Measurement Error in Exposure Doses and Cancer Risk Estimation
Masiuk S1, Kukush A2* and Shklyar S2
1Ukrainian Radiation Protection Institute, Ukraine
2Taras Shevchenko National University of Kyiv, Ukraine
Submission: December 29, 2017; Published: January 18, 2018
*Corresponding author: Alexander Kukush, Professor, The Faculty of Mechanics and Mathematics, Taras Shevchenko National University of Kyiv, Volodymyrska Str. 64, Kyiv 01601, Ukraine, Tel:+380-44-259-059; Email:alexander.kukush@gmail.com
How to cite this article: Masiuk S, Kukush A* and Shklyar S. Measurement Error in Exposure Doses and Cancer Risk Estimation. Biostat Biom Open Access J. 2018; 4(2): 555635. DOI: 10.19080/BBOAJ.2018.04.555635
Abstract
The data used in estimation of radiation exposure dose are not known exactly. We discuss different types of errors which are present in radio-epidemiological models we work with. We illustrate these types or errors in simplified models and share our experience on how the errors of different types affect the estimates of radiation risk parameters.
Keywords: Berkson error; The classical error; Measurement error; Radiation risk estimation; Attenuation effect
Abbreviations: ERR: Excess Relative Risk; EAR: Excess Absolute Risk; GSD: Geometric Standard Deviation
Opinion
Until recently the most common methods for estimation of radiation risks that associated with human exposure use the assumption of no uncertainty in exposure dose, i.e., it is assumed that we have a determined value for the individual dose of a subject. But now it is clear that such a statement is fundamentally wrong, since in practice there are no situations in which the dose estimated by any method would not have some uncertainty. The nature of the dose uncertainty can be very diverse. E.g., the measurement error can be systematic or random. Systematic under- or overestimation of true values of the exposure dose is known as bias. As a rule a systematic error is not considered as a component of the total error due to the following reason: if a source of such an error is known, then it would be eliminated. In turn a random error can be either shared or unshared. Assume that in a mathematical model used to dose reconstruction for a group of subjects (cohort), some parameters can be the same for subjects of some subgroup. Suppose also that these parameters have uncertainty due to a lack of knowledge of the true value. Then for the subjects of this subgroup, this lack of knowledge is a source of the shared error. In contrast, some parameters can vary stochastically among the subjects of the subgroup, and this is a source of unshared error. So, if parameters are shared, then errors in the parameters are shared as well, leading to a systematic error in dose estimates for the subjects of the subgroup [1].
In addition, the random error can be either classical or Berkson [2]. Let Dtr stand for the true value of exposure dose 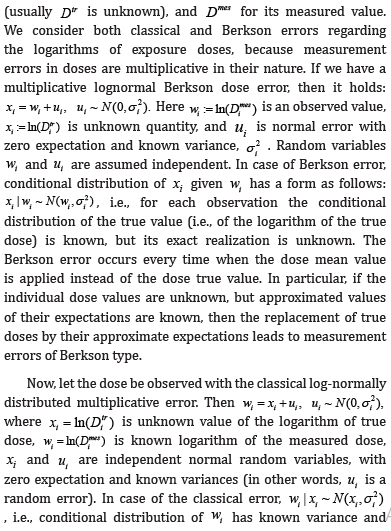 unknown expectation, the latter is regarded as the logarithm
of true dose. The classical error occurs when the measured
dose value (i.e., some computational and instrumental dose
realization including the error) is used instead of the true dose
value. E.g., instrumental measurements of radioactivity fluctuate
just because of the presence of the classical error.
unknown expectation, the latter is regarded as the logarithm
of true dose. The classical error occurs when the measured
dose value (i.e., some computational and instrumental dose
realization including the error) is used instead of the true dose
value. E.g., instrumental measurements of radioactivity fluctuate
just because of the presence of the classical error.
One of the consequences from the assumption of the absence of errors in exposure doses is the bias of risk estimates and distortion of the shape of the curve “dose – effect”. Notice that such distortions of the risk estimates can be caused not only by systematic errors in dose estimates which is obvious, but by random errors as well. Even though there have been many attempts to include dose errors in the risk analysis lately, the problem is not fully resolved until now. In many cases estimation of dose uncertainty can be expressed as a combination of the classical and Berkson types of error. However, at the moment there is no final conclusion on the impact of the classical, Berkson, or mixed error in dose estimates to the final result of risk analysis, usually being expressed in values of either excess relative risk (ERR) or excess absolute risk (EAR).
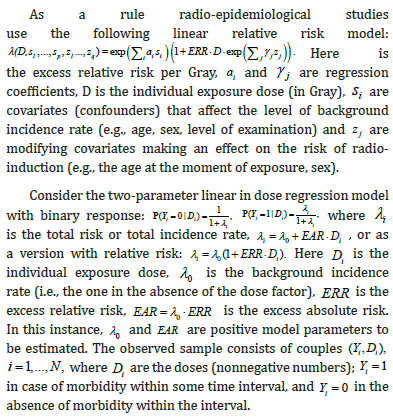
To check the influence of classical and Berkson errors in
exposure doses on the cancer risk estimation, a simulated
stochastic experiment was made. The simulation was performed
based on epidemiological studies of thyroid cancer morbidity in
Ukraine [3,4]. The absorbed doses of internal thyroid exposure
correspond to the published in [5] doses for a real subpopulation
of children and adolescents aged from 0 to 18 years (N=13204
persons in total) resided in settlements of Zhytomyr, Kyiv, and
Chernihiv oblasts of Ukraine, where direct measurements of
thyroid radioactivity were conducted in May–June 1986 [6]. In
simulation of the thyroid cancer incidence rate at fixed time
interval, the two-parameter logistic linear model of absolute
risk was used. The true model parameters were chosen being
close to the estimates obtained during epidemiological studies
of thyroid cancer in Ukraine [3], namely 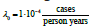 and
and
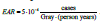 ⋅ Berkson and the classical errors in exposure
doses were simulated separately. It was assumed that both
Berkson and classical multiplicative errors in dose are
distributed by log-normal law. The error value was set so that
its geometric standard deviation GSD = exp(σi) varied from 1.5
to 5, for all i =1,..., N in the case of the classical error, as well as
in the case of Berkson error. In both simulation scenarios, 1,000
data sets were generated for each error value. To estimate the
regression parameters λ0
and EAR the naive estimation method
(i.e., the one that ignores the presence of errors in doses) was
used, Figure 1 & 2.
⋅ Berkson and the classical errors in exposure
doses were simulated separately. It was assumed that both
Berkson and classical multiplicative errors in dose are
distributed by log-normal law. The error value was set so that
its geometric standard deviation GSD = exp(σi) varied from 1.5
to 5, for all i =1,..., N in the case of the classical error, as well as
in the case of Berkson error. In both simulation scenarios, 1,000
data sets were generated for each error value. To estimate the
regression parameters λ0
and EAR the naive estimation method
(i.e., the one that ignores the presence of errors in doses) was
used, Figure 1 & 2.
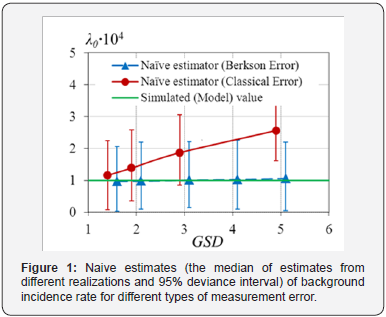
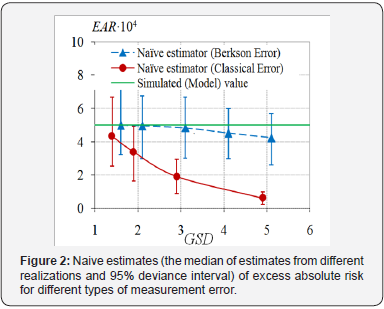
Figures 1 & 2 demonstrate the attenuation effect, i.e., underestimation of excess absolute risk and overestimation of background incidence rate. The effect is more contrast as the error variance is increasing. In the case of Berkson errors, the bias of naive estimates is not significant, compared with the case of the classical errors. Though for large enough Berkson errors the bias of radiation risk estimates will be significant as well. And the bias of ERR estimates will be larger than the one of EAR estimates.
Our experience tells the following:
o Neglecting of the presence of measurement errors in doses leads to underestimation of both ERR and EAR, if the classical errors are not vanishing.
o Under the absence of the classical errors, Berkson errors do not affect the estimates of regression parameters. Only for huge values of GSD of Berkson errors, the estimates of EAR and ERR are imprecise.
o The larger the variance of the classical error, the worse the estimates of EAR and ERR.
We infer that a close analysis of measurements process is important. Not only the level of measurement errors is important, but also it is crucial which part of the errors constitute the classical ones. The classical errors should be taken into account in construction of the estimates of regression parameters. Berkson errors typically can be neglected if they are not very large. For instance, in radio-epidemiological studies of ecological type, Berkson errors in exposure doses can be quite large, and in this case ignoring Berkson errors will cause significant bias of the EAR and especially ERR estimates.
Notice that standard statistical and epidemiological packages like EPICURE, SAS, SPSS, etc. cannot estimate the affect of measurement errors on the final result. Therefore, in the presence of such errors, one should use specific estimation methods [2] for evaluation of correct estimates of radiation risks. A choice of estimation method depends on the type of error (shared or unshared, multiplicative or additive, the classical or Berkson or their mixture) and on the shape of its distribution. Our experience suggests that a particular method works well for one type of error, but for error of another type, the method can perform even worse than the naive estimation. Thus, in concrete situations, especially under a mixture of errors of different types, it is necessary to make preliminary simulation studies in order to compare the efficiency of estimation methods.
References
- Simon SL, Hoffman FO, Hofer E (2015) The Two-Dimensional Monte Carlo: A New Methodologic Paradigm for Dose Reconstruction for Epidemiological Studies. Radiation Res 183(1): 27-41.
- Masiuk S, Kukush A, Shklyar S, Chepurny M, Likhtarov I (2017) Radiation risk estimation based on measurement error models. De Gruyter, Berlin.
- Tronko MD, Howe GR, Bogdanova TI, Bouville AC, Epstein OV, et al. (2006) A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: Thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst 98(13): 897-903.
- Bogdanova TI, Zurnadzhy LY, Nikiforov YE, Leeman-Neill RJ, Tronko MD, et al. (2015) Histopathological features of papillary thyroid carcinomas detected during four screening examinations of a Ukrainian-American cohort. Br J Cancer 113(11): 1556-1564.
- Likhtarov I, Kovgan L, Masiuk S, Talerko M, Chepurny M, et al. (2014) Thyroid cancer study among Ukrainian children exposed to radiation after the Chornobyl accident: Improved estimates of the thyroid doses to the cohort members. Health Phys 106(3): 370-396.
- Likhtarov IA, Kovgan LM, Chepurny MI, Masiuk SV (2015) Interpretation of results of radioiodine measurements in thyroid for residents of Ukraine (1986). Probl Radiac Med Radiobiol 20: 185-203.






























