Patient Retention Stewardship in Real world Evidence Studies: the Pentad of P's Model
Amit Kumar1*, Sanjay Kalra2 and Vandna Rana3
1AstraZeneca Pharma India Ltd, India
2Bharti Hospital, Karnal, India
3Indegene Lifesystems Pvt Ltd, India
Submission: March 08, 2017; Published: April 17, 2017
*Corresponding author: Amit Kumar, Astra Zeneca Pharma India Ltd, India, Email: Amit.KumarAK@astrazeneca.com
How to cite this article: Amit K, Sanjay K, Vandna R. Patient Retention Stewardship in Real world Evidence Studies: the Pentad of Pís Model. Curre Res Diabetes & Obes J. 2017; 2(1): 555578. DOI: 10.19080/CRDOJ.2017.02.555578
Abstract
Real World Evidence (RWE) studies generate data that helps fill the knowledge gap between clinical trials and actual clinical practice setting. RWE studies generate data across different age groups, races, ethnicities, varying severities, unstudied co-morbid conditions, differing concomitant drugs (including OTC medications), lifestyle variations and differing adherence/compliance patterns. RWE studies are across very large number of patients and therefore may help unearth rarer ADRs which are not evident in relatively smaller populations in clinical trials.
By definition the RWE studies are across larger and more diverse populations than the more controlled setting of Clinical trials which are conducted across a relatively smaller number of subjects. Given that controls are lesser in real world setting of actual practice, it is difficult to enrol and more importantly retain patients in these real world studies. The very basis of these real world studies is to generate rich data from a larger population and patient retention being a challenge, the results RWE study would lose significance if the challenges of retention are not addressed at the very beginning. This article is an endeavour to discuss various risk factors affecting patient retention and suggests a practical approach by the entire study team to effectively address and mitigate these risks.
Introduction
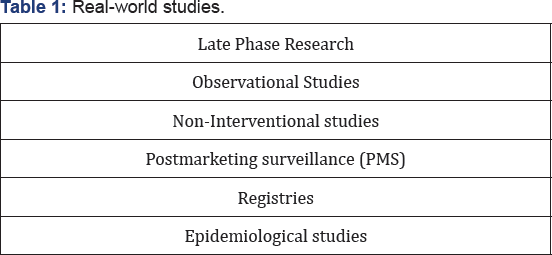
The main purpose of drug development is to bring drugs and devices to market faster and improve patient's health outcomes. But what happens once those drugs and devices are in the market? Real World Evidence (RWE) provides deep insight into how a drug is actually used and how it performs in the "real world" with all its variety of settings and circumstances. RWE is obtained from real world evidence studies, which may be of various types (Table 1)
Factors Affecting Study Success
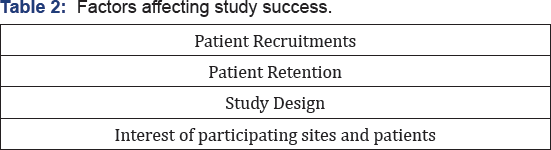
There Are Many Factors, Which Can Affect Study Success (Table 2)
Importance of retention
While patient recruitment is often highlighted as the key aspect in ensuring study success, the area of patient retention in RWE studies is often overlooked. Retention of patients throughout the life of study is however critical from scientific as well as economic point of view. Retaining as many patients as possible should be a priority since it directly affects the statistical power of the study. Some reasons for subject dropout are within the control of study or can be addressed through careful study design and during conduct of the study.
Factor Affecting Retention
Retention Stewardship
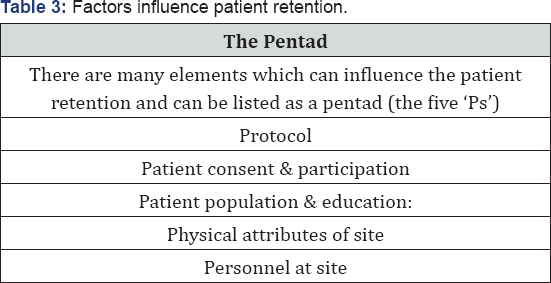
A good retention plan needs stewardship by Sponsor, CRO & Sites. This is done through a good patient retention plan, which can be developed at the initiation of the study and that can be implemented throughout the life of the study to mitigate patient retention risks. There paper emphasizes on 5 key elements Table 3 which can lead to good retention stewardship.
Protocol
A study protocol is a document that describes, in detail, the plan for conducting the study. The study protocol explains the purpose and function of the study as well as how to carry it out. During protocol development, sponsors should consider how the study design will translate into patient burden for study participants. The number of study visits, complexity of the tasks the patient must perform, and the convenience of the site location can all build into unrealistic patient burden that leads to early discontinuation from the study.
A proactive approach to integrate into the study design phase is to conduct a feasibility or Panel discussion with NCI/ study chair person & SC members (National Co-ordinating Investigator & Steering Committee Members), which comprises of experience Investigators of the country. This group can offer the sponsor insights into patient perceptions & practical challenges of the study. A Sponsor can pen down common questions and seek clarity during the discussion. There are few questions mentioned in Table 4.

The investigator meeting can also provide an interactive forum for all PIs & site staff to exchange successful strategies for addressing patient concerns.
Patient consent & participation
At the time a patient consents to participate in a study, the consent process should be a detailed discussion or explanation of what will occur during the conduct of the study, risks and benefits, study duration, Number of follow-ups and timing of study visits and potential side effects. The principal investigator is key to setting the right tone at this first visit. Recognizing and addressing a patient's concern during consent process and throughout the study is important to retain the patient till study completion. During the consent process if a patient has a fear, it has to be addressed by the principal investigator who listens to and answers their questions
Patient population and education
Patient population for a study depends on the disease prevalence in the surrounding areas of the site. For example, there are good transport facilities in plain areas versus hilly areas, so studies which require frequent follow up will have better retention in the plains. At present almost all the studies require educated patients. For example, diabetes studies design includes patient's diary or questionnaires for collection of information regarding blood glucose values and other patient reported outcomes such as quality of life. This means that site with lot of illiterate patients will not be able to retain patients because they need support to fill the details and in long term they can feel it's a burden or pressure
Physical attributes of site
Recruiting a large number of participants and retaining them in the study usually requires involvement of a considerable number of centres. However, all centres need to have a patient pool of adequate size and the infrastructure and resources to recruit and manage the projected numbers of patients efficiently
Site resources: One should assess personnel resources including the number and type of personnel available, their functional responsibilities, and their relationships to other institutional departments, referring physicians and community organizations. Participating research clinics need to be centrally located, easily accessible, well organised and efficient in scheduling tests, collecting information and so on. They should offer flexible appointment times and sufficient time with the clinical trial staff for participants to adequately understand the study's rationale, its requirements and risks and have all their questions answered.
Site facilities and procedures: Site facilities & procedures can also affect patient's retention like
• Availability and responsiveness of site staff; Patients will feel more secure having 24/7 access to trained study staff.
• Convenient timing of appointments: Will facilitate patient’s compliance with the study schedule and prevent missed visits.
• Logistic issues can also lead to study procedure's fatigue and patients may lose their interest in participation further.
Personnel at site
Though the PI is responsible for the conduct of the study however the Study Co-ordinator (SC) is the heart and soul of the study and that, ultimately, it is the SC who carries forward the research goals and play a significant role in the success of the study. Employing an experienced and dedicated SC at each participating centre is key to successful recruitment, retention, and reduces the time demanded of investigators. Same site personnel/SC throughout the study duration helps to facilitate trusting relationship with the patient. SC should be skilled at verbal communication (in languages) and responding to subjects’ questions and concerns.
Site experience: Past experience in conducting studies in a similar patient population and assessment of past enrolment & retention performance metrics is important in deciding whether a site can fulfil the need.
Communications: Strategy of maintaining a strong communication with the patient over extended periods of time during the study is essential. Patient retention involves great customer relationships therefore SC should focus on creating a pleasant "Soft Skills" experience for the subject. Patients are people too and treating them in the same way as you would want to be treated will build trust and confidence required to retain a patient in the study.
Use of Technology in Retention Plan
Technology solutions can provide a supportive role in retention during the patient participation phase, ensuring the patient has a positive experience at the clinical study site and this remains critical for ongoing patient participation.

Some key points to keep patient engaged and motivated are listed in Table A.

Conclusion
Patient motivation for participation in the study is multifactorial and is a continuous process. Part of this process includes reinforcing the study purpose and the value of their participation at each study visit. The practices described above, may be quite effective in identification and fixing the problem of patient dropouts at the right time. The “Pentad of P's model” is a simple method that Study Team (sponsors, CRO and sites) can use to prepare a good retention plan, minimise the retention risks and ensure effective retention stewardship.
Acknowledgement
We would like to thank you Dr Bhavesh Kotak and Dr Ammar Raza, AstraZeneca Pharma India for their review and valuable suggestions.
References
- Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades RM, et al. (2015) Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 3(2): 105-113.
- Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, et al. (2013) ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 34(39): 3035-3087.
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375(9133): 2215-2222.
- Centers for Disease Control and Prevention (2014) Number (in Millions) of Civilian, Non-Institutionalized Persons with Diagnosed Diabetes, United States, 1980-2014. Diabetes Public Health Resource, USA.
- Beckman JA, Creager MA, Libby P (2002) Diabetes and atherosclerosis: epidemiology, pathophysiology, and management JAMA 287(19): 2570-2581.
- Gu K, Cowie CC, Harris MI (1998) Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care 21(7): 1138-1145.
- Lancet (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Group. Lancet 352(9131): 837-853.
- Holman RR, Paul SL, Bethel MA, Matthews DR, Neil HA (2008) 10 years follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359(15): 1577-1589.
- Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, et al. (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358(24): 2545-2559.
- Chow E, Bernjak A, Williams S, Fawdry RA, Hibbert S, et al. (2014) Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 63(5): 1738-1747.
- Nordin C (2010) The case for hypoglycaemia as a proarrhythmic event: basic and clinical evidence. Diabetologia 53(8): 1552-1561.
- Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356(24): 2457-2471.
- Final Guidance for Industry: Guidance for Industry: Diabetes Mellitus - Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. U.S. Department of Health and Human Resources; December 2008. European Medicines Agency Guideline on the Investigation of Drug Interactions.
- American Diabetes Association (2017) Pharmacologic Approaches to Glycemic Treatment. Diabetes Care 40(Suppl 1): S64-S74.
- Garber AJ (2011) Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability Diabetes Care 34(supply 2): S279-S284.
- Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, et al. (2015) Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med 373(23): 2247-2257.
- Marso SP, Daniels GH, Brown FK, Kristensen P, Mann JF, et al. (2016) Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 375: 311-322.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, et al. (2016) Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes N Engl J Med 375(19): 1834-1844.
- AstraZeneca (2017) Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus. ClinicalTrials, USA.
- Intarcia Therapeutics (2017) A Study to Evaluate Cardiovascular Outcomes in Patients With Type 2 Diabetes Treated With ITCA 650. ClinicalTrials, USA.
- Eli Lilly and Company (2017) Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND). ClinicalTrials.gov, USA.






























