Hypertriglyceridemia in Type 1 Diabetes Children During Diabetic Ketoacidosis; Relation to DKA Severity and Glycemic Control
Lubna Fawaz1, Noha Musa1*, Sahar Abdel Atty2 and Ahmed Nassef3
1 Diabetes, Endocrine and Metabolism Pediatric Unit, Cairo University, Egypt
2Professor of Clinical and Chemical Pathology, Cairo University, Egypt
3Pediatric resident, Children and Women hospital, Egypt
Submission: March 03, 2019; Published: April 04, 2019
*Corresponding author: Noha Musa, Lecturer of Pediatric Endocrinology, Diabetes, Endocrine and Metabolism Pediatric Unit, Pediatric Department, Cairo University, 15 Naser El Thawra street, Haram, Giza, Cairo, Egypt
How to cite this article: Lubna F, Noha M, Sahar A A, Ahmed N. Hypertriglyceridemia in Type 1 Diabetes Children During Diabetic Ketoacidosis; Relation to DKA Severity and Glycemic Control. Curre Res Diabetes & Obes J. 2019; 10(3): 555787. DOI: 10.19080/CRDOJ.2019.10.555787
Abstract
Background: Diabetic ketoacidosis (DKA) is a common, life-threatening complication of type 1 diabetes (T1D). Insulin deficiency impairs lipoprotein lipase (LPL) resulting in elevated serum triglycerides (TG) that usually normalize after establishing IV insulin.
Objectives: To study the prevalence of hypertriglyceridemia during DKA in T1D patients and assess its relation to DKA severity and glycemic control after 3 months.
Methodology: This cohort study included 84 children with T1D presenting with DKA at the Diabetes, Endocrine and Metabolism Pediatric Unit (DEMPU), Cairo University. Patients were evaluated for serum TG on admission and 48 h after initiating insulin therapy. HbA1c was assessed 3 months later.
Results: In our cohort, 74 patients (88.1%) had hypertriglyceridemia at onset of DKA that resolved in 41 of them after 48 h, while 33 patients still had hypertriglyceridemia. There was significant improvement in TG after 48 h of DKA management (p<0.001). When basal serum TG was correlated with other study parameters, a significant positive correlation was found with BG (p=0.005) and duration of ICU stay (p< 0.001), while a significant negative correlation was found with serum bicarbonate and GCS (i.e. Conscious level) with a p value of 0.012 & 0.022 respectively. No significant correlation was found between TG (basal & after 48 h) and glycemic control or insulin requirements after 3 months.
Conclusion: Hypertriglyceridemia was detected in most patients of T1D during DKA that significantly improved with insulin therapy. TG correlated with the DKA severity and BG levels. However, it did not affect glycemic control or insulin dose later.
Keywords:Hypertriglyceridemia; Diabetic ketoacidosis; Insulin; Glycemic control; Insulin therapy; Lipid metabolism; Circulation; Pancreatic capillaries; Acute pancreatitis; Cerebral edema; Plasmapheresis; Heparin
Abbreviations: DKA: Diabetic Ketoacidosis; T1D: Type 1 Diabetes; LPL: Lipoprotein Lipase; TG: Triglycerides; DEMPU: Diabetes, Endocrine and Metabolism Pediatric Unit; FFA: Free Fatty Acids; TC: Total Cholesterol; TINIA: Turbidimetric Inhibition Immunoassay; DCCT: Diabetes Control and Complications Trial; SPSS: Statistical Program for Social Science; SD: Standard Deviation
Introduction
Regulation of lipid metabolism is greatly influenced by insulin through inhibiting the hormone-sensitive lipase in adipose tissue [1]. The anti-lipolytic action of insulin promotes excessive storage of triglycerides (TG) in the adipocytes with reduction of free fatty acids (FFA) release into the circulation [2]. Diabetic ketoacidosis (DKA) is by far a serious and potentially life-threatening complication of T1D [3]. During an episode of DKA, an increase in TG (in 30-50% of cases) and total cholesterol levels (TC) was reported that probably results from temporary impairment of lipoprotein lipase (LPL) activity [4]. This increase in serum TG is also associated with increase in chylomicron level leading to milky blood serum [5]. In DKA, insulin deficiency activates lipolysis in adipose tissue releasing FFA accelerating the formation of VLDL in the liver. Reduced activity of LPL in peripheral tissue decreases the removal of VLDL from the plasma as well, causing hypertriglyceridemia [6]. The incidence of acute pancreatitis and cerebral edema is increased in patients with DKA and hypertriglyceridemia [7]. Severe hypertriglyceridemia can increase risk of acute pancreatitis, especially with TG levels higher than 1,000–1,772 mg/dl [8,9].
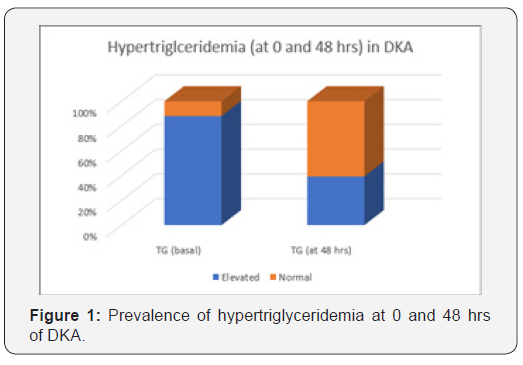
This can be attributed to the fact that TG and chylomicrons are hydrolyzed by lipase in the pancreatic capillaries and the released FFA activate the trypsinogen, triggering the capillary damage by free radicals [10,11]. On the other hand, DKA may mask coexisting acute pancreatitis occurring in 10–15% of cases. Ranson’s prognostic criteria can overestimate the severity of acute pancreatitis during DKA, so it’s not beneficial to use it for assessment of severity. Severity index based on CT findings can be used instead with better prognostic value. Elevation of serum lipase and amylase occurs in DKA. However, elevation in lipase levels appears to be less specific than amylase [12]. Insulin is considered to be the drug of choice in severe hyperlipidemiainduced pancreatitis with concomitant hyperglycemia, while plasmapheresis and heparin can also be used during emergency [13]. The aim of our study was to evaluate the prevalence of hypertriglyceridemia at onset of DKA in T1D patients and assess its relation to DKA severity and glycemic control after 3 months (Figure 1).
Methodology
This cross-sectional observational study was conducted on random cohort of 84 children with T1D presenting with DKA episode at the Diabetes, Endocrine and Metabolism Pediatric Unit (DEMPU), Children hospital, Cairo University during the period from January to August 2016. This study was approved by the Research Ethics Committee of Cairo University. The diagnosis of DKA was established according to the ISPAD Clinical Practice Consensus Guidelines 2014 (BG > 200mg/dl, Venous pH < 7.3 or bicarbonate <15 mmol/L, ketonemia and ketonuria) [14]. Children with T2D, with diabetes secondary to post-surgical pancreatectomy, cystic fibrosis, or steroid therapy, or those using thyroxin therapy or any lipid lowering medications as well as those who had family history of dyslipidemia were excluded from the study.
All patients included in the study (after taking informed consents from their legal guardians) were evaluated clinically for conscious level assessment (using Glasgow coma scale) as well as biochemical analysis for BG, ABG, serum electrolytes (Na, K) kidney functions (urea, creatinine). Serum TG were measured initially during DKA presentation then at 48 h of DKA management (insulin therapy). 2ml blood was drawn into vacutainer tube containing no anticoagulant then incubated in an upright position at room temperature for 20-30 min to allow clotting. Centrifugation for 10 minutes was done. Serum was aspirated (using a clean pipette for each tube) and TG levels (mg/ dl) were measured by glycerol phosphate oxidase/peroxidase method by fully automated chemistry analyzer Beckman coulter Inc, USA [15].
A TG level below 150 mg/dl was considered normal. HbA1c (%) levels were measured 3 months later. The HbA1c% measurement is based on a turbidimetric inhibition immunoassay (TINIA) principle, and the measurement of total hemoglobin is based on a modification of the alkaline hematin reaction. Using the values obtained for each of these two analytes in (g/dl), the percentage of the total hemoglobin that is glycated is calculated and reported as %HbA1c. The final HbA1c% result has been standardized to the results obtained in the Diabetes Control and Complications Trial (DCCT).
Data was analyzed using Statistical Program for Social Science (SPSS) version 20.0. Quantitative data were expressed as mean± standard deviation (SD). Qualitative data was expressed as frequency and percentage. Mann Whitney U test was used for two-group comparisons in non-parametric data. Spearman’s rank correlation coefficient was used to assess the degree of association between two sets of variables. Linear regression was used to test and estimate the dependence of a quantitative variable based on its relationship to one or more independent variables. P-value <0.05 was considered significant and <0.001 was considered highly significant.
Results
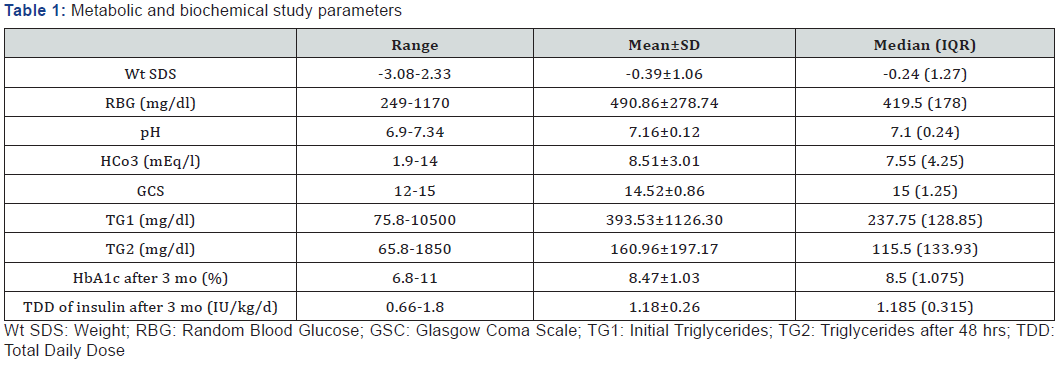
Among the study group (84 cases),43 (51.19%) were males and 41 (48.81%) were females with mean age of 7.1±2.65 years (ranging between 2.5 and 13 years). Sixty-eight (80.95%) were newly diagnosed and 16 (19.05%) were known to be diabetic. Mean BG level at presentation was 490.86±278.74 mg/dl and median TG was 237.75 mg/dl (Table 1). There was a significant difference in prevalence of hypertriglyceridemia at onset of DKA and after 48 h of management (p<0.001). In our cohort, 74 patients (88.1%) had hypertriglyceridemia at onset of DKA ranging from 75.8-10500 mg/dl with significant improvement in TG after 48 h of DKA management (p<0.001). Hypertriglyceridemia completely resolved in 41 of them after 48 h, while 33 patients still had hypertriglyceridemia. One of our patients had high TG level reaching 10500 mg/dl (with milky serum), but acute pancreatitis was ruled out by serum lipase, amylase and CT abdomen, his TG level improved after 48 h of DKA management reaching a level of 1860 mg/dl and resolved completely after 14 days (140 md/dl). When basal serum TG were correlated with other study parameters in our cohort, a significant positive correlation was found with BG level (r= 0.703, p=0.005) and duration of ICU stay (r=0.37, p<0.001), while a significant negative correlation was found with serum bicarbonate and GCS (i.e. conscious level) with a p value of 0.012 & 0.022 respectively. On the other hand, correlating TG after 48 h with different study parameters showed a significant positive correlation with BG level (r= 0.704, p=0.005) and a significant negative correlation with pH, serum bicarbonate and GCS (p=0.01, 0.004 & 0.013 respectively) (Table 2). When insulin requirements and HbA1c were assessed 3 months later in the study group, no significant correlation was found between triglycerides (either basal or after 48 h of DKA) and glycemic control or insulin requirements. When we compared between different study parameters in relation to the onset of DKA (newly diagnosed with first attack of DKA & those known to be diabetic presenting with DKA), we found a significant difference between both groups in mean difference in TG at 0 and at 48 h (p=0.049) (Table 3).


Discussion
Moderate hypertriglyceridemia is common during episodes of DKA [16]. However, severe hypertriglyceridemia, which is defined as a TG level >2,000 mg/dl is rare. In severe hypertriglyceridemia, there is an increased risk of developing acute pancreatitis [11].
In our cohort, 74 patients (88.1%) had hypertriglyceridemia at onset of DKA that significantly improved after 48 hrs of DKA management. Our results were similar to Weidman et al. [17] who studied 13 patients with DKA (15-60 yrs) and found that before insulin treatment, plasma TG levels ranged from 63-2800 mg/dl and that 12 patients out of 13 demonstrated dramatic decreases in plasma TG levels after 24 h of insulin therapy. Similarly, Fulop & Eder [16] measured plasma TG and cholesterol concentrations in 50 episodes in 46 adults hospitalized on a Municipal hospital medical service and reported that 32 patients (64%) had TG levels above the 95th percentile (adjusted for age and sex), and 18 patients (36%) had cholesterol levels above the 95th percentile. Severe hypertriglyceridemia (levels above 5.65 mmol/L) was found in 14 patients (28%) and that treatment of the ketoacidosis was associated with a rapid decrease in plasma lipid levels.
Fulop & Eder [6] concluded that 12 of the 15 patients with DKA and hypertriglyceridemia did not have an underlying genetic hyperlipidemia contributing to their original severe hypertriglyceridemia during DKA episode. However, Karagianni et al. [18] analysed the lipoprotein lipase coding gene sequence in a 10-year-old girl with new-onset T1D, ketoacidosis and severe hypertriglyceridemia (TG > 112.9 mmol/l) and revealed that the patient was a compound heterozygote for two mutations, D9N in exon 2 and S447X in exon 9. Although these two mutations usually did not considerably impair lipolytic enzyme activity, the combination of both in that patient may have played a role in the development of severe hypertriglyceridemia. Similar findings were reported also by Rodríguez Santana et al. [19].
In our cohort, despite high TG that reached 10500 mg/dl in one patient, none of our patients developed acute pancreatitis. Similarly, a case report in Brazil had TG level during DKA episode of 11578 mg/dl, yet she did not develop acute pancreatitis and the levels of TG decreased significantly with hydration and intravenous insulin [20]. Many case reports showed the association of DKA with severe hypertriglyceridemia and acute pancreatitis [21-28]. Nair et al. [12] studied 100 patients with DKA and hypertriglyceridemia and confirmed that the prevalence of acute pancreatitis was 11%.
When basal serum TG were correlated with other study parameters, a significant positive correlation was found with BG level and duration of ICU stay, while a significant negative correlation was found with serum HCO3 (i.e, severity of DKA) and GCS (i.e. conscious level). This could be attributed to the fact that the insulin deficiency is the cause of hypertriglyceridemia and that the more severe the insulin deficiency, the higher the BG level and the more severe the DKA (more acidosis) and the higher the TG will be and the longer the duration of ICU stay (need for IV insulin therapy for correction of acidosis and dehydration). When we compared between different study parameters in relation to the onset of DKA (newly diagnosed with first episode of DKA & those known to be diabetic presenting with DKA), we found a significant mean difference in TG (p=0.049). This also can be attributed to the degree of insulin deficiency as those with first episode of DKA are supposed to be more insulin deficient that those who are known to be diabetic on insulin therapy but developed DKA due to relative insulin deficiency.
When insulin requirements and HbA1c were assessed 3 months later in the study group, no significant correlation was found between triglycerides (either basal or after 48 hrs of DKA) and glycemic control or insulin requirements (TDD). This could be explained by the fact that the severity of DKA (and insulin deficiency) that affected serum TG during DKA episodes was not persistent after establishing insulin therapy, hence not affecting glycemic control after 3 months.
Conclusion
Hypertriglyceridemia was detected in most patients of T1D during episodes of DKA (reaching values as high as 10500 mg/ dl without developing acute pancreatitis) that significantly declined with insulin therapy. Serum TG correlated with the DKA severity and BG levels. However, it did not affect glycemic control or insulin dose later on.
Acknowledgement
We acknowledge the patients, the nursing staff and all members of Diabetes, Endocrine and Metabolism Pediatric Unit (DEMPU) ICU.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Cairo University and National Research Center and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed Consent
Informed consent was obtained from every individual participant included in the study.
References
- Vergès B (2009) Lipid disorders in type 1 diabetes. Diabetes Metab 35(5): 353-360.
- Vergès B (2001) Insulin sensitiviy and lipids. Diabetes Metab 27(2): 223-227.
- Kearney T, Dang C (2007) Diabetic and endocrine emergencies. Postgrad Med J 83(976): 79-86.
- Nocoń-Bohusz J, Wikiera B, Basiak A, Śmigiel R, Noczyńska A (2016) LPL gene mutation as the cause of severe hypertriglyceridemia in the course of ketoacidosis in a patient with newly diagnosed type 1 diabetes mellitus. Pediatr Endocrinol Diabetes Metab 21(2): 89-92.
- McLean AG, Petersons CJ, Hooper AJ, Burnett JR, Burt MG, et al. (2009) Extreme diabetic lipaemia associated with a novel lipoprotein lipase gene mutation. Clinica Chimica Acta 406(1): 167-169.
- Fulop M, Eder H (1990) Severe hypertriglyceridemia in diabetic ketosis. Am J Med Sci 300(6): 361-365.
- Saengkaew T, Sahakitrungruang T, Wacharasindhu S, Supornsilchai V (2016) DKA with severe hypertriglyceridemia and cerebral edema in an adolescent boy: A case study and review of the literature. Case Rep Endocrinol 2016: 7515721.
- Athyros VG, Giouleme OI, Nikolaidis NL, Vasiliadis TV, Bouloukos VI, et al. (2002) Long-term follow-up of patients with acute hypertriglyceridemia-induced pancreatitis. J Clin Gastroenterol 34(4): 472-475.
- Sandhu S, Al-Sarraf A, Taraboanta C, Frohlich J, Francis GA (2011) Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis 11(10): 157.
- Benifla M, Weizman Z (2003) Acute pancreatitis in childhood: analysis of literature data. J Clin Gastroenterol 37(2): 169-172.
- Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A (2009) Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol 104(4): 984-991.
- Nair S, Yadav D, Pitchumoni CS (2000) Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol 95(10): 2795-2800.
- Lutfi R, Huang J, Wong HR (2012) Plasmapheresis to treat hypertriglyceridemia in a child with diabetic ketoacidosis and pancreatitis. Pediatrics 129(1): e195-198.
- Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser N, et al. (2014) A Consensus Statement from the International Society for Pediatric and Adolescent Diabetes: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 15(20): 154-179.
- Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28(10): 2077-2080.
- Fulop M, Eder HA (1989) Plasma triglycerides and cholesterol in diabetic ketosis. Arch Intern Med 149(9): 1997-2002.
- Weidman SW, Ragland JB, Fisher JN, Kitabchi AE, Sabesin SM (1982) Effects of insulin on plasma lipoproteins in diabetic ketoacidosis: evidence for a change in high density lipoprotein composition during treatment. J Lipid Res 23(1): 171-182.
- Karagianni C, Stabouli S, Roumeliotou K, Traeger-Synodinos J, Kavazarakis E, et al. (2004) Severe hypertriglyceridemia in diabetic ketoacidosis: clinical and genetic study. Diabet Med 21(4): 380-382.
- Rodríguez Santana Y, Nimo Román A, García Sáez I, López Alvarez JM, Consuegra Llapur E, et al. (2011) Treatment of severe hypertriglyceridemia with continuous insulin infusion. Case Rep Crit Care 2011: 293917.
- Lucchetti G, Granero AL, Almeida LGC, Battistella VM (2009) Severe hypertriglyceridemia in diabetic ketoacidosis: case report. Arq Bras Endocrinol Metab 53(7): 880-883.
- Rius RF, Pizarro LE, Reverter CJ, Gener RJ, Bechini J, et al. (1993) Acute pancreatitis in newly diagnosed type 1 diabetes mellitus with diabetic ketoacidosis and hypertriglyceridemia. Medicina Clinica 101(16): 622-624.
- Soejima S, Umeno Y, Fujita T, Dohmen K, Miyamoto Y (2000) A case of diabetic ketoacidosis complicated by severe hypertriglyceridemia and acute pancreatitis. JDS 43: 561-566.
- Bae JH, Baek SH, Choi HS, Cho KR, Lee HL, et al. (2005) Acute pancreatitis due to hypertriglyceridemia: report of 2 cases. Korean J Gastroenterol 46(6): 475-480.
- Hahn SJ, Park J, Lee JH, Lee JK, Kim KA (2010) Severe Hypertriglyceridemia in Diabetic Ketoacidosis Accompanied by Acute Pancreatitis: Case Report. J Korean Med Sci 25(9): 1375-1378.
- Kota SK, Jammula S, Krishna SV, Modi KD (2012) Hypertriglyceridemia-induced recurrent acute pancreatitis: A case-based review. Indian J Endocr Metab 16(1): 141-143.
- Argueta EE, Nugent KM (2013) Acute pancreatitis in a patient with diabetic ketoacidosis and normal lipase levels. ICU Director 4(4): 166-169.
- Seth A, Rajpal S, Saigal T, Bienvenu J, Sheth A, et al. (2014) Diabetic Ketoacidosis-induced Hypertriglyceridemic Acute Pancreatitis Treated with Plasmapheresis—Recipe for Biochemical Disaster Management. Clin Med Insights Gastroenterol 7: 51-53.
- Singla AA, Ting F, Singla A (2015) Acute pancreatitis secondary to diabetic ketoacidosis induced hypertriglyceridemia in a young adult with undiagnosed type 2 diabetes. JOP 16(2): 201-204.






























