children with type 1 diabetes compared to controls: ARYA J 2(1): 36-38.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes. Diabetes Care 27: 1047-1053.
- (2012) IDF Diabetes Atlas. (5th), International Diabetes Federation.
- Feely J, Isles TE (1979) Screening for thyroid dysfunction in diabetics. Br Med J 1(6179): 1678.
- Perros P, Mc Crimmon RJ, Shaw G, Frier BM (1995) Frequency of thyroid dysfunction in diabetics patients: value of annual screening. Diabet Med 12(7): 622-627.
- Smithson MJ (1998) Screening for thyroid dysfunction in a community population of diabetic patients. Diabet Med 15(2): 148-150.
- Celani MF, Bonati ME, Stucci N (1994) Prevalence of abnormal thyrotropin concentrations measured by a sensitive assay in patients with Type 2 diabetes mellitus. Diabete Res 27(1): 15-25.
- Udoing CEJA, Udoh E, Etukudoh ME (2007) Evaluation of thyroid function in diabetes mellitus in Calabar, Nigeria. Indian J Clin Biochem 22(2): 74-78.
- Akbar DH, Ahmed MM, Al-Mughales J (2006) Thyroid dysfunction and thyroid autoimmunity in Saudi type 2 diabetics. Acta Diabetologica 43(1): 14-18.
- Metab Al-Geffari, Najlaa A Ahmad, Ahmad H Al-Sharqawi, Amira M Youssef, Dhekra AlNaqeb, et al. (2013) Risk Factors for Thyroid Dysfunction among Type 2 Diabetic Patients in a Highly Diabetes Mellitus Prevalent Society. Int J Endocrinol 2013: 1-6.
- Tunbridge WMG, Evered DC, Hall R, Appleton D, Brewis M, et al (1977) The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol 7(6): 481-493.
- Shah SN (2007) Thyroid disease in diabetes mellitus. J Assoc Physicians India 32(12): 1057-1059.
- (2019) American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 42(Suppl 1): S13-S28.
- dos Remedios LV, Weber PM, Feldman R, Schurr DA, Tsoi TG (1980) Detecting unsuspected thyroid dysfunction by the free thyroxine index. Arch Intern Med 140(8): 1045-1049.
- Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I (2012) Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 18(6):988-1028.
- Brijesh K, Mahantesh G (2018) A Study of Prevalence and Spectrum of Thyroid Dysfunction in Type II Diabetes Mellitus. IOSR Journal of Dental and Medical Sciences 17(8): 42-46.
- Pasupathi P, Chandrasekar V, Kumar US (2009) Evaluation of oxidative stress, antioxidant and thyroid hormone status in patients with diabetes mellitus. J Medicine 10: 60-66.
- Papazafiropoulou A (2010) Prevalence of thyroid dysfunction among Greek Type 2 diabetic patients attending an outpatient clinic. J Clin Med Res 2(2): 75-78.
- Radaideh ARM, Nusier MK, Amari FL, Bateiha AE, El-Khateeb MS, et al. (2004) Thyroid dysfunction in patients with type 2 diabetes mellitus in Jordan. Saudi Med J 25(8): 1046-1050.
- Swami RM, Kumar N, Srinivasa K, Manjunath GN, Byrav PDS (2012) Evaluation of hypothyroidism as a complication in Type II Diabetes Mellitus. J Biomed Res 23(2): 170-172.
- Hage M, Mira S, Zantout MS, Azar ST (2011) Thyroid disorders and diabetes mellitus. J Thy Res 10: 2-7.
- Bharat Hijam D, Gangate D, Lalnunpui, Premchand, Devi I (2013) Thyroid status in diabetes mellitus. J Glycomics Lipidomics 3: 106.
- Rai S, Kumar AJ, Prajna K, Shetty SK, Rai T, et al. (2013) Thyroid Function in Type 2 Diabetes Mellitus and in Diabetic Nephropathy. J Clin Diagn Res 7(8): 1583-1585.
- Suresh DR, Silvia CR, Murthy UK (2014) Inter-relationship of glycemic control and thyroid status in type-2 diabetes mellitus. International journal of clinical and diagnostic research 2(4).
- Bassyouni A, Ebrashy IE, Ismiel A, Amara I, Mahfouz M (2010) Profile of the thyroid function and ultrasound among patients with type-2 diabetes mellitus. Sci Med J 22(2): 15-28.
- Shaikh AW, Memon AS, Sirichand (2009) Frequency of hypothyroidism in type 2 diabetic patients. Pakistan J Med Health Sci 2(4).
- Boucai L, Hollowell JG, Surks MI (2011) An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid 21(1): 5-11.
- Schlienger JL, Anceau A, Chabrier G, North ML, Stephan F (1982) Effect of diabetic control on the level of circulating thyroid hormones. Diabetologia 22(6): 486-488.
- Bagchi N, Brown TR, Parish RF (1990) Thyroid dysfunction in adults over age 55 years. A study in an urban US community. Arch Intern Med 150(4): 785-787.
- Verga Falzacappa C, Panacchia L, Bucci B, Stigliano A, Cavallo MG, et al. (2006) 3,5,30-Triiodothyronine is a survival factor for pancreatic beta cells undergoing apoptosis. J Cell Physiol 206(2): 309-321.
- Kim MK, Kwon HS, Baek K, Lee JH, Park WC, et al. (2010) Effects of thyroid hormone on A1C and glycated albumin levels in nondiabetic subjects with overt hypothyroidism. Diabetes Care 33(12): 2546-2548.






























