Diabetes Mellitus and Its Existing Oral Drug Therapies (ODT)
Ighodaro, O.M.1* (Ph.D), Asejeje, F.O.1 (Ph.D), Ujomu, T.A.2 (Ph.D), Adeosun A.M.1 (M.Sc.)
1Department of Biochemistry, Lead City University, Nigeria
2Department of Pharmacology & Therapeutics, University of Ibadan, Nigeria
Submission: June 20, 2018; Published: November 06, 2018
*Corresponding author: Ighodaro OM,, Department of Biochemistry, Faculty of Sciences, Lead City University, Ibadan, Nigeria
How to cite this article: Ighodaro OM, Asejeje FO, Ujomu TA, Adeosun AM. Diabetes Mellitus and Its Existing Oral Drug Therapies (ODT). Curre Res Diabetes & Obes J. 2018; 9(2): 555756. DOI:10.19080/CRDOJ.2018.09.555756.
Abstract
Diabetes mellitus (DM) is an endocrine disorder that is characterized by high level of blood glucose due to either insulin deficiency or defectiveness or both. DM has been associated with both micro- and macro- vascular complications, and these are arguably the principal actors in its morbidity and mortality. Over the years, several classes of Oral Antidiabetic Drugs (OADs) ranging from the sulfonylureases to the dipeptidyl peptidase IV inhibitors have been introduced besides insulin therapy. Diabetes management has no doubt been improved with the use of these therapies. Nonetheless, obvious limitations associated with these drugs have made DM a long standing and increasing global health challenge. It is therefore imperative that in search for perfect antidiabetic agents, the limitations of the existing remedies should be noted and taken into cognizance as vital pointers in the direction to go.
Keywords: Diabetes mellitus; health challenge; oral antidiabetic drugs
Introduction
Diabetes mellitus (DM) remains a global health challenge due to lack of curative therapy. Most available therapies are for management of the diseases and are associated with various side effects. The incidence of the disease is alarming and threatens a worldwide epidemic, particularly among the upper age cadre [1,2]. Its prevalence as at 2010 was 5.2 % of the global populace, affecting over 285 million people worldwide. As at 2015, this number has increased to 415 million people, with over 5.0 million deaths attributable to diabetes, and the total global health expenditure due to diabetes estimated at 673 billion US dollars [3]. Diabetes obviously has a great economic impact on individuals, healthcare systems and economies of nations [4]. The number of people affected by diabetes is still on the rise, with the number rising to 422 million in 2016, and expected to escalate to over 642 million by 2040 [3,5]. Global estimates predict that the proportion of adult population with diabetes will increase by 69% by the year 2030 [2].
The morbidity and mortality associated with diabetes mellitus often occur as a result of the complications of the disease [6]. These complications which are usually termed diabetic complications are categorized into micro-vascular and macro- vascular complications, and they are arguably the principal actors in the pathogenesis and mortality of the disease [7].
Management of diabetes without any side effect is still a challenge for the medical system. Home et al., (2007) reported that despite many oral hypoglycemic agents available to manage type 2 diabetes, 5 to 10 % of the population with diabetes experience secondary failure [8]. This bottleneck can be arrested if the limitations of some therapies currently in use are acknowledged and improved upon. The currently used antidiabetic drugs are associated with multiple undesirable side effects which include haematological, cutaneous and gastrointestinal reactions, hypoglycemic coma and impairment of liver and kidney functions. This scenario has greatly encouraged a fast-growing interest in search of perfect antidiabetic drugs.
Epidemiology and Prevalence of Diabetes Mellitus
The prevalence of diabetes mellitus is increasing with ageing of the population and lifestyle changes associated with rapid urbanization and westernization. The disease is found in all parts of the world and is rapidly increasing in its scope, affecting both the old and the young [9]. Globally, the prevalence of diabetes, without type distinction, is on the increase. According to the World Health Organization, it was estimated in 2010 that 6.4 % (285 million adults) of the world’s population have diabetes and the prevalence is expected to double by the year 2030 to rise to7.7%, with 439 million adults affected [2,10]. This scary prevalence is to escalate to 14.19 % by the year 2040 [3].
Economic burden of diabetes mellitus
Diabetes Mellitus has a great economic burden on the individual as well as the health care systems and economies of nation [4]. It is documented by the American Diabetes Association (ADA) that individuals with diabetes have about 2.3 times higher medical expenditures compared to those without diabetes in USA [11]. Diabetes mellitus is a disproportionately expensive disease and has profound implications on global economy [12,13]. According to Kirigia et al. (2009) diabetes mellitus poses a big economic burden with regards to direct health care costs, indirect costs engendered by patient disability and premature mortality as well as intangible costs of psychological and emotional trauma experienced by the family and loved ones of the diabetics [14]. Studies have shown that the annual national costs of managing diabetes are severely biting deep into the fabric of budgets of nations with high prevalence of the disease. As at 2003; Barceló et al. reported US $65.2 billion as the total annual cost for diabetes care in Latin America and the Caribbean [15]. As at 2007, the total annual costs (direct and indirect) for diabetes care in the United States of America (USA) was pegged at $174 billion [11]. India with a prevalence of 200,000 type 1 diabetics in 2002 reported the cost of diabetes treatment to be as high as US $50 million. AS at 1994, there were over 1.4 million known diabetics in Spain and the total direct cost of the disease was reported to be over US$650 million. The list goes on and on for countries with adequate and up to data base.
Diabetes and oxidative stress
Several studies have shown that oxidative stress is a key element in the development and progression of diabetes and its associated complications [16-18] had earlier proposed oxidative stress as a major participant in the pathophysiology of diabetic complications [19]. Although, oxidative stress is not the primary cause of diabetes, it nonetheless facilitates the induction of multiple cellular pathways which ultimately lead to both the onset and complications of diabetes [20-22]. Moreover, oxidative stress has been shown to affect the two major mechanisms failing during diabetes: insulin action and insulin secretion [22-24].
Existing drug therapies for diabetes
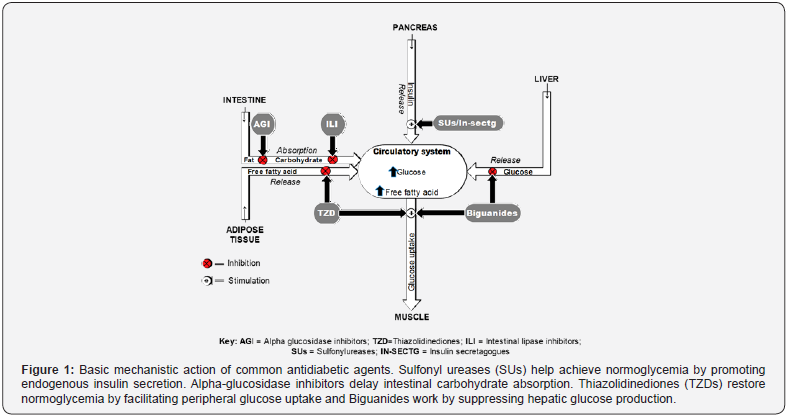
Life style management is apparently the cornerstone of management of diabetes mellitus. It is recognized as being an essential part of diabetes and cardiovascular disease prevention. Meta-analyses demonstrate that lifestyle interventions, including diet and physical activity, led to a 63% reduction in diabetes incidence in those at high risk [25]. In spite of the underscored importance of lifestyle measures in diabetes management, the importance of drug therapy in achieving glycemic control and homeostasis in the diabetics cannot be undermined. Sequel to the limitation of insulin therapy, different oral antihyperglycemic drugs (OADs) with distinct mechanisms have been in use till date. They include the sulphonylureas (e.g., glimepiride), α-glucosidase inhibitors (e.g., acarbose), meglitinides (e.g., repaglinide), thiazolidinediones (e.g., pioglitazone), biguanides (e.g., metformin) and dipeptidyl peptidase IV inhibitors (e.g., sitagliptin). Sulfonylureases and the non-sulfonylurea secretagogues establish normoglycemia by up regulating endogenous insulin secretion. Alpha-glucosidase inhibitors work by delaying intestinal carbohydrate absorption. Thiazolidinediones (TZDs) maintain normoglycemia by enhancing insulin sensitivity primarily by increasing peripheral glucose disposal and suppressing hepatic glucose production. Biguanides such as metformin function by decreasing hepatic gluconeogenesis while at times also increasing peripheral glucose mobilization and disposal [26] (Figure 1).
The Sulphonylureases
Sulfonylureases (SUs) are the oldest and most widely used medications for the treatment of type 2 diabetes mellitus. Although SU therapy effectively lowers blood glucose concentrations by stimulating insulin secretion from β-cells, treatment with SUs is associated with a progressive linear decline in β-cell function [27-28]. Hypoglycemia and weight gain are the common adverse effect associated with SUs therapy. Weight gain is thought to result from an anabolic effect of increased insulin concentration [29].
The Alpha (α) -Glucosidase Inhibitors (AGIS)
Alpha glucosidase inhibitors, such as acarbose, are competitive inhibitors of membrane-bound intestinal α-glucosidases that hydrolyze oligosaccharides, trisaccharides and disaccharides to glucose and other monosaccharides in the small intestine and thereby delay postprandial glucose absorption [30]. These agents are available as a first-line treatment in patients with slightly raised basal glucose concentrations and marked postprandial hyperglycaemia [31]. AGIs have an average decrease capacity in HbA1c of 0.5–1%. Derosa et al., (2009) demonstrated that both repaglinide and acarbose had a similar effect in reducing postprandial glucose levels (-14.9%, p < 0.05; -16.2%, p < 0.05; compared to baseline, respectively [32]. Treatment of diabetes with AGIs has been associated with significant risk reduction of cardiovascular disease via suppression of oxidative stress induced by postprandial hyperglycemia [33]. Despite their good safety record, limited gastrointestinal tolerability has substantially restricted their use.
The Glinides
Meglitinides such as repaglinide and nateglinide are prandial insulin releasers that stimulate rapid insulin secretion [34]. Repaglinide (NovoNorm®, Prandin®, GlucoNorm®) is the first clinically available insulin secretagog that specifically enhances early-phase prandial insulin response by increasing the sensitivity of β-cells to elevated glucose levels, producing a greater insulin release under hyperglycemic conditions [35,36]. In this regard, it has been shown in vitro that repaglinide is fivetimes more potent than glibenclamide in stimulating insulin secretion, with half-maximal stimulation observed at 40 and 200 nmol/L, respectively [37]. Lower risk of hypoglycemia makes these agents attractive option for some elderly patients, in particular when other agents may be contraindicated.
Thiazolidinediones
The thiazolidinediones are insulin-sensitizing drugs that improve whole-body insulin sensitivity through gene regulation [31]. These agents increase glucose uptake via glucose transporter-4 in skeletal muscle and reduce rates of gluconeogenesis in the liver. Reductions in plasma insulin concentration and lowering of circulating triglycerides are additional indirect mechanisms that may help improve wholebody insulin sensitivity. Thiazolidinediones have also been known to improve β-cell function and reduce insulin resistance; however, they are associated with weight gain and can cause peripheral edema [38].
The Biguanides
The biguanides such as Metformin, which act directly against insulin resistance, are regarded as insulin sensitizing drugs and are considered to be a cornerstone in the treatment of type 2 diabetes mellitus [39]. Metformin acts on the liver to produce a hypoglycemic effect. Available formulations include Glucophage®, Glucophage XR®, Riomet®, Fortamet®, Glumetza®, Obimet®, Dianben®, Diabex® and Diaformin®. Metformin lowers blood glucose levels without causing overt hypoglycemia or stimulating insulin secretion. In a study of the metformin mechanism of action, it was postulated that metformin decreases endogenous glucose production in type 2diabetes patients. Treatment with the drug decreases fasting plasma glucose concentrations by 25% and reduced hepatic gluconeogenesis by interfering with respiratory oxidation in mitochondria. Despite being the most widely used oral antidiabetic drug (OAD) in the world, metformin can reach a plateau of effectiveness due to progressive β-cell failure [40,41]. Metformin is only effective when there is sufficient endogenous or exogenous insulin and, because of this, patients are unable to maintain tight glycemic control as their disease progresses [42].
Dipeptidyl Peptidase-iv (DPP-IV) Inhibitors
Dipeptidyl peptidase-IV (DPP-IV) inhibitors are compounds which suppress the degradation of a variety of bioactive peptides, including DPP-IV and subsequently increasing the endogenous concentration of incretins, such as GLP-1glucagonlike peptide-1 [43,44]. Bioavailability of GLP-1 evidently improves glycemic control [43]. DPP-IV inhibitors are orally administered drugs with a significant effect on glucose tolerance and lasting improvement of HbA1c [45]. Several agents are in different stages of clinical development. Sitagliptin is a good example of selective DPP-IV inhibitor, it was approved by the Food and Drug Agency in 2006 in United States as an adjunct to diet and exercise in patients with type 2 diabetes. In 2009, 2011 and 2013, Saxagliptin, Linagliptin, Alogliptin (other selective DPP-IV inhibitors) were respectively approved in the US [38].
Medicinal plant approach in management of diabetes mellitus
The effective roles of some plants in the management of diabetes mellitus are well documented. According to Piero et al. (2012), plant derived medications have in recent times found immense use in the management of diabetes mellitus [46]. To date, the catalogue of antidiabetic medicinal plants is growing at a pleasantly high rate particularly in the African continent. The antidiabetic potential of some medicinal plants extracts has been demonstrated in human and animal models of type 2 diabetes. The increasing reliance on antidiabetic medicinal plants is a major motivation for scientists to investigate more plants in a bid to elucidate more hypoglycemic agents of natural origin.
Conclusion
Diabetic conditions have no doubt been improved by existing therapies. However, their associated limitations have made DM a long standing and increasing global health challenge. In search for perfect antidiabetic agents, it is therefore important for scientists to continuously ride on the strength and weaknesses of the available therapeutic options.
References
- Ighodaro OM, Akinloye OA (2017) Anti-diabetic potential of S apium ellipticum (Hochst) Pax leaf extract in Streptozotocin (STZ)-induced diabetic Wistar rats. BMC Complement Altern Med 17(1): 525.
- Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice 87(1): 4-14.
- Ogurtsova K, da Rocha Fernandes J, Huang Y (2017) IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes research and clinical practice 128: 40-50.
- Simmons R, Unwin N, Griffin S (2010) International Diabetes Federation: An update of the evidence concerning the prevention of type 2 diabetes. Diabetes research and clinical practice 87(2): 143-149.
- Jaacks LM, Siegel KR, Gujral UP, Narayan KV (2016) Type 2 diabetes: A 21st century epidemic. Best Practice & Research Clinical Endocrinology & Metabolism 30(3): 331-343.
- Daneman D (2006) Type 1 diabetes. The Lancet 367(9513): 847-858.
- Ighodaro O, Adeosun A (2017) Vascular Complications in Diabetes Mellitus. Kidney 4: 16.
- Home P, Jones N, Pocock S (2007) Rosiglitazone RECORD study: glucose control outcomes at 18 months. Diabetic Medicine 24(6): 626-634.
- Rathmann W, Giani G (2004) Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030: Response to Wild et al. Diabetes care 27(10): 2568-2569.
- Andrade-Cetto A, Heinrich M (2005) Mexican plants with hypoglycaemic effect used in the treatment of diabetes. Journal of ethnopharmacology 99(3): 325-348.
- Association AD (2010) Diagnosis and classification of diabetes mellitus. Diabetes care 33(Suppl 1): S62–S69
- Votey SR, Peters A (2005) Diabetes mellitus, Type 2-A review.
- Sobngwi E, Mauvais-Jarvis F, Vexiau P (2001) Diabetes in africans. Studies 6: 34.
- Kirigia JM, Sambo HB, Sambo LG (2009) Economic burden of diabetes mellitus in the WHO African region. BMC international health and human rights 9(1): 6.
- Barcelo A, Aedo C, Rajpathak S (2003) The cost of diabetes in Latin America and the Caribbean. Bulletin of the world health organization 81(1): 19-27.
- Tangvarasittichai S (2015) Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World journal of diabetes 6(3): 456.
- Domingueti CP, Dusse LMSA, de Sousa LP (2016) Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. Journal of Diabetes and its Complications 30(4): 738-745.
- Thornalley PJ (1996) Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification-a role in pathogenesis and antiproliferative chemotherapy. General Pharmacology: The Vascular System 27(4): 565-573.
- Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6): 1615-1625.
- Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circulation research 107(9): 1058-1070.
- Maritim A, Sanders a, Watkins J (2003) Diabetes, oxidative stress, and antioxidants: a review. Journal of biochemical and molecular toxicology 17(1): 24-38.
- Rains JL ,Jain SK (2011) Oxidative stress insulin signaling, and diabetes. Free Radical Biology and Medicine 50(5): 567-575.
- Ogihara T, Asano T, Katagiri H (2004) Oxidative stress induces insulin resistance by activating the nuclear factor-κB pathway and disrupting normal subcellular distribution of phosphatidylinositol 3-kinase. Diabetologia 47(5): 794-805.
- Bloch-Damti A, Bashan N (2005) Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxidants & redox signaling 7(11-12): 1553-1567.
- Appuhamy JRN, Kebreab E, Simon M, Yada R, Milligan LP, et al. (2014) Effects of diet and exercise interventions on diabetes risk factors in adults without diabetes: meta-analyses of controlled trials. Diabetology & metabolic syndrome 6(1): 127.
- Salpeter SR, Greyber E, Pasternak GA, Salpeter EE (2010) Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. The Cochrane Library.
- Gillani SW, Sulaiman SAS, Baig M (2012) Pharmacist intervention in home care program for diabetes patients. Journal of Diabetes Mellitus 2(03): 279.
- Inzucchi SE, Bergenstal RM, Buse JB (2012) Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55(6): 1577-1596.
- Bailey SH, Bull DA, Harpole DH (2003) Outcomes after esophagectomy: a ten-year prospective cohort. The Annals of thoracic surgery 75(1): 217-222.
- van de Laar FA (2008) Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vascular health and risk management 4(6): 1189.
- Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents. Drugs 65(3): 385- 411.
- Derosa G, Putignano P, Bossi AC (2011) Exenatide or glimepiride added to metformin on metabolic control and on insulin resistance in type 2 diabetic patients. European journal of pharmacology 666(1-3): 251- 256.
- Delorme S, Chiasson J-L (2005) Acarbose in the prevention of cardiovascular disease in subjects with impaired glucose tolerance and type 2 diabetes mellitus. Current opinion in pharmacology 5(2):184- 189.
- Black C, Donnelly P, McIntyre L, Royle P, Shepherd JJ, et al. (2007) Meglitinide analogues for type 2 diabetes mellitus. The Cochrane Library.
- Johansen OE, Birkeland KI (2007) Defining the role of repaglinide in the management of type 2 diabetes mellitus. American journal of cardiovascular drugs 7(5): 319-335.
- Raskin P (2008) Oral combination therapy: repaglinide plus metformin for treatment of type 2 diabetes. Diabetes, Obesity and Metabolism 10(12): 1167-1177.
- Fuhlendorff J, Rorsman P, Kofod H (1998) Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes 47(3): 345-351.
- Del Prato S, Taskinen M-R, Owens DR (2013) Efficacy and safety of linagliptin in subjects with type 2 diabetes mellitus and poor glycemic control: pooled analysis of data from three placebo-controlled phase III trials. Journal of Diabetes and its Complications 27(3): 274-279.
- Bosi E (2009) Metformin–the gold standard in type 2 diabetes: what does the evidence tell us? Diabetes, Obesity and Metabolism 11(s2): 3-8.
- Nichols GA, Alexander CM, Girman CJ, Kamal-Bahl SJ, Brown JB (2006) Treatment escalation and rise in HbA1c following successful initial metformin therapy. Diabetes Care 29(3): 504-509.
- Butler PC, Meier JJ, Butler AE, Bhushan A (2007) The replication of β cells in normal physiology, in disease and for therapy. Nature Reviews Endocrinology 3(11): 758-68.
- Hundal RS, Inzucchi SE (2003) Metformin. Drugs 63(18): 1879-1894.
- Conarello SL, Li Z, Ronan J (2003) Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proceedings of the National Academy of Sciences 100(11): 6825-6830.
- Drucker DJ (2001) Minireview: the glucagon-like peptides. Endocrinology 142(2): 521-527.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, et al. (2006) Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes care 29(12): 2632-2637.
- Piero NM, Njagi MJ, Kibiti MC (2012) Herbal management of diabetes mellitus: A rapidly expanding research avenue.






























