Chronic Facial Pain in Female Patients with Rheumatoid Arthritis of The Temporomandibular Joint: A Clinical and Radiographic Study
Kamal Effat G* and Abeer Berty
1Department of Otolaryngology, El-Sahel Teaching Hospital, Cairo, Egypt
2Department of Rheumatology, St. Mark Center, Cairo, Egypt
Submission: July 07, 2024; Published: July 15, 2024
*Corresponding author: Kamal Effat G, Department of Otolaryngology, El-Sahel Teaching Hospital, Ahmed EL-Dardiri Street, Heliopolis, Cairo, Egypt
How to cite this article: Kamal Effat G, Abeer B. Chronic Facial Pain in Female Patients with Rheumatoid Arthritis of The Temporomandibular Joint: A Clinical and Radiographic Study. Adv Dent & Oral Health. 2024; 17(4): 555969. DOI: 10.19080/ADOH.2024.17.555969
Abstract
Objective: The incidence and causes of chronic facial pain in patients with temporomandibular disorder have previously been characterized. The current study aims to reveal the causes of chronic facial pain in patients with clinical involvement of the temporomandibular joint (TMJ) in female patients with rheumatoid arthritis (RA).
Methods: This prospective study involved 82 female RA patients and TMJ involvement who complained of chronic facial pain. A control group consisting of 61 female patients with chronic facial pain who did not have evidence of RA or TMJ involvement was recruited.
Results: Evidence of chronic rhinosinusitis (CRS) on computed tomography (CT) scan of the sinuses was documented in 31.8% of RA patients, versus 47.5% of the control group, (P=0.051). In the patients where CT scans of the sinuses were clear, midfacial segment pain (MFSP) was clinically diagnosed in 40.2% of the RA patients, versus 27.9% of the control group patients, (P=0.12).
Conclusion: The incidence of CRS in RA patients is in accordance with previous literature. The high incidence of MFSP in RA patients suggests central sensitization as an important pathophysiological mechanism in facial pain associated with TMJ involvement by RA.
Keywords: Rheumatoid arthritis- Facial pain; Temporomandibular joint; Temporomandibular disorder; Tension-Type Headache; Chronic Rhinosinusitis
Abbreviations: TMJ: Temporomandibular Joint; RA: Rheumatoid Arthritis; CRS: Chronic Rhinosinusitis; CT: Computed Tomography; MFSP: Midfacial Segment Pain; TMD: Temporomandibular Disorder; TTH: Tension-Type Headache; CNS: Central Nervous System; POMP: Persistent Orofacial Muscle Pain; DC/TMD: Diagnostic Criteria for Temporomandibular Disorders
Introduction
Chronic facial painful conditions are a multi-faceted problem associated with sensitization of peripheral nociceptors, as well as a central sensitization process [1]. The trigeminal subnucleus caudalis in the brain stem and upper cervical spinal cord is regarded as being crucial in the development of the central sensitization in chronic craniocervical pain [2]. In addition to its role in integrating nerve impulses from trigeminal and upper cervical nociceptive afferents, the subnucleus caudalis receives inputs from higher brain centers, especially those concerned with emotions (the corticolimbic circuitry). This may lead to the intensification of pain in patients with psychological distress and lack of sleep [3,4]. The convergent afferent nociceptive nerve fibers onto the subnucleus caudalis explain different chronic pain presentations, such as referred, heterotopic, or radiating pain patterns in the head and neck [5,6]. Indeed, chronic painful temporomandibular disorder (TMD), headache, facial pain, eye pain, otalgia, and neck pain are frequently comorbid conditions [7- 12]. Data from longitudinal studies reveal that there are major risk factors for the variable constellation of chronic pain conditions in the head and neck. These are psychological factors, physical and emotional trauma, and genetic factors that contribute to neurotransmission and inflammation [13]. It should be noted that trigeminal nerve input represents more than 40% of the sensory cortex in the brain, making cranial and facial pain a special type of pain [14].
Chronic facial pain poses a diagnostic and therapeutic challenge [15]. Sino-nasal causes of facial pain, such as chronic rhinosinusitis (CRS) and contact point headache, are readily diagnosed by objective means, including nasal endoscopy and computed tomography (CT) [16,17]. Patients with chronic facial pain, being referred as (sinus headache), are increasingly being diagnosed by neurologists as facial migraine [18,19]. An important issue in facial pain, characterized by N.S. Jones, is midfacial segment pain (MFSP) [20]. MFSP has a similar pathophysiology as tension-type headache (TTH), and readily responds to low-dose amitriptyline [21]. Notably, MFSP can be a referred pain, especially from painful temporomandibular disorder (TMD) [9,12]. It is typically diagnosed when nasal endoscopy and CT of the sinuses are normal [22]. Contributing to the diagnostic challenge, MFSP may be associated with nasal congestion, rhinorrhea, and lacrimation due to activation of the trigemino-autonomic reflex, thereby clinically mimicking CRS [15]. Recently, an international panel of workers suggested the novel term: persistent orofacial muscle pain (POMP) [23]. They emphasized that the facial muscle tension is mediated by aberrant pathways in the central nervous system (CNS).
Rheumatoid arthritis (RA) is a systemic and chronic disease characterized by persistent inflammation of synovial joints. It represents the most frequent autoimmune human disease, affecting about 1% of the adult population [24]. It is more common in females than in males. Involvement of the temporomandibular joint (TMJ) in RA has been studied from clinical, biochemical, arthroscopic, histopathologic, and radiographic perspectives [25- 29].The incidence of clinical involvement of the TMJ in patients with RA is approximately 50%, although up to 80% of the patients have radiographic evidence of TMJ abnormalities [24]. In contrast to osteoarthrosis of the TMJ, which is classified as a low-grade inflammatory condition, RA of the TMJ is considered a high-grade inflammatory joint disease [30]. In RA of the TMJ, the characteristic synovial pannus may cause progressive destruction of the articular cartilage, disc, and subchondral bone. This may ultimately result in the formation of fibrous adhesions, fibrous or bony ankyloses, and condylar resorption, which may manifest clinically as trismus and/or anterior open bite, in advanced stages [31,32]. These changes may develop within a relatively short period of time, compared to osteoarthrosis of the TMJ [33]. Pathologically, RA of the TMJ may be considered as a more severe form of arthrogenous TMD [34,35]. Previous studies have recently investigated the issue of facial pain in patients with TMD. It has been reported that the incidence of facial pain/eye pain in nasal endoscopy- negative and sinus CT- negative patients with TMD is 57%. Moreover, 36.3% of these TMD patients with normal nasal endoscopy and CT had MFSP as a referred, radiating, or heterotopic facial pain from painful jaw structures [9,12]. As far as the authors of the current study are aware, the incidence and characteristics of facial pain/eye pain in patients with clinical involvement of the TMJ in RA patients had not been previously reported. The current study addresses this issue; and, furthermore, documents the status of the sino-nasal unit in the patients and control subjects by CT, which is safe, screening tool for objectively assessing the status of the paranasal sinuses in patients with facial pain [36,37].
Materials and Methods
The current study was a prospective clinical and radiographic study on chronic facial pain. The study was performed by a consultant rheumatologist and a consultant otolaryngologist; both having expertise in the management of TMD, as well as having international publications on this topic. The study period was 9 months, starting on the 1st of June, 2023. Ethical approval for the study was obtained from the General Organization of Teaching Hospitals and Institutes in Cairo (Approval number: 87/2023). Informed consent was obtained from patients of the study group and the control group to participate in the study and to undergo CT-scanning of the paranasal sinuses. A standard clinical history and examination findings sheet was prospectively filled by the two consultants, and the results of the CT-scan were included in each sheet. The study group comprised adult female RA patients who complained of chronic facial pain (of more than 6 months); and on subsequent examination were found to have objective signs of TMJ affection, according to the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD). Objective evidence of TMJ affection required, at least, eliciting joint noises on mandibular movements. The minimum tests performed for diagnosis of RA were rheumatoid factor, anti-cyclic citrullinated peptide antibodies, C-reactive protein, and erythrocyte sedimentation rate. The control group comprised adult female subjects, presenting to the otolaryngology clinic, and complaining of chronic facial pain, who did not have symptoms or signs of RA, and who did not present objective signs of TMJ affection. Both individuals from the study and control groups were from similar socioeconomic conditions. Patients with acute facial pain and male subjects were not included in the study.
The age of the subjects was recorded. In the RA group, the duration of the disease was noted. Any associated rheumatological disorders, especially secondary Sjögren syndrome, was assessed. Notably, all the patients with RA in the current study had their TMD symptoms developing after the diagnosis of RA by a variable period of time. For both cohorts in the study, the characteristics and laterality of the facial pain were noted. From the description of pain, a presumptive categorization of the facial pain was made. A throbbing, unilateral facial pain, associated with migraine headache was assigned as facial migraine. Paroxysms of severe unilateral electric shock-like pain were diagnosed as trigeminal neuralgia. Frequently, a symmetric nasal, perinasal, periorbital and cheek pain of a pressing quality was encountered. In the absence of evidence of chronic rhinosinusitis (CRS), it was assigned as midfacial segment pain (MFSP), especially if there was prompt response to low-dose amitriptyline. Next, the presence of headache and its location were noted. The presence of autonomic symptoms, associated with the facial pain (nasal congestion, clear rhinorrhea, and lacrimation) were asked about. The loss of sense of smell, nasal obstruction and presence of mucopurulent discharge suggested the diagnosis of CRS. Clinical symptoms pertaining to TMD (depressive symptoms, bruxism, disturbed sleep quality, and a previous history of open-lock) were asked about.
Examination included nasal endoscopy, to reveal any mucopurulent discharge, nasal polyps, or a septal spur impacting the lateral nasal wall. Examination of the TMJ region firstly included manual palpation of the TMJ for elicited tenderness (arthralgia). Audible and/or palpable joint sounds (click or crepitus or both) were noted on movement of the mandible. The presence of anterior open bite was assessed, which could be due to condylar resorption. Trismus was defined as an interincisal distance of less than 40 mm on maximal mouth opening, which could be due to TMJ ankylosis. Next, manual palpation of the temporalis and masseter muscles was done to examine for elicited tenderness (myalgia) and any referral of pain. The results of CT-scan of the sinuses were documented. Marked mucosal thickening or opacification of the sinuses, without bone erosion, indicated CRS. The presence of heterogeneous opacities indicated presence of fungal elements in the sinuses. Documenting the presence of a septal spur impacting the lateral nasal wall, especially in the context of a concha bullosa, could be a cause for contact point headache. The presence of nasal polyps was assessed on CT. Finally, a formal diagnosis of the cause of the facial pain was made, and compared between both groups in the study, based on clinical and radiographic data.
Statisticalanalysis
Results were expressed as mean ± standard deviation or number percent (n%). Comparison between categorical data [n(%)] was performed using Chi square test or Fisher exact test whenever it was appropriate. Comparison between mean values of age in the two groups was performed using unpaired t test. Statistical analysis was performed using SPSS computer program (version 19 windows). P value ≤ 0.05 was considered significant.
Results
The current prospective study involved 82 female patients with chronic facial pain who had rheumatoid arthritis (RA) and objective evidence of temporomandibular joint (TMJ) affection, as well as a control group consisting of 61 female patients with chronic facial pain, but without evidence of RA or TMJ affection. Both cohorts were from a similar socioeconomic background. The RA patients were recruited from a rheumatology clinic, while the control group patients were recruited from an otolaryngology clinic. The otolaryngologist in the study reviewed both cohorts of patients, and performed the necessary nasal endoscopy procedures.

Data are expressed as number (%).
RA= rheumatoid arthritis.
p> 0.05= not significant;*p≤ 0.05= significant.
The mean age of the RA patients was 49.59 years (range: 30-76 years), while the mean age of the patients in the control group was 33.97 years (range: 18-70 years); (P=0.001). In the RA group, the duration of systemic arthritis affection was 10.39 years (range: 1-45 years). Symptoms of temporomandibular disorder (TMD) developed after the diagnosis of RA by a variable period of time in all RA patients. The inclusion criteria for the RA patients required, at least, eliciting TMJ sounds (click or crepitus or both) on mandibular movement. Notably, TMJ tenderness (arthralgia) was also detected in all RA patients. The main clinical findings pertinent to TMD in both cohorts of patients are shown in Table 1. Table 2 illustrates the pertinent symptoms reported by the patients of the two groups. Notably, symptoms of dry eyes and dry mouth were reported by 41.5% of RA patients, versus 4.7% of control subjects (P=0.001), suggesting a high incidence of secondary Sjögren syndrome in the RA patients.
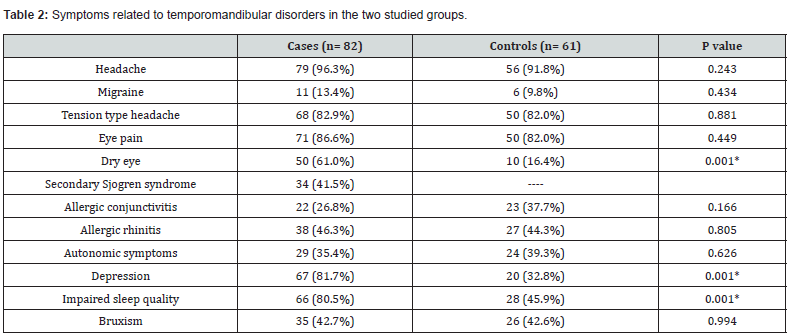
Data are expressed as number (%).
RA= rheumatoid arthritis.
*p≤ 0.05= significant.

Data are expressed as number (%).
RA= rheumatoid arthritis.
p> 0.05= not significant;*p≤ 0.05= significant.
Regarding CT-scan findings, 68.3% of RA patients had clear sinuses, while 45.97% of the control group patients had clear CT-scans (P=0.007). Of the patients with clear sinuses on CT-scanning, midfacial segment pain (MFSP) was diagnosed in 40.2% of RA patients, and in 27.9% of the control group patients (P=0.12). In the groups which showed sinus pathology on CT-scans, chronic rhinosinusitis (CRS) was documented in 31.8% of RA patients and in 47.5% of the control group patients (P=0.051). Table 3 reveals the nature of the facial pain in both groups, according to the CT-scan findings.
Discussion
Facial pain in patients with temporomandibular joint disorders had first been described by James Costen in 1934 [38]. The patients in his series also complained of nasal congestion and rhinorrhea, mimicking chronic sinusitis, although the sinus radiographs were normal. Costen attributed these symptoms to irritation of the chorda tympani and auriculotemporal nerve from a displaced mandibular condyle. More recently, a prospective study involving 132 patients with both TMD and facial pain utilized CT-scanning of the sinuses for investigation [9]. More than half of the patients in that report had clear CT-scans of the paranasal sinuses. After excluding other causes of facial pain in that cohort (especially facial migraine and contact point headache), 36.3% of the patients were diagnosed as MFSP as heterotopic, radiating, or referred pain from painful jaw structures [9]. MFSP is a recently described term by N.S. Jones, that is analogous to tension-type headache, with a similar pathophysiology [20]. Although it had been postulated by the author, that it could be a referred pain from other cranial painful sites, TMD was not specifically addressed in the original reports [20-22]. MFSP typically occurs as a bilateral, symmetric, facial pain of a pressing quality, involving the nose, periorbital regions, and the cheeks. Nasal endoscopy and CT-scans in these patients show no features of chronic rhinosinusitis, such as mucopurulent discharge or opacification of the sinuses, respectively. It is postulated that this pattern of pain in TMD is due to convergence of various nociceptive nerve fibers of the trigeminal nerve onto the subnucleus caudalis [6]. Furthermore, in cases with more severe pain, patients may experience nasal congestion, rhinorrhea, and lacrimation due to activation of the trigemino-autonomic reflex, thereby erroneously being diagnosed as CRS [39].
The current study involved 82 patients with rheumatoid arthritis (RA) and evidence of TMJ affection, who complained of chronic facial pain. The control group consisted of 61 patients presenting with chronic facial pain, but without evidence of RA or TMJ affection. In the RA patients, TMJ complaints occurred after the diagnosis of RA by a variable period of time. Objective evidence of TMJ affection in the RA group was found in all patients in the form of palpable and/or audible joint sounds (click or crepitus or both); and in some cases, trismus and anterior open bite. Arthralgia of the TMJ, classically indicating inflammation in the joint [40], was noted in 100% of the RA patients, versus 26.2% of control subjects (P=0.001). In the RA patients, CT-scan of the sinuses was clear in 68.3%, indicating absence of inflammation in the sinuses. In the control group, CT-scan of the sinuses was clear in 45.9% of the subjects (P=0.007). In both groups of patients with clear sinuses on CT-scanning, facial migraine, contact point headache, and trigeminal neuralgia were diagnosed based on clinical criteria. Facial migraine was diagnosed by the unilateral throbbing quality of the facial pain associated with migraine headache [41]. Contact point headache was diagnosed when nasal endoscopy revealed impaction of a septal spur onto the lateral nasal wall, that was confirmed by reviewing the CT [17]. Trigeminal neuralgia was diagnosed by the patient reporting paroxysms of severe, electric shock-like facial pains [42]. Facial migraine, contact point headache, and trigeminal neuralgia were encountered in 13.4%, 7.3 %, and 7.3% of the RA patients, respectively. In the control group, the diagnosis of the facial migraine, contact point headache, and trigeminal neuralgia were encountered in 9.8%, 6.6%, and 1.6% of the subjects, (P=.513),(P=.860),(P=.120), respectively. MFSP was diagnosed, based on the character of pain, normal nasal endoscopy, clear sinus CT scan, and absence of any other identifiable cause for the facial pain [20-22].
Such a type of facial pain presents as a symmetric pressing pain, associated frequently with tension-type headache, and might be referred from a regional cranial source of pain, such as the TMJ [9, 12]. Many cases with MFSP have previously been erroneously diagnosed as (atypical facial pain) or (persistent idiopathic facial pain) [43]. MFSP was diagnosed in 40.2% of RA patients, versus 27.9% of control subjects (P=0.12). The diagnosis of MFSP was confirmed by the prompt response to low-dose amitriptyline [20- 22]. The results of the current study confirms that a pathological, painful TMJ could indeed be an important cause of MFSP. Therefore, patients presenting with MFSP, should have the stomatognathic system clinically assessed for signs of TMD [9,12]. Recent studies suggest that the major culprits of MFSP are psychological stress and impaired sleep pattern [44,45]. This is due to disturbances in central serotonin neurotransmission, which augment pain pathways [46]. Both depression and lack of sleep are highly prevalent in RA patients [47,48]. In the current study, reports of depression and impaired sleep pattern were found in 81.7% and 80.5% of RA patients and in 32.8% and 45.9% of control subjects, (P=0.001),(P=0.001), respectively.
Chronic rhinosinusitis (CRS) is a common inflammatory disorder, with predisposing factors including allergens, bacterial biofilms, and inhaled irritants [49]. Facial pressure and facial pain are commonly associated with CRS [50]. The gold standard with respect to diagnosis of CRS is CT-scanning of the sinuses, because a negative nasal endoscopy does not rule out the diagnosis [51]. CT-scans showing marked opacities in the sinuses are correlated with greater symptom severity and a worse quality of life in CRS patients [52]. Additionally, CT is necessary if surgery for CRS is being contemplated [52]. Recently, the issue of CRS in patients with RA had gained wide interest. Epidemiological studies suggest that the inflammatory focus in CRS might act as a trigger for the development of RA by generating specific RA antibodies, such as anti-cyclic citrullinated peptide antibodies [53-55]. On the other hand, RA is significantly associated with the development of infectious disorders, including CRS, due to altered innate and adaptive immunity in the disease, or as a consequence of medications used for RA, such as steroids or biological agents [56,57]. A previous study quoted the incidence of CRS in RA patients as 29%, two-fold greater than in the general population [58]. It had been suggested that screening for CRS in RA patients could prevent or ameliorate lower airway disease; a frequent extra-articular morbidity in RA patients [56,57]. In the current study, evidence of radiologically confirmed CRS was found in 31.8% of RA patients, whereas in control subjects, in the otolaryngology clinic, CRS was diagnosed in 47.5% of patients (P=0.05). The incidence of CRS in RA patients, documented by CT-scanning, is comparable with that in previous studies [56-58].
Chronic facial pain and chronic headache are strongly correlated [59]. Chronic headache is one of the diagnostic categories of TMD, according to the DC/TMD [60]. In a recent prospective study, the incidence of chronic headache in patients with temporomandibular joint disorder was 88%, of which 13% were migraines and the remainder were analogous to tension-type headache (TTH) [61]. In the same study, the incidence of chronic facial pain was 52% [61]. In the current study, on patients with chronic facial pain associated with RA and TMJ affliction, chronic headache was reported by 96.3% of the patients, while the incidence of chronic headache in control subjects was 91.8% (P=.243). The pathogenesis of TTH in temporomandibular joint disorder had been attributed to entrapment of sensory nerve fibers of the trigeminal and upper cervical nerves by over contracting pericranial muscles [62-65]. Named implicated nerves are the supraorbital and supratrochlear nerves in fronto-orbital headache, auriculotemporal and zygomaticotemporal nerves in temple headache, and greater and lesser occipital nerves in occipital headache [63-65]. It is postulated that the pericranial muscle tension in TMD is due to central sensitization, with increased action potential barrages to the muscles [66,67]. On the other hand, the throbbing pain of migraine is attributed to activations of mechanoreceptors, in the context of dural inflammation [68]. In the current study on RA patients, the incidence of TTH was 82.9%, while the incidence of migraine headache was 13.4%. In the control subjects, the incidence of TTH was 82.6%, and the incidence of migraine was 28% (P=.881) (P=.434), respectively.
In the current study, eye pain was noted in 86.6% of the RA patients with TMJ involvement, and in 82% of the control subjects (P=0.449). The main factors responsible for eye pain in the study were MFSP, dry eye disease, allergic conjunctivitis, facial migraine, and CRS. A previous recent study revealed that 73% of patients with arthrogenous TMD had eye pain as part of their symptoms [12]. Of these, 22% of the patients were diagnosed by ophthalmologists as having dry eye disease [12]. The current study revealed that 61% of the RA patients had a diagnosis of dry eye disease. The incidence of dry eye disease in RA patients is roughly double that in the general population and it is multifactorial in its pathogenesis [69]. Dry eye disease causes a burning and stinging pain in the eye [70]. When combined with mucosal dryness (such as dry mouth), it could be labeled as Sjögren syndrome, although biopsy of the mucosal glands would be confirmatory. The incidence of secondary Sjögren syndrome in RA patients is as high as 53% [71]. In the current study, the combination of dry eyes and mouth, in RA patients was 41.5%, while in the control subjects it was 4.7% (P=0.001). Allergic conjunctivitis was reported in 26.8% of RA patients, versus 37.7% of the control subjects (P=0.166).
The major limitation of the current study was that it involved patients with quite heterogeneous pathologies, both in the study and control groups. However, a common putative unifying factor in all the patients with chronic facial pain is a central nervous system sensitization of nociceptive pathways involving the trigeminal system [1-4]. Notably, MFSP was a common feature in both the patients and study groups, which could be due to the central sensitization process. A further limitation of the study was that it did not include control subjects who did not complain of chronic facial pain. In the current prospective study, the mean age of the study group was higher than that of the control group. However, the CT-scans of the paranasal sinuses showed pathology in the control group more than in the RA group.
Conclusion
In the current study, the most frequent cause of chronic facial pain in patients with RA and TMJ involvement was MFSP, followed by CRS. MFSP probably reflects a central sensitization process at the level of subnucleus caudalis, where referred pain to facial structures develops due to convergence of trigeminal nociceptive afferents. The incidence of CRS, documented by CT-scanning agrees with previous reports on RA patients. In the control subjects, the most frequent cause of chronic facial pain was CRS, followed by MFSP. MFSP is a frequent cause of chronic facial pain that is associated with a dysfunction in central serotonin neurotransmission.
References
- Pak DJ, Yong RJ, Kaye AD, Richard DU (2018) Chronification of pain: mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep 22(2): 9.
- Sagripanti M, Viti C (2018) Primary headaches in patients with temporomandibular disorders: diagnosis and treatment of central sensitization pain. Cranio 36(6): 381-389.
- Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tétreault P, et al. (2016) The emotional brain as a predictor and amplifier of chronic pain. J Dent Res 95(6): 605-612.
- Cole H A, Carlson CR (2018) Mind-body considerations in orofacial pain. Dent Clin N Am 62(4): 683-694.
- De Rossi SS (2013) Orofacial pain: a primer. Dent Clin N Am 57(3): 389-392.
- Sessle BJ (2011) Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol 97(1): 179-206.
- Effat KG (2016) Otological symptoms and audiometric findings in patients with temporomandibular disorders: Costen’s syndrome revisited. J LaryngolOtol 130(12): 1137-1141.
- Effat K G, Berty A (2019) A comparative clinical study of temporomandibular disorder patients in the otolaryngology clinic versus a rheumatology clinic. Cranio 37(5): 329-334.
- Effat KG (2021) A comparative clinical and radiographic study on patients with chronic facial pain with and without temporomandibular disorder presenting to an otolaryngology clinic. Cranio 39(1): 5-11.
- Effat KG (2021) A comparative clinical study of arthrogenous versus myogenous temporomandibular disorder in patients presenting with Costen’s syndrome. Cranio 39(5): 433-439.
- Effat KG (2021) A minireview of the anatomical and pathological factors pertaining to Costen’s syndrome symptoms. Cranio 42(4): 445-449.
- Effat KG (2020) Eye pain in patients with arthrogenous versus myogenous temporomandibular disorder presenting to an otolaryngology clinic. J ENT Care Otolaryngol Res 2(1): 1002.
- Mcfate T, Scher AJ (2009) Chronic pain disorders and headache chronification. Curr Pain Headache Rep 13(4): 308-313.
- Renton T (2017) Chronic orofacial pain. Oral Dis 23(5): 566-571.
- De Corso E, Kar M, Cantone E, Lucidi D, Settimi S, et al. (2018) Facial pain: sinus or not? Acta Otorhinolaryngol Ital 38(6): 485-496.
- De Conde A S, Mace J C, Ashby S, Timothy LS, Richard RO (2015) Characterization of facial pain associated with chronic rhinosinusitis using validated pain evaluation instruments. Int Forum Allergy Rhinol 5(8): 682-690.
- Maniaci A, Merlino F, Cocuzza S, Giannicola I, Claudio V, et al. (2021) Endoscopic surgical treatment for rhinogenic contact point headache: systematic review and meta-analysis. Eur Arch Otorhinolaryngol 278(6): 1743-1753.
- Straburzyński M, Gryglas-Dworok A, Nowaczewska M, et al. (2021) Etiology of ‘sinus headache’- moving the focus from rhinology to neurology. A systematic review. Brain Sci 11(1): 79.
- Lukic N, Ettlin D (2020) Midfacial pain. Oral Surg 13(4): 415-421.
- Jones NS (2004) Midfacial segment pain: Implications for rhinitis and sinusitis. Curr Allergy Asthma Rep 4(3): 187-192.
- Agires A M, Jones N S, Muscat R (2014) Prospective three-year follow up of a cohort study of 240 patients with chronic facial pain. J LaryngolOtol 128(6): 518-526.
- West B, Jones NS (2001) Endoscopy-negative, computed tomography- negative facial pain in a nasal clinic. Laryngoscope 111: 581-586.
- Bendeil R, Svensson P, Heir G M, et al. (2011) Persistent orofacial muscle pain. Oral Dis 17(suppl 1): 23-41.
- Klasser GD, Balasubramaniam R, Epstein J (2007) Topical review-connective tissue diseases: orofacial manifestations including pain. J Orofac Pain 21(3): 171-184.
- Sidebottom AJ (2013) How do I manage a restricted mouth opening secondary to problems with temporomandibular joint? Br J Oral Maxillofac Surg 51(6): 469-472.
- Kroese J M, Kopp S, Lobbezoo F, et al. (2020) TMJ pain and crepitus occur early whereas dysfunction develops over time in rheumatoid arthritis. J Oral Facial Pain Headache 34(4): 398-405.
- Detamore M S, Athanasiou KA (2003) Structure and function of the temporomandibular joint disc: implications for tissue engineering. J Oral Maxillofac Surg 61(4): 494-506.
- Gynther G W, Holmlund A B, Reinholt F P, et al. (1997) Temporomandibular joint involvement in generalized osteoarthritis and rheumatoid arthritis: a clinical, arthroscopic, histologic, and immunohistochemical study. Int J Oral Maxillofac Surg 26(1): 10-16.
- Witulski S, Vogl T J, Rehart S, Peter O (2014) Evaluation of the TMJ by means of clinical TMD examination and MRI diagnostics in patients with rheumatoid arthritis. Biomed Res Int 2014: 328560.
- Tanaka E, Detamore MS, Mercuri LG (2008) Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res 87(4): 296-307.
- Israel H A, Langevin C-J, Singer MD, David AB (2006) The relationship between temporomandibular joint synovitis and adhesions: pathogenic mechanisms and clinical implications for surgical management. J Oral Maxillofac Surg 64(7): 1066-1074.
- Larheim T A, Storhaug K, Tveito L (1983) Temporomandibular joint involvement and dental occlusion in a group of adults with rheumatoid arthritis. Acta Odontol Scand 41(5): 301-309.
- Broussard JS (2005) Derangement, osteoarthritis, and rheumatoid arthritis of the temporomandibular joint: implications, diagnosis, and management. Dent Clin N Am 49(2): 327-342.
- Holmlund A B, Gynther G, Reinholt FP (1992) Rheumatoid arthritis and disk derangement of the temporomandibular joint. A Comparative arthroscopic study. Oral Surg Oral Med Oral Pathol 73(3): 273-277.
- Bjørnland T, Refsum SB (1994) Histopathologic changes of the temporomandibular joint disk in patients with chronic arthritic disease. A comparison with internal derangement. Oral Surg Oral Med Oral Pathol 77(6): 572-578.
- Fraczek M, Guzinski M, Morawska-Kochman M, Kamil HN, Tomasz K (2016) Nasal endoscopy: an adjunct to patient selection for preoperative low-dose CT examination in chronic rhinosinusitis. Dentomaxillofac Radiol 45(8): 20160173.
- Sedaghat AR (2017) Chronic rhinosinusitis. Am Fam Physician 96(8): 500-506.
- Costen JB (1997) A syndrome of ear and sinus symptoms dependent upon disturbed function of the temporomandibular joint. Ann OtolRhinolLaryngol 106(10 pt1): 805-819.
- Möller M, May A (2019) The unique role of the trigeminal autonomic reflex and its modulation in primary headache disorders. Curr Opin Neurol 32(3): 438-442.
- Chang H, Israel H (2005) Analysis of inflammatory mediators in temporomandibular joint synovial fluid lavage samples of symptomatic patients and asymptomatic controls. J Oral Maxillofac Surg 63(6): 761-765.
- Sharav Y, Haviv Y, Benoliel R (2023) Orofacial migraine or neurovascular orofacial pain from pathogenesis to treatment. Int J Mol Sci 24(3): 2456.
- Cruccu G, Finnerup NB, Jensen TS, Joachim S, Marc S, et al. (2016) Trigeminal neuralgia: New classification and diagnostic grading for practice and research. Neurology 87(2): 220-228.
- Benoliel R, Gaul C (2017) Persistent idiopathic facial pain. Cephalalgia 37(7): 680-691.
- Joo Y-H, Cho H-J, Jeon Y-J, Kim RB, Kim SW (2023) Chronic rhinitis and stress: the possible culprits of midfacial segment pain. Rhinology 61(3): 214-220.
- Leong SC, Tsang HK, Banhegyi G (2015) The utility of the sino-nasal outcome test (SNOT) as a treatment outcome measure in nonsinogenic facial pain syndrome. Ann Otol Rhinol Laryngol 124(4): 317-321.
- Agius AM, Jones NS, Muscat R (2013) A randomized controlled trial comparing the efficacy of low-dose amitriptyline, amitriptyline with pendolol and surrogate placebo in the treatment of chronic tension-type facial pain. Rhinology 51(2): 143-153.
- Luyster F S, Chasens E R, Wasko MCM, Jacqueline DJ (2011) Sleep quality and functional disability in patients with rheumatoid arthritis. J Clin Sleep Med 7(1): 49-55.
- Hughes M, Chalk A, Sharma P, Sandeep P, James G (2021) A cross-sectional study of sleep and depression in a rheumatoid arthritis population. Clin Rheumatol 40(4): 1299-1305.
- Lam K, Schleimer R, Kern RC (2015) The etiology and pathogenesis of chronic rhinosinusitis: a review of current hypothesis. Curr Allergy Asthma Rep 15(7): 41.
- De Conde AS, Mace JC, Ashby S, Timothy LS, Richard RO, et al. (2015) Characterization of facial pain associated with chronic rhinosinusitis using validated pain evaluation instruments. Int Forum Allergy Rhinol 5(8): 682-690.
- Cohen-Kerem R, Marshak T, Uri N, Maayan G, Ori H, et al. (2021) Is nasal endoscopy of diagnostic value in chronic rhinosinusitis without nasal polyps? Ear Nose Throat J 100(3): 172-176.
- Brooks SG, Trope M, Blasetti M, Laurel D, Arjun P, et al. (2018) Preoperative Lund-Mackay computed tomography score is associated with preoperative symptom severity and predicts quality-of-life trajictouis after sinus surgery. Int Forum Allergy Rhinol 8(6): 668-675.
- Kronzer V L, Huang W, Zaccardelli A, Cynthia SC, John MD, et al. (2022) Association of sinusitis and upper respiratory tract diseases with incident rheumatoid arthritis: A case-control study. J Rheumatol 49(4): 358-364.
- Kronzer VL, Huang W, Crouson CS, John MD, Robert V, et al. (2022) Timing of sinusitis and other respiratory tract diseases and risk of rheumatoid arthritis. Semin Arthritis Rheum 52: 151937.
- Lee IIH, Yang HG, Ha SS, Gil MS, Dae Woo K (2023) Effect of chronic rhinosinusitis on the risk of development of rheumatoid arthritis. Allergy Asthma Immunol Res 15(5): 647-658.
- Descamps E, Gorlier C, Ottaviani S, Elisabeth P, Philippe D, et al. (2021) Screening of dental and sinus infections in rheumatoid arthritis. Eur J Clin Invest 51(4): e 13437.
- Liu J, Zhang H, Dong Z (2017) The prevalence of dental and sinus infection in patient with rheumatoid arthritis before biologic therapy initiation: usefulness of a systematic screening? Ann Rheum Dis 2017.
- Michaud K, Wolfe F (2006) The association of rheumatoid arthritis and its treatment with sinus disease. J Rheumatol 33(12): 2412-2415.
- Van Deun L, de Witte M, Goessens T, Stijn H, Marie Christine K, et al. (2020) Facial pain: A comprehensive review and proposal for a pragmatic diagnostic approach. Eur Neurol 83(1): 5-16.
- Peck CC, Goulet JP, Lobbezoo F (2014) Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J Oral Rehabil 41: 2-23.
- Effat KG (2024) Characterization of headache in patients with temporomandibular joint disorders. Adv Dent Oral Health 17(1): 555954.
- Svensson P (2007) Muscle pain in the head: overlap between temporomandibular disorders and tension-type headache. Curr Opin Neurol 20(3): 320-325.
- Karl HW, Trescot AM (2019) Nerve entrapment headaches at the temple: zygomaticotemporal and/or auriculotemporal nerve? Pain physician 22(1): E15-E36.
- Blake P, Burstein R (2019) Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain 20(1): 76.
- Hagan RR, Fallucco MA, Janis JE (2016) Supraorbital rim syndrome: definition, surgical treatment, and outcomes for frontal headache. PlastReconstr Surg Glob Open 4(7): e 795.
- Moseley GL (2003) A pain neuromatrix approach to patients with chronic pain. Man Ther 8(3): 130-140.
- Murray GM, Peck CC (2007) Orofacial pain and jaw muscle activity: a new model. J Orofac Pain 21(4): 263-278.
- Strassman A M, Raymond SA, Burstein R (1996) Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384(6609): 560-564.
- Ma W, Wang G, Li X, Huping W, Zuguo L, et al. (2020) Study of factors influencing dry eye in rheumatoid arthritis. J Opthalmol 2020: 5768679.
- Petris C K, Almony A (2012) Ophthalmic manifestations of rheumatologic disease: Diagnosis and management. Mo Med 109(1): 53-58.
- Sebastian A, Szachowicz A, Wiland P (2019) Classification criteria for secondary Sjögren’s syndrome. Current state of knowledge. Rheumatologia 57(5): 277-280.






























