Advances in Neutrophil Testing In Type 2 Diabetes Mellitus
Han Wei Hou1, Hui Min Tay1, Rinkoo Dalan1,2 and Bernhard O Boehm1,2,3*
1Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore
2Endocrine and Diabetes, Tan Tock Seng Hospital, Singapore
3Imperial College London, UK
Submission: February 7, 2017; Published: March 27, 2017
*Corresponding author: Bernhard Otto Boehm, Lee Kong Chian School of Medicine, Nanyang Technological University and Imperial College London, Experimental Medicine Building, level 3, anyang Drive, Singapore 636921, Tel: +65-65923637; Email: Bernhard.boehm@ntu.edu.sg
How to cite this article: Han W H, Hui M T, Rinkoo D, Bernhard O B. Advances in Neutrophil Testing In Type 2 Diabetes Mellitus. Curre Res Diabetes & Obes J 2017; 1(5): 555572. DOI: 10.19080/CRDOJ.2017.1.555572
Abstract
Patients with type 2 diabetes mellitus (T2DM) suffer from impaired glucose metabolism which results in low-grade inflammation and activation of the innate immune system. Neutrophils the key effector cells of the innate immune system and heavily implicated in the pathogenesis of T2DM, are promising cell-based inflammatory biomarkers for immune health profiling, provided that they can be rapidly purified and measured with sufficient precision. In this review, we highlight recent advances in neutrophil isolation and functional assay using microfluidics technologies and the potential of their functional phenotype as a novel biomarker of vascular risk in diabetes.
Keywords: Neutrophils; Diabetes; Point-of-care; Microfluidics; Immunology
Introduction
With the increasing aging population worldwide, metabolic disorders such as diabetes mellitus (DM) and cardiovascular diseases (CVDs) have become the main public health challenges with rising premature morbidity and associated mortality, as well as escalating healthcare costs [1]. DM is characterized by chronic hyperglycemia resulting in increased oxidative stress, inflammation and endothelial dysfunction [2,3]. Patients with CVDs or type 2 diabetes mellitus (T2DM) often exhibit low- grade inflammation, and are assessed based on established cardiovascular risk factors (glycemic control, blood pressure and lipids). Immune health is evaluated by differential leukocyte count and circulating biomarkers (cytokines and C-reactive protein (CRP), which are suboptimal for monitoring stage- dependent pathogenesis, advocating the need to develop new cell-based biomarkers that can quantity specific immune functions in addition to leukocyte enumeration.
Neutrophils, the key effector cells of the innate immune system, play a pivotal role in T2DM and CVDs pathogenesis [4]. Various neutrophil dysfunctions have been reported in T2DM patients including cell stiffening [5,6] impaired chemotaxis [7,8] and phagocytosis which lead to increased susceptibility to bacterial infections [9]. Despite the adverse changes of leukocytes in diabetes, there are currently no specific measurements to assess patient's leukocyte phenotypes or inflammatory status. As distinct neutrophil subsets exhibit functional and phenotypic differences [10] a better understanding of their phenotype and pathophysiological relevance requires novel neutrophil separation tools (independent of surface markers) to improve their predictive capabilities as novel biomarkers [11]. Microfluidics, also known as "lab-on-a-chip" technologies, is a powerful toolbox for rapid sample preparation and detection with its low consumption of sample and reagents, device miniaturization, and single-cell analysis [12]. In this short review, we will highlight recent advances in microfluidics- based neutrophil testing technologies, and the potential of neutrophilfunctional phenotype as biomarkers for diabetes testing.
Discussion
Neutrophil isolation
Neutrophil polymorphonuclear granulocytes (PMN) are the most abundant leukocytes (~50-70%) in humans, with ~2-5x106 neutrophils per mL of whole blood (~109 RBCs). They are short-lived (~5-24hr), prone to activation [13] and should be processed quickly within 2-4 hours of collection. Conventional neutrophil isolation methods include density gradient centrifugation and RBCs lysis, which are laborious (~1- 3hr) and require large blood volume (~10mL). Commercial kits based on magnetic bead-based affinity binding (MACS xpress® Neutrophil Isolation Kit (Miltenyi Biotec) and Easy SepTM neutrophil enrichment kit (STEMCELL Technologies) provide high neutrophil yield and purity by negative selection, but is expensive for large volume processing.
Microfluidics technologies for neutrophil isolation have been developed based on affinity binding to functionalized surfaces using common neutrophil markers (CD66b, P-selectin) [14,15]. However, these methods require on-chip cell analysis as it is non-trivial to elute the purified neutrophils off-chip for downstream assays. Our group has previously developed an efficient size-based cell sorting technique known as Dean Flow Fractionation (DFF) based oninertial focusing phenomenon in micro channels [16]. In DFF systems, fluid flowing through a curvilinear (spiral) channel experiences centrifugal acceleration directed radially outward, leading to the formation of two counter-rotating vortices known as Dean vortices [17]. Besides inertial lift forces (FL) particles experience lateral Dean drag force (FD) due to these transverse Dean flows, which results in superior separation resolution as both forces (FL and FD) scale non-linearly with particle size [18-20].
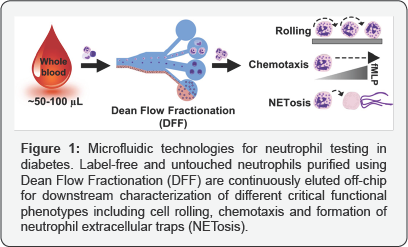
We first applied DFF technology to isolate circulating tumor cells (CTCs) [21] and microorganisms from whole blood whereby the size ranges of the target cells are distinctly different from RBCs. By exploiting the subtle size differences between major leukocyte subtypes (neutrophils/monocytes~10-12μm lymphocytes~7-9μm), we recently developed a novel DFF spiral micro device to purify neutrophils rapidly from whole blood for functional phenotyping in T2DM [22]. The developed technology enables single-step neutrophil isolation (>90% purity) without immune-labeling, saving both time and cost. In addition, the sorted "untouched" neutrophils are continuously eluted off-chip with simultaneous buffer exchange, facilitating user operation and eliminating the need for centrifugation. Moreover, as the method only requires small blood volumes (finger prick ~50- 100μL) it can be easily integrated with other cellular assays or detection modules for point-of-care (POC) testing (Figure 1).
Neutrophil rolling
During endothelial inflammation, leukocyte adhesion cascade is a multi-step process involving cell rolling, adhesion and transmigration through blood vessel walls to the site of injury [23]. Neutrophil rolling is widely considered a critical step as it can affect cell adhesion with impaired cell tethering or increased rolling speeds [24,25]. Several microfluidics- based cell rolling assays have been reported previously to study rolling behavior under physiological flow conditions (~1-10dynecm-2), but not in disease-specific context [26-28]. In our study, we combined DFF neutrophil sorting method and microfluidics assay to measure neutrophil rolling speed on E-selectin, a cell adhesion molecule expressed by activated endothelium to initiate leukocyte recruitment. This neutrophilendothelial interaction is mediated by several sialyl Lewisx presenting ligands expressed on leukocytes including P-selectin glycoprotein ligand 1 (PSGL1), glycosylated CD44 and E-selectin ligand 1 (ESL1) [29].
In our clinical validation, we observed a significant down regulation of neutrophil PSGL-1 expression in T2DM patients. Using automated cell tracking algorithm, we further showed that rolling trajectories ofT2DM neutrophils were more discontinuous and irregular as compared to healthy neutrophils. Interestingly, diabetic neutrophils had ~20% higher rolling speeds, which correlated with neutrophil activation, PSGL-1 expression, as well as established cardiovascular risk factors (cholesterol, CRP and HbA1c). Taken together, the data support the hypothesis that neutrophil-endothelial interactions are impaired in T2DM patients which can lead to defective neutrophil recruitment, and thus increased patient susceptibility to infection.
Neutrophil chemotaxis
Chemotaxis, a dynamic process where cells sense and move in response to chemical gradients, is traditionally studied using Boyden chamber (transwell), Dunn chamber and micropipette assay [30]. However, these methods suffer from poor reproducibility and ill-defined chemical gradients, which could be overcome by using microfluidics technologies to generate stable and linear chemo attractant gradient in small length scale (~μm) [15]. Moreover, most microfluidic chemotaxis assays only require ~102-3 neutrophils, and facilitate real-time imaging of cell movement at single cell resolution [31]. First performed clinical testing of patients with burn injury using microfluidics, and observed that neutrophils suffered from impaired directionality or slower migration speed, which were associated with degree of burn injury.
Similarly neutrophils from asthmatic patients also displayed significantly slower migration speed as compared to healthy subjects, suggesting its use as a novel diagnostics marker [32]. As impaired neutrophil chemotaxis behavior was reported previously in diabetic patients our group has developed an integrated micro device for neutrophil chemotaxis assay using a drop of blood. The novelty lies in the single-step enrichment of neutrophils using biomimetic cell margination [33] and affinity capture, followed by simultaneous exposure to chemotactic gradient without requiring additional user manipulation [34]. In our preliminary clinical data we also observed signification suppression of chemotaxis behavior in T2DM patient, which can be mitigated by short exposure to metformin in vitro. Besides diagnostics applications, microfluidics chemotaxis assays also enable study of complex chemoattractant gradients with high precision [35], well-controlled spatial and temporal gradients to probe cell migration pattern [36,37], as well as effect of inflammatory mediators in neutrophil-monocyte interactions [38].
Neutrophil extracellular traps (NETs)
First discovered in 2004, formation of neutrophil extracellular traps (NETs) is an innate key defense mechanism against bacterial infections through the release of nuclear and granular contents to contain and kill pathogens [39]. Upon activation or exposure to bacteria, histones undergo citrullination, followed by chromatin decondensation. Nuclear membrane will degrade, leading to DNA release into the cell, and subsequently extrusion out of neutrophils. Secreted NETs (process known as NETosis) then form a sticky scaffold consisting mainly of microbicidal proteases/elastase and cytotoxic molecules (histones). Interestingly, recent work have shown that diabetic neutrophils were more susceptible to NETosis [40], which can mediate delayed wound healing [41].
NETs components (elastase, histones, neutrophil gelatinase- associated lipocalin, and proteinase-3) are also elevated in the blood of patients with diabetic foot ulcers, and were associated with infection or worsening of ulcer [42]. Overall these clinical evidences suggest a major role of NETosis in diabetes pathophysiology and endothelial damage making it a novel biomarker for early detection of diabetes-related vascular or end-organ complications. Compared to chemotaxis development of microfluidics NET osis assay is still at its early infancy with a recent reported assay based on fluorescent imaging of nucleus degradation [43]. Nevertheless given the increasing importance of NETosis and easy quantification using imaging, we expect more development of novel tools to measure NETosis phenotype in POC settings.
Conclusion
Multidimensional neutrophil phenotypic markers will significantly improvetheir predictive capabilitiesasinflammatory biomarkers provided that they can be rapidly purified and measured with sufficient precision. Microfluidics technologies are not only useful for efficient neutrophil purification but they can also be readily developed and integrated into POC testing plat forms to look at the sum effects of diabetes, hypertension and hyperlipidemia. This enables proper identification of high risk patients with appropriate follow up, reduces the risks in different aspects of the endothelial activation pathway and in time, the effects of therapeutics can also be studied in diabetes and other dysmetabolic diseases.
Acknowledgment
B.O.B. is supported by Lee Kong Chian School of Medicine, Nanyang Technological University Start Up Grant, MOE AcRF Tier 1 (2015-T1-001-258) and NTU-NHG Metabolic Diseases Collaboration Grant (MDCG/15006).H.W.H would like to acknowledge the financial support from the Lee Kong Chian School of Medicine Postdoctoral Fellowship, NTU-NHG Metabolic Diseases Collaboration Grant (MDCG/15004), and the Singapore National Research Foundation under CBRG NIG, and administered by the Singapore Ministry of Health's National Medical Research Council (NMRC-08/2015-BNIG).
References
- Roger VL, Go AS, Lolyd-JDM, Benjamin EJ, Berry JD, et al. (2012) Heart disease and stroke statistics-2012 update: A report from the american heart association. Circulation 125(1): e2-e220.
- Galkina E, Ley K (2006) Leukocyte Recruitment and Vascular Injury in Diabetic Nephropathy. J Am Soc Nephrol 17(2): 368-377.
- Hartge MM, Unger T, Kintscher U (2007) The endothelium and vascular inflammation in diabetes. fDiab Vasc Dis Res 4(2): 84-88.
- Bagdade JD, Root RK, Bulger RJ (1974) Impaired Leukocyte Function in Patients with Poorly Controlled Diabetes. Diabetes 23(1): 9-15.
- Ernst E, Matrai A (1986) Altered red and white blood cell rheology in type II Diabetes. Diabetes 35(12): 1412-1415.
- Pecsvarady Z, Ficher TC, Darwin CH, Fabok A, Maqueda TS, et al. (1994) Decreased polymorphonuclear leukocyte deformability in NIDDM. Diabetes Care 17(1): 57-63.
- Mowat AG, Baum J (1971) Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N Engl J Med 284(12): 621-627.
- Delamaire M, Maquendre D, Moreno M, Legoff MC, Allanic H, et al. (1997) Impaired leucocyte functions indiabetic patients. Diabet Med 14(1): 29-34.
- Shah BR, Hux JE (2003) Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 26(2): 510-513.
- Beyrau M, Bodkin JV, Nourshargh S (2012) Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol 2(11): 120134.
- Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13(3): 159-175.
- Sackmann EK, Fulton AL, Beebe DJ (2014) The present and future role of microfluidics in biomedical research. Nature 507(7491): 181-189.
- Dorward DA, Lucas CD, Alessandri AL, Marwick JA, Rossi F, et al.(2013) Technical advance: autofluorescence-based sorting: rapid and nonperturbing isolation of ultrapure neutrophils to determine cytokine production. J Leukoc Biol 94(1): 193-202.
- Kotz KT, Xio W, Miller-GC, Qian WJ, Russom A, et al. (2010) Clinical microfluidics for neutrophil genomics and proteomics. Nat Med 16(9): 1042-1047.
- Sackmann EK, Berthier E, Young EW, Shelef MA, Wernimont SA, et al. (2012) Microfluidic kit-on-a-lid: a versatile platform for neutrophil chemotaxis assays. Blood 120(14): e45-e53.
- Di Carlo D, Irimia D, Tompkins RG, Toner M (2007) Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc Natl Acad Sci 104(48): 18892-18897.
- Dean W (1928) The stream-line motion of fluid in a curved pipe. Philosophical Magazine Series 5(30): 673-695.
- Bhagat AAS, Kuntaegowdanahalli SS, Papautsky I (2008) Continuous particle separation in spiral microchannels using dean flows and differential migration. Lab Chip 8(11): 1906-1914.
- Kuntaegowdanahalli SS, Bhagat AAS, Kumar G, Papautsky I (2009) Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 9(20): 2973-2980.
- Wu L, Guan G, Hou HW, Bhagat AAS, Han J (2012) Separation of leukocytes from blood using spiral channel with trapezoid crosssection. Analytical Chemistry 84(21): 9324-9331.
- Hou HW, Majid EW, Bee LK, Zee RL, Ross A, et al. (2013) Isolation and retrieval of circulating tumor cells using centrifugal forces. Scientific Reports 3: 1259.
- Hou HW, Petchakup C, Tay HM, Tam ZY, Dalan R, et al. (2016) Rapid and label-free microfluidic neutrophil purification and phenotyping in diabetes mellitus. Sci Rep 6: 29410.
- Lawrence MB, Springer TA (1993) Neutrophils roll on E-selectin. J Immunol 151(11): 6338-6346.
- Rijcken EM, Mike GL, Christoph A, Stephanie M, Rudolf M, et al. (2004) Immunoblockade of PSGL-1 attenuates established experimental murine colitis by reduction of leukocyte rolling. Am J Physiol Gastrointest Liver Physiol 287(1): G115-G124.
- Jung U, Norman KE, Scharffetter-KK, Beaudet AL, Ley K (1998) Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest 102(8): 1526-1533.
- Schaff UY, Malcolm MQX, Kathleen KL, Ning P, Noo LJ, et al. (2007) Vascular mimetics based on microfluidics for imaging the leukocyte- endothelial inflammatory response. Lab Chip 7(4): 448-456.
- Chang WC, Lee LP, Liepmann D (2005) Biomimetic technique for adhesion-based collection and separation of cells in a microfluidic channel. Lab Chip 5(1): 64-73.
- Bose S, Rishi S, Mikhail HH, Chong S, Chia-HL, et al. (2013) Affinity flow fractionation of cells via transient interactions with asymmetric molecular patterns. Scientific Reports 3: 2329.
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7(9): 678-689.
- Kim B, Wu M (2012) Microfluidics for mammalian cell chemotaxis. Ann Biomed Eng 40(6): 1316-1327.
- Butler KL, Ambravaneswaran V, Agrawal N, Bilodeau M, Toner M, et al. (2010) Burn injury reduces neutrophil directional migration Speed in microfluidic devices. PLoS ONE 5(7): e11921.
- Sackmann EKH, Erwin B, Elizabeth A, Paul SF, Michael D, et al.(2014) Characterizing asthma from a drop of blood using neutrophil chemotaxis. PNAS 111(16): 5813-5818.
- Hou HW, Bhagat AA, Chong AG, Mao P, Tan KS, et al. (2010) Deformability based cell margination-A simple microfluidic design for malaria-infected erythrocyte separation. Lab Chip 10(19): 2605-2613.
- Hou HW, Tay HM, Boehm BO (2015) The 19th International conference on miniaturized systems for chemistry and life sciences, Korea.
- Boneschansker L, Yan J, Wong E, Briscoe DM, Irimia D (2014) Microfluidic platform for the quantitative analysis of leukocyte migration signatures. Nat Commun 5: 4787.
- Aranyosi AJ, Wong EA, Irimia D (2015) A neutrophil treadmill to decouple spatial and temporal signals during chemotaxis. Lab Chip 15(2): 549-556.
- Li Jeon N, Baskaran H, Dertinger SK, Whitesides GM, Van de Water L, et al. (2002) Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol 20(8): 826-830.
- Jones CN, Dalli J, Dimisko L, Wong E, Serhan CN, et al. (2012) Microfluidic chambers for monitoring leukocyte trafficking and :humanized nanoproresolving medicines interactions. Proc Natl Acad Sci USA 109(50): 20560-20565.
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663): 1532-1535.
- Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, et al.(2015) NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 52(3): 497-503.
- Wong SL, Demers M, Martinod K, Gallant M, Wang Y, et al. (2015) Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 21(7): 815-819.
- Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, et al. (2016) NETosis delays diabetic wound healing in mice and humans. Diabetes 65(4): 1061-1071.
- Moussavi-HSF, Mladinich KM, Sackmann EK, Shelef MA, Starnes TW, et al. (2016) Microfluidic device for simultaneous analysis of neutrophil extracellular traps and production of reactive oxygen species. Integr Biol (Camb) 8(2): 243-252.






























