Association between 25-Hydroxyvitamin D Levels and Glycemic Status
Georgios Papadakis1* and Vasiliki Villiotou2
1Department of Endocrinology, Metaxa Anti-Cancer Hospital, Greece
2Departmentof Biochemistry, Metaxa Anti-Cancer Hospital, Greece
Submission: December 01, 2016; Published: March 09, 2017
*Corresponding author: Georgios Papadakis, Department of Endocrinology, Metaxa Anti-Cancer Hospital, Botasi 51, Pireaus, Athens, Greece, Tel: +30 2132079670; Email; george.papadakis.md@gmail.com
How to cite this article: Georgios P, Vasiliki V. Association between 25-Hydroxyvitamin D Levels and Glycemic Status. 2017; 1(4): 555567. DOI:10.19080/CRDOJ.2017.01.555567
Abstract
Aim: A significant body of literature supports that 25(OH) vitD deficiencies is associated with insulin resistance.
Methods: We studied the prevalence of 25(OH) vit D deficiencies across the spectrum of glucose metabolism. The study participants 441 total, 362 females (82.1%) had a mean age (±SD) of 64.59 (±9.44) years. The study population was divided into 2 groups: Individuals with Diabetes (n=184), and controls with normal glucose levels (n=257).
Results: Results: The mean 25(OH) vitD (±SD) levels were significantly lower in subjects with diabetes (18.6±10.6mg/ml) compared to normal subjects (24.2±12.2mg/ml), p=0.035. 25(OH) vitD deficiency was observed in 49.7% of the entire study population and was significantly more frequent in patients with diabetes compared to controls (60.9 vs 41.6% respectively).
Conclusion:This study illustrates the higher prevalence of 25(OH) vitD deficiencies among patients with diabetes.
Keywords: Diabetes; Vitamin D; HbA1c
Introduction
Diabetes is widely prevalent globally and around 285 million people have diabetes this number is expected to reach 438 million by the year 2030 [1]. Vitamin D deficiency is also an important public health problem globally [2]. Exposure to ultraviolet light and consumption of foods rich in fat-soluble vitamin D (egg, oily fish, meat and eggs) are the main sources of vitamin D and its levels can be measured by concentration of serum 25-hydroxyvitamin D (25(OH) vitD) [3]. 25(OH) vitD levels are positively related to bone mineral density [4], muscle mass and strength [5]. Moreover, there is a possible association of 25(OH) vitD with certain types of cancer [6,7] infections [8], autoimmune diseases [9] and cardiovascular disease [10]. 25(OH) vitD levels vary with age [11], obesity [12], skin type [13] and ethnicity [14], season [15], liver and kidney disease, medication, nutritional habits and others [16]. Moreover, there is an association of 25(OH) vitD levels with type 2 diabetes mellitus [17,18]. The aim of this study was to examine the association of vitamin D levels with glycemic status in two groups of subjects: patients with diabetes and normal subjects.
Methods
This study was conducted at the Outpatient and Inpatient Departments of the Endocrinology Unit of a tertiary cancer center (Metaxa Anticancer Hospital). The study was approved by the Scientific Committee of the hospital. A total of 441 randomly selected Greek Caucasian individuals, 79 males (17.9%) and 362 (82.1%) females were recruited between August 2013 and October 2015 including all four seasons. Their mean age was 64.59±9.44 (range=32-92 years).
A fasting venous blood sample after an overnight fast of over 8 hours was collected to determine blood glucose, HbA1c and serum 25(OH) vitD levels. Plasma glucose was estimated by the Photometric method, Abbott’s ARCHITECT Abbott’s ARCHITECT c16000 Clinical Chemistry Analyzer, Abbott Diagnostics, North Chicago, Illinois, USA. HbA1c was estimated by high pressure liquid chromatography method Menarini’s ADAMS A1c HA-8160 Analyzer. The prevalence of vitamin deficiency (<20mg/ml), insufficiency (20–<30mg/ml) and sufficiency (≥30mg/ml) was estimated, as described by the Endocrine Society Clinical Practice guidelines [19]. The analytical assay for the determination of total 25(OH)D was the Roche electrochemiluminescence binding essay (ECL) on Elecsys immunoassay analyzers, Roche Diagnostics GmbH, Mannheim, state D-68305, Germany.
Exclusion criteria were major illness (end stage kidney disease, cirrhosis, pancreatitis, cancer and cardiovascular disorders), recent surgery or diabetic keto acidosis in the last 6 months, use of glucocorticoids and secondary diabetes. The control population was derived from patients of the obesity and thyroid clinic with normal glucose and HbA1c levels (Glu<100mg/dl and HbA1c<5.7%) and without any systemic illness. Data were analyzed using the SPSS analytical software 22 (SPSS Inc-IBM Corporation, New York, United States). Data are presented as mean, standard deviation (SD) and descriptive statistics were used for the data analysis. Variables with p-values of less than 0.05 were considered significant.
Results
The study population was divided into 2 groups
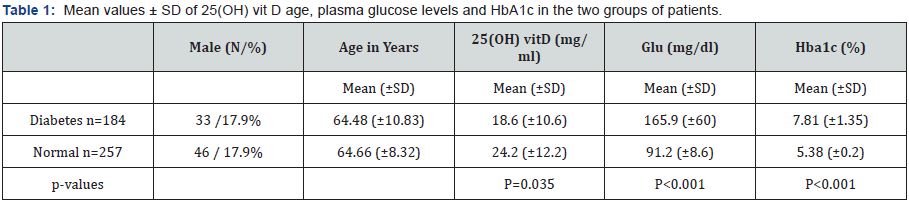
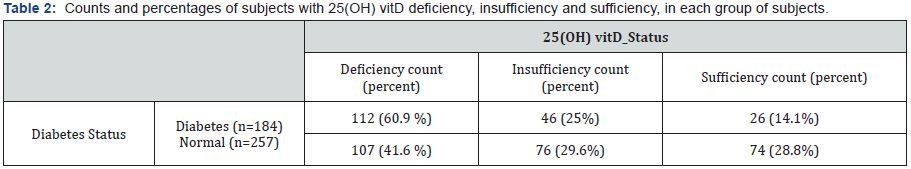
Patients with Diabetes Type 2 (n=184, 33 males) and subjects with normal fasting blood glucose and HbA1c (n=257, 46 males). The mean 25(OH) vitD values for males and females patients was 22.9±11.6 and 21.6 ±11.9mg/ml respectively, p=0.815. The mean 25(OH) vitD levels were higher in the control group when compared with patients with diabetes (24.20±12.2, vs 18.6±10.6mg/ml, p=0.035, respectively). There was no difference in the mean age of patients and sex distribution between the two groups. Table 1 shows the mean values±SD for 25(OH) vitD, age, plasma glucose levels and HbA1c for each group. 25(OH) vitD deficiencies were seen in 49.7% of the entire study population. The majority of diabetic patients studied had 25(OH) vitD serum levels below the recommended threshold of 30mg/ml and only 26 of 184 (14.1%) of patients with diabetes had 25(OH) vitD sufficiency, compared to 28.8% of subjects with normal glucose (p<0.001). Table 2 shows the counts and percentages of subjects with 25(OH) vitD deficiency, insufficiency and sufficiency, in each group of subjects. There is statistically significant difference in the prevalence of vitD deficiency and insufficiency between the three groups (p-value is <0.001).
Discussion
There is a relationship between 25(OH) vitD deficiency and risk of diabetes mellitus [20-23]. A high prevalence of 25(OH) vitD deficiencies has been reported in association with the metabolic syndrome [24]. Type 1 and Type 2 Diabetes Mellitus are multifactorial diseases and both genetic predisposition and environmental factors contribute to their development. 25(OH) vitD deficiency is linked to type 1 and type 2 diabetes development, and receptors for its activated form-1alpha, 25-dihydroxyvitamin D3-have been identified in beta cells and immune cells. 25(OH) vitD deficiencies impair insulin synthesis and secretion in humans and in animals, suggesting a role in the development of type 2 diabetes. Besides, epidemiological studies suggest a link between vitamin D deficiency in early life and the later onset of type 1 diabetes [25].
There is also compelling evidence that 1, 25(OH)2D regulates beta-cell function by different mechanisms, such as influencing insulin secretion by regulating intracellular levels of Ca2+, increasing beta-cell resistance to apoptosis, and possibly also increasing beta-cell replication. The capacity of vitamin D, more specifically 1, 25(OH)2D, to modulate immune responses is of particular interest for the prevention and the therapy of diabetes [26]. Diabetes itself can also result in lower circulating 25(OH) vitD concentrations [27]. Moreover, lower serum 25(OH) vitD levels are associated with prediabetes [28]. Vitamin D supplementation in prediabetes subjects has been shown todecrease fasting plasma glucose, 2-h plasma glucose and HbA1c levels [29].
Another important observation of our study is that the majority of the study population has 25(OH) vitD deficiency in general and respective of the underlying glycemic status. Greece is considered a country of high sunlight levels. Based on the important contribution of sunlight exposure to the production and maintenance of serum 25(OH) vitD levels, one might consider that 25(OH) vitD deficiency may be a problem limited to countries located at higher latitudes and not Greece (Athens, Greece: 37.9°C north of the equator). Nevertheless, we observed a high prevalence of 25(OH) vitD deficiency and insufficiency, especially among patients with diabetes.
The sample of our study represents Greek individuals and there are some limitations. The data are aggregated and do not permit analysis by body mass index, personal medical history, complete medication of each patient or other covariates, multivitamin use, skin pigmentation, clothing style, place, duration of outdoor stay of each patient. Finally, we only had one spot measurement for each participant.
However, our study has an important strength: We included a large sample of individuals and divided them in two separate groups, taking into account the underlying glycemic status. Prospective studies with a large number of patients including a representative Greek sample are required to study the link between vitamin D deficiency and other factors related to the diabetes mellitus. Since many individuals are vitamin D deficient and at high risk of diabetes, vitamin D supplementation may contribute to prevent diabetes and insulin resistance. Recent observational data reports a positive effect of vitamin D on preventing the onset of diabetes [18]. Nevertheless the possible benefits of vitamin D supplementation on glycemic control are still questioned. There is a need to conduct further studies to evaluate the impact of vitamin D deficiency in relation to many chronic diseases such as diabetes, and answer whether this link is causative or not.
Conclusion
This study examined the relation between 25(OH) vitD levels and glycemic status in Greek patients with diabetes and normal subjects. We found that patients with diabetes had a significantly higher prevalence of vitamin D deficiency compared to normal subjects. From a clinical standpoint, specific advice needs to be provided especially to people with diabetes. Vitamin D supplements on a regular basis over the year and adequate sun exposure could be also recommended in order to achieve sufficient levels of 25(OH) vitD.
References
- Hu FB (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34(6): 1249-1257.
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, et al. (2014) A systematic review of vitamin D status in populations worldwide. Br J Nutr 111(1): 23-45.
- Holick MF (2003) Evolution and function of vitamin D. Recent Results Cancer Res 164: 3-28.
- Tanzy ME, Camacho PM (2011) Effect of vitamin D therapy on bone turnover markers in postmenopausal women with osteoporosis and osteopenia. Endocr Pract 17(6): 873-879.
- Rizzoli R, Stevenson JC, Bauer JM, van Loon LJ, Walrand S, et al. (2014) The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 79(1): 122-132.
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, et al. (2006) Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354(7): 684-696.
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ (2014) The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 14(5): 342-357..
- Adams JS, Hewison M (2010) Update in vitamin D. J Clin Endocrinol Metab 95(2): 471-478.
- Cantorna MT, Mahon BD (2004) Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med Maywood 229(11): 1136-1142.
- Al Mheid I, Patel RS, Tangpricha V, Quyyumi AA (2013) Vitamin D and cardiovascular disease: is the evidence solid? Eur Heart J 34(48): 3691-3698.
- Mac Laughlin J, Holick MF (1985) Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 76(4): 1536-1538.
- Wortsman J, Matsuoka LY, Chen TC, Lu Z , Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72(3): 690-693.
- Gilchrest BA (2008) Sun exposure and vitamin D sufficiency. Am J Clin Nutr 88(2): 570S-577S.
- Papadakis G, Zambelis T, Villiotou V, Dogkas N, Pappas A (2015) Lower
Levels of Vitamin D Among Bangladeshi Immigrants with Diabetes in
Greece Compared to Indigenous Greek Patients with Diabetes. In Vivo
29(5): 541-545.
- Papadakis G, Keramidas I, Kakava K, Pappa T, Villiotou V, et al. (2015) Seasonal variation of serum vitamin D among Greek female patients with osteoporosis. In Vivo 29(3): 409-413.
- Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3): 266-281.
- Kampmann U, Mosekilde L, Juhl C, Moller N, Christensen B, et al. (2014) Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency - a double-blind, randomized, placebo-controlled trial. Metabolism 63(9): 1115-1124.
- Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, et al. (2013) Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a metaanalysis of prospective studies. Diabetes Care 36(5): 1422-1428.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7): 1911-1930.
- Pittas AG, Nelson J, Mitri J, Hillmann W, Garganta C, et al. (2012) Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care 35(3): 565-573.
- Mitri J, Dawson-Hughes B, Hu FB, Pittas AG (2011) Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 94: 486-494.
- Chagas CE, Borges MC, Martini LA, Rogero MM (2012) Focus on vitamin D, inflammation and type 2 diabetes. Nutrients 4(1): 52-67.
- Lim S, Kim MJ, Choi SH, Shin CS, Park KS, et al. (2013) Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. Am J Clin Nutr 97(3): 524-530.
- Kayaniyil S, Harris SB, Retnakaran R, Vieth R, Knight JA, et al. (2014) Prospective association of 25(OH)D with metabolic syndrome. Clin Endocrinol Oxf 80(4): 502-507.
- Mathieu C, Gysemans C, Giulietti A, Bouillon R(2005) Vitamin D and diabetes. Diabetologia 48(7): 1247-1257.
- Takiishi T, Gysemans C, Bouillon R, Mathieu C (2012) Vitamin D and diabetes. Rheum Dis Clin North Am 39(2): 179-206.
- Schneider LE, Schedl HP, McCain T, Haussler MR (1977) Experimental diabetes reduces circulating 1,25-dihydroxyvitamin D in the rat. Science 196(2497): 1452-1454.
- shankar A, Sabanayagam C, Kalidindi S (2011) Serum 25-hydroxyvitamin d levels and prediabetes among subjects free of diabetes. Diabetes Care 34(5): 1114-1119.
- Kuchay MS, Laway BA, Bashir MI, Wani AI, Misgar RA, et al. (2015) Effect of Vitamin D supplementation on glycemic parameters and progression of prediabetes to diabetes: A 1-year, open-label randomized study. Indian J Endocrinol Metab 19(3): 387-392.






























