Characterization of Headache in Patients with Temporomandibular Joint Disorders
Kamal G Effat*
Consultant Otolaryngologist, Department of Craniofacial pain clinic, El-Sahel Teaching Hospital, Cairo, Egypt
Submission: December 20, 2023; Published: January 09, 2024
*Corresponding author: Kamal G Effat, Consultant Otolaryngologist, Department of Craniofacial pain clinic, El-Sahel Teaching Hospital, Cairo, Egypt
How to cite this article: Kamal G E. Characterization of Headache in Patients with Temporomandibular Joint Disorders. Adv Dent & Oral Health. 2024; 17(1): 555954. DOI: 10.19080/ADOH.2024.17.555954
Abstract
Objective: The incidence and characteristics of headache in patients with temporomandibular joint disorder (TMJD) had not been described previously. The aim of the current study was to discuss this issue.
Materials and methods: The study involved 200 patients with TMJD and 200 control subjects. The incidence and characteristics of headache, as well as associated symptoms were compared between the two groups.
Results: The incidence of chronic headache was significantly higher in the TMJD patient group (88%) than in the control group (37%); (P=0.001). The headache patterns included unilateral throbbing migraine headache, as well as bilateral regional headache.
Conclusion: The results lend support to the theory of overactivity of pericranial muscles in TMJD, as well as extracranial –intracranial cross-talk in sensory nociceptive nerve fibers.
Keywords: Headache; Temporomandibular Joint Disorder; Chronic pain
Abbreviations: TMJD: Temporomandibular joint disorder; CNS: Central nervous system; TTH: Tension-type headache; TMD: Temporomandibular disorder; TMJs: Temporomandibular joints; DC: Diagnostic Criteria; MRI: Magnetic resonance imaging; ICHD: International Classifications of Headache Disorders; CT: Computed tomography
Introduction
The pathogenesis of headaches, especially primary headaches, is probably multifactorial. Various structures in the cranium have been implicated in the causation of headache; with central nervous system (CNS) plastic changes contributing to the chronicity of the condition [1,2]. The cranial calvarial bones are covered from outside by skin and pericranial myofascial structures. Myofascial trigger points are prevalent in both tension-type headache (TTH) and migraine [3,4]. Underneath the calvarial bones is the dura mater. The dura mater has a rich supply of nociceptors and mechanoreceptors; both of which are implicated in the pathogenesis of headaches [5,6]. Animal studies, as well as human imaging studies, have stressed the importance of sterile dural inflammation as a universal finding in primary headaches; thereby sensitizing the receptors [7,8]. The dural inflammation is associated with an increased barrage of action potentials, along nociceptive fibers to the trigeminal ganglion [9,10]. Furthermore, non-trigeminal nociceptive afferents from the occipital dura mater and cervical structures contribute to headaches; being mediated through the upper three cervical nerves [11,12]. Nociceptive afferents from head and neck structures converge onto the subnucleuscaudalis in the brain stem and upper cervical spinal cord [13].
Temporomandibular disorder (TMD) is an umbrella term for pain and dysfunction involving the masticatory muscles and the temporomandibular joints (TMJs) [14]. Most studies on TMD patients report that headache is a frequently associated symptom [15-17]. The Diagnostic Criteria for Temporomandibular Disorders (DC / TMD) considers headache as one of the diagnostic categories of TMD; in addition to TMJ and masticatory muscle disorders [18]. Temporomandibular joint disorder (TMJD) represents a major category of TMD; although, occasionally, it is not associated with painful symptoms [19]. Radiological studies, especially magnetic resonance imaging (MRI) have confirmed the various clinical signs elicited in patients with TMJD [20]. Pathophysiologically, a dysfunctional, abnormal TMJ cannot be considered as an isolated disorder. Many factors, including bruxism, malocclusion, lateral pterygoid muscle dysfunction (which is centrally-mediated) contribute to the joint dysfunction [21,22]. These factors cause abnormal stresses on the TMJ, which are associated with ligament laxity, a low-grade joint inflammation, and deterioration in joint lubrication; all of which predispose to a dysfunctional joint [23,24]. Pain in TMJD is an intringuing issue, and there is often a poor correlation between the degree of pain over the joint and the degree of joint pathology [25,26]. A recent systematic review on structural and functional brain MRI studies revealed aberrant CNS changes in TMJD with pain. The CNS aberrations involved sensory, motor, and affective regions in the brain. Interestingly, the imaged brain aberrations were frequently resolved after intra-oral splint therapy [27].
From a clinical perspective, headache disorders tend to be under-recognized and under-treated, especially in developing populations [28]. The reason for this is that the diagnosis of primary headaches, which is clinically based, frequently does not fulfill the strict criteria provided by the International Classifications of Headache Disorders (ICHD). In these situations, the diagnoses of (probable TTH) and (probable migraine) are often made [29,30]. Furthermore, TTH and migraine frequently coexist [31]. In contrast to the rather diffuse head pain in primary headaches, TMJD pain is localized infront of the ear, being frequently associated with joint noises and functional limitations [32,33]. Additionally, TMJD is commonly associated with otoneurological and alleged sinus symptoms; known as Costen’s syndrome [34-36]. Although the headache associated with masticatory myofascial pain had been previously characterized, headache associated with TMJD had not been previously specifically addressed. Notably, headache associated with masticatory myofascial pain most commonly resembles TTH with a bilateral temporal location [37-39]. On the other hand, the TMJ contains; in addition to nociceptors, mechanoreceptors (proprioceptors), which guide mandibular movements through their inputs to the central pattern generator in the brain stem [40]. The distribution of the proprioceptors in the joint is altered in cases with joint pathology [41]. According to the (gate control theory), altered proprioceptive input to the CNS can alter pain sensation experienced in patients with TMJDs [42]. In addition to exaggerated nociceptive input from the TMJ in TMJD, altered proprioceptive input to the CNS may also contribute to CNS plastic changes causing a peculiar type of pain, in addition to abnormal jaw mechanical behavior [43]. From these perspectives, the aim of the current study was to characterize the incidence and pattern of headache in patients with documented TMJD. As far as the author is aware, and after a Pub Med literature search from 1970 till 2023, this issue had not been previously addressed.
Materials and Methods
The current study was a clinical study conducted at the craniofacial pain clinic at El-Sahel Teaching Hospital in Cairo, Egypt. The study period was 6 months, starting on the 1st of January 2023. Ethical approval was obtained from the Ethics Committee of the General Organization for Teaching Hospitals and Institutes in Cairo (Approval number: 83- 2022). Informed consent was obtained from the patients and control subjects to participate in the study. The patient group consisted of consecutive adult subjects with chronic jaw pain, who reported the experience of joint sounds during mandibular movements. The presence of joint sounds was later confirmed on examination. This criterion allowed objective evidence of TMJD for inclusion in the study. Patients who had jaw pain (arthralgia and/or myalgia) without joint sounds were not included in the patient group. The control group consisted of adult relatives of the patients presenting to the clinic, who experienced no sounds elicited on movement of the mandible. The absence of joint sounds in the control group was later confirmed on examination, revealing clinical evidence of normal TMJs. Exclusion criteria in both groups consisted of any acute disorder in the head and neck, as well as previous macrotrauma to the jaw.
A questionnaire and examination findings sheet were filled in by the author. The main criterion investigated in the current study was the reporting of chronic headache for at least 6 months in both cohorts of participants. In the participants suffering from headache, the characteristics of the head pain were noted according to the description of the subject (pressing or throbbing). Furthermore, the laterality of the head pain was noted (unilateral versus bilateral). The most commonly invloved region in the head was addressed (diffuse, frontoorbital, temporal, occipital, or at the vertex). The presence of otalgia, facial pain, and/or neck pain in association with the headache was reported. Questioning also included the presence or absence of autonomic symptoms accompanying the headache, including nasal congestion, rhinorrhea, and/or lacrimation. Additionally, conditions possibly predisposing to TMJD were asked about (bruxism and dental status). Relevant to TMD, a diagnosis of depression was noted if present. For the reporting of depressive symptoms, the patient had to have been diagnosed by a psychiatrist or reported daily weeping. A previous history of open-lock was addressed. Otological symptoms pertaining to Costen’s syndrome (subjective hearing loss, subjective tinnitus, and/or vertigo) were explored.
Examination of the subjects followed a standard protocol. The TMJ was palpated, noting for any exquisite tenderness (by observing a palpebral reflex). Tenderness over the TMJ was assigned as arthralgia. Any TMJ noises elicited during opening and closing the mouth were classified as click (snapping noise), or crepitus (grating noise). An audible noise was confirmed by palpation of the joint. The presence of joint noises was used to categorize the subject as a TMJD patient. Next, the temporalis and masseter muscles were palpated, noting for any exquisite tenderness (eliciting a palpebral reflex), and any referral pattern for the pain (denoted as myalgia). The oral cavity was observed for the dental status; and accordingly, the subjects were classified as having normal dentition, being partly edentulous, or totally edentulous. While the mouth was fully opened, any trismus was noted (inter-incisal distance less than 40 mm). While the mouth was closed, any open-bite deformity was observed. Any gross deviation of the mandible during opening and closing the mouth was noted. Following examination of the stomatognathic system, examination of the ears, nose, pharynx, and eyes was performed.
Statistical analysis
Results were expressed as mean ± standard deviation or number percent (n %). Comparison between categorical data (n %) was performed using Chi square test. Comparison between mean values of age in the two groups was performed using unpaired t test. Statistical analysis was performed using SPSS computer program (version 19 windows). P value ≤ 0.05 was considered significant.
Results
The current study comprised 200 TMJD patients and 200 control subjects. The mean age of the patient group was 40.34 ± 12.36 years, while the mean age of the control group was 39.1 ± 12.38 years (P= 0.319). There was no significant difference in the male-to-female ratio between the two groups (Table 1). The incidence of chronic headache in the patient group (88%) was significantly higher than in the control group (37%); (P= 0.001). Migraine headache was unilateral and throbbing. Its incidence was significantly higher in the patient group (13%) than in the control group (6%); (P= 0.017). The incidence of bilateral regional headache was significantly higher in the patient group than in the control group for temporal, fronto-orbital, and occipital locations (Table 2). Table 3 reveals clinical features pertinent to TMJD in patients versus controls. Notably, clicking was noted in 85 % of TMJD patients, while crepitus was elicited in 15 % of TMJD patients. The control subjects showed clinically normal TMJs. All other symptoms and signs related to the stomatognathic system had higher incidence in TMJD patients versus control subjects (Table 3). Table 4 reveals the incidence of symptoms related to Costen’s syndrome in patients and control subjects. All otological and alleged sinus symptoms had a higher incidence in TMJD patients versus controls.
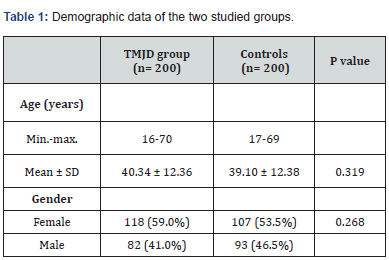
Data are expressed as mean ± SD or number (%).
TMJD= temporomandibular joint disorder.
p> 0.05= not significant.
Discussion
Temporomandibular joint disorder (TMJD) involves a set of functional and pathological changes affecting the TMJ [44]. As the two TMJs cannot function independently during mandibular movements, TMJD is very frequently a bilateral disorder [45]. The current concept is that the pathogenesis of TMJD involves excessive or abnormal stresses on the TMJ, that exceed the adaptive capacity of the joint tissues [46]. These abnormal stresses acting on the TMJ lead to laxity of the joint ligaments, thereby predisposing to disc displacement [47]. Furthermore, an associated low-grade inflammation in the TMJ has been reported to initiate a cascade of events, including impaired lubrication and increased friction coefficient in the joint [48,49]. The result of increased friction in the joint is a disturbance in disc-condyle complex harmony and predisposition to disc displacement and degenerative joint disease [50,51]. Moreover, increased tension in the lateral pterygoid muscle had been postulated to account for the most common form of disc displacement (anteromedially) [52]. Clinically, reducing disc displacement manifests as clicking, whereas degenerative joint disease manifests as crepitus [53]. In the current study, the TMJD patient group demonstrated clicking in 85% of the patients, whereas crepitus was present in 15% of the patients. In the control group, there was absence of joint noises during mandibular movement. A painful, tender TMJ is designated as TMJ arthralgia. The current view is that the degree of TMJ arthralgia is correlated with the levels of inflammatory mediators and neuropeptides, which sensitize and activate afferent nociceptive nerve fibers in the joint [54,55]. The current study revealed TMJ arthralgia in 75.5% of patients, versus 9% of control subjects (P= 0.001).
A continuous barrage of action potentials from the sensitized and activated TMJ nociceptors contribute to the chronicity of TMJ pain [56]. In pain literature, chronic pain is defined as a multidimensional experience involving negative sensory, affective, and cognitive aspects [57]. These aspects are associated with corresponding abnormal structural and functional brain regions, seen on neuroimaging [58]. Currently, there are two proposed theories for these brain changes in chronic painful TMJD. The first theory involves sensitization and activation of CNS pathways relevant to chronic pain in its three dimensions, consequent on a continuous barrage of action potentials from the peripheral TMJ nociceptors [59,60]. The alternative hypothesis is the activation of a pre-set brain pain neuromatrixby stress and peripheral pain [61-64]. In both theories, the result of chronic painful TMJD is the unwanted experience of pain, alteration in homeostatic mechanisms; and importantly, an altered motor output to the masticatory and cranial muscles [65-67].
The altered motor output to these muscles contributes to trigger points, commonly observed in patients with TMJD associated with headache [68,69]. Temple headaches, frontoorbital headaches, and occipital headaches represent common presentations of headache disorders. Various studies propose that these regional headache disorders primarily arise due to entrapment of respective sensory nerve fibers by the overcontracting pericranial muscles. These nerve fibers include various trigeminal and upper cervical nerve fibers which course through the muscles [70-72]. In the current study, 26% of patients with TMJD had headache at the temples; 21% had headache with a fronto-orbital location; and 12% had headache at the occipital region (Table 2). In the control subjects, 12.5% had headache at the temples, 10.5% had headache at the fronto-orbital region; and 2% had headache at the occipital region. These regional headaches had a pressing, tightening quality and were of bilateral location. The difference in incidence of these respective headaches between the TMJD patient group and the control group was statistically significant (Table 2).
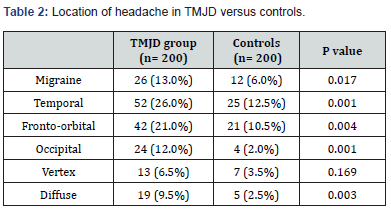
Data are expressed as number (%).
TMJD= temporomandibular joint disorder.
p> 0.05= not significant; p≤ 0.05= significant.
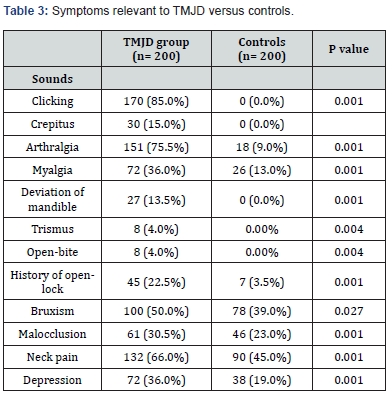
Data are expressed as number (%).
TMJD= temporomandibular joint disorder.
p≤ 0.05= significant
TMJD may act as a migraine trigger in genetically predisposed individuals [73]. A recent large longitudinal population study had suggested that the relation between TMJD and migraine is bidirectional [74]. A biological explanation for the association between TMJD and migraine is that extracranial and intracranial trigeminal innervation forms a functional unit [75]. This concept is supported by numerous animal studies, which reveal that dural afferents project through the calvarial bones to innervate extracranial structures [76]. It is hypothesized that there would be a physiological and pathological cross-talk between trigeminal nociceptive nerve fibers in both the intracranial and extracranial compartments [77,78]. Activation of the meningeal and meningovascular nociceptive afferents, with the release of various neuropeptides, results in a sterile dural inflammation [79]. Activation of dural mechanoreceptors, in the context of dural inflammation, explains the throbbing pain, experienced in some forms of headache, especially migraine [5]. Migraine headache is typically a unilateral throbbing headache, associated with photophobia, phonophobia and nausea/vomiting [80]. In the current study, headache typical of migraine was experienced in 13% of TMJD patients versus 6% of controls (P=0.017).
Totally, 88% of TMJD patients in the current study reported chronic headache, in contrast to only 37% of control subjects (P=0.001). Importantly, 66% of TMJD patients also reported chronic neck pain versus 45% of control subjects (P=0.001). Neck pain is mediated via the upper cervical nerves, and it is commonly associated with headache and TMD [12]. Indeed, the cranio-mandibular-cervical complex has been considered as an anatomical and functional unit, with neuromuscular restoring forces stabilizing this unit against the force of gravity [81,82]. Epidemiological, clinical, and imaging studies have revealed a significant association between TMJD and neck pain [83-86]. It is postulated that this association might be due to the convergent nociceptive input from the TMJ and neck structures onto the subnucleuscaudalis in the brain stem [87]. An alternative theory that was also postulated involves abnormal proprioceptive afferents from the TMJ that activate the trigemino-cervical reflex, resulting in abnormal neck muscle contraction and neck pain [88]. The trigemino-cervical reflex could also be implicated in migraine and TTH [89,90], thereby providing a putative explanation for the association of TMJD, headache, and neck pain.
Further adding to the morbidity in patients with TMJD, is the frequent association of symptoms pertaining to Costen’s syndrome. In 1934, James Costen reported (a syndrome of ear and sinus symptoms dependent upon disturbed function of the temporomandibular joint) [91]. These symptoms have recently been validated by audiometric tests and computed tomography (CT) scans of the paranasal sinuses [34-36]. In the current study, hearing loss, subjective tinnitus, and vertigo were reported in 39%, 40.5%, and 56.5% of TMJD patients, respectively. The incidence of these symptoms in control subjects was 20.5%, 21%, 32%, respectively (Table 4). The difference in incidence of these symptoms between the two groups is statistically significant. The origin of these symptoms in TMJD patients was postulated to be due to an increased tension on the malleus of the middle ear from a displaced TMJ disc, leading to abnormal position of the stapes at the oval window [92-94]. This, in turn, would lead to altered firing patterns from inner-ear hair cells [95]. Additionally, inputs from higher brain centers onto relevant brain stem nuclei may exaggerate these symptoms in conditions of pain, stress, anxiety, and lack of sleep, via top-down mechanisms [96,97]. Alleged sinus symptoms, especially facial pain, are also common in patients with TMJD [36]. In the current study, facial/eye pain was present in 52% of TMJD patients, versus 29% of control subjects (P=0.001). Facial/ eye pain had been attributed to the convergence of nociceptive branches of the trigeminal nerve onto the subnucleuscaudalis in the brain stem [98]. Jones described (midfacial segment pain), as a condition analogous to tension-type headache, with a similar pathophysiology, in patients with normal nasal endoscopy and clear sinus CT scans [99-101]. He postulated that this condition was attributed to abnormal nociceptive afferents from a regional source. Interestingly, Costen, in his original reports, mentioned that the otologic and alleged sinus symptoms were alleviated by correction of dental bite, in his patients with malocclusion [91]. Correction of the dental bite probably also ameliorates TMJD pain and headache [102,103]. The incidence of malocclusion in the current study was 30.5% in TMJD patients, versus 23% in control subjects (P=0.001).
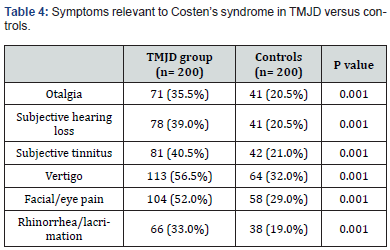
Data are expressed as number (%).
TMJD= temporomandibular joint disorder.
p≤ 0.05= significant
Physical and emotional stress appear to be a common denominator in patients with headache, neck pain, and TMJD [104,105]. The stress response involves a pronounced sympathetic outflow and the release of stress hormones, and it is associated with a documented overactivity of head and neck muscles [106,107]. Moreover, the stress response is associated with peripheral and central sensitization in pain pathways, thereby contributing to the chronicity of headache, neck pain, and jaw pain [108,109]. Biomechanically, overactivity of masticatory muscles, as in bruxism, causes abnormal stresses on the TMJ, leading to its deterioration [110]. Overactivity of the lateral pterygoid muscle leads to the most common form of disc displacement (anteromedially) [111]. Clinically, bruxism, as a model of stress, is associated with TMJD [112,113]. In the current study, a report of bruxism was found in 50% of TMJD patients, versus 39% of control subjects (P=0.027).
The main strength of the current study was that it shedded light on the patterns of headache and associated symptoms in patients with TMJD, a previously undiscussed topic. The presence or absence of TMJ noises allowed an objective clinical distinction between TMJD patients and control subjects, respectively. Furthermore, the cohorts of TMJD patients and control subjects in the current study were of comparable socio-economic conditions, thereby allowing reliable distinctions to be made between both groups. The major limitation of the current study was that the reporting of symptoms depended entirely on the recalling of symptoms by the participants, which was totally subjective. From this view, the diagnosis of headache depends mainly on the verbal description of the patient. Apart from migraine, which has a classic presentation, no attempt was made to classify the headache from a diagnostic point of view. Rather, a description of headache sites was used in the current study, which was generally of a pressing, tightening quality and of bilateral location. The variable locations of headache in the study lends support to the theory of sensory nerve entrapment by respective over-contracting muscles. This notion emphasizes the recently-documented fact that extracranial and intracranial nociceptive nerve fibers act as a functional unit. A further limitation of the current study was that the author was not blinded, as regards the participant’s clinical features. However, the author has several publications regarding TMJD, which may validate the clinical signs elicited in the current study, as well as the recording of the relevant symptoms.
Conclusion
The current study revealed a higher incidence of headache in patients with TMJD, compared to control subjects. In addition to the typical migraine unilateral, throbbing headache, the patterns of headache frequently revealed a bilateral regional distribution with a pressing, tightening quality. This lends support to the theory of overactivity of pericranial muscles in TMJD, causing entrapment of isolated sensory trigeminal or cervical nerve branches, thereby explaining the regional headache patterns. Moreover, the connections between the extracranial and intracranial sensory nerve fibers account for the frequency of migraine in several participants.
References
- McFate T, Scher AJ (2009) Chronic pain disorders and headache chronification. Curr Pain Headache Rep 13(4): 308-313.
- Pak DJ, Yong RJ, Kaye AD (2018) Chronification of pain: mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep 22(2): 9.
- Do TP, Heldarskard GF, Kolding LT (2018) Myofascial trigger points in migraine and tension-type headache. J Headache Pain 19(1): 84.
- Aaseth K, Grande RB, Lundqvist C (2014) Pericranial tenderness in chronic tension-type headache: the Akershus population-based study of chronic headache. J Headache Pain 15(1): 58.
- Strassman AM, Raymond SA, Burstein R (1996) Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384(6609): 560-564.
- Ahn AH (2012) The neurobiology of throbbing pain in migraine. Headache 52(suppl 1): 12-14.
- Filipović B, Matak I, Lacković Z (2014) Dural neurogenic inflammation induced by neuropathic pain is specific to cranial region. J Neural Transm (Vienna) 121(5): 555-563.
- Glinskii OV, Huxley VH, Glinsky VV (2017) Estrogen-dependent changes in dura mater microvasculature add new insights to the pathogenesis of headache. Front Neurol 8: 549
- Shimazaki K, Yajima T, Ichikawa H (2022) Distribution and possible function of galanin about headache and immune system in the rat dura mater. Sci Rep 12: 5206.
- Harriott AM, Gold MS (2009) Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. J Neurophysiol 101(6): 3126-3134.
- Noseda R, Melo-Carvillo A, Nir RR (2019) Non-trigeminal nociceptive innervation of the posterior dura: Implications to occipital headache. J Neurosci 39(10): 1867-1880.
- Kraus S (2007) Temporomandibular disorders, head and orofacial pain: cervical spine considerations. Dent Clin North Am 51(1): 161-193.
- Sessle BJ (2011) Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol 97: 179-206.
- List T, Jensen RH (2017) Temporomandibular disorders: Old ideas and new concepts. Cephalagia 37(7): 692-704.
- Franco AL, Gonçalves DAG, Casatonharo SM (2010) Migraine is the most prevalent primary headache in individuals with temporomandibular disorders. J Orofac Pain 24(3): 287-292.
- Graff-Radford SB, Bassiur JP (2014) Temporomandibular disorders and headaches. Neurol Clin 32(2): 525-537.
- Emshoff R, Bertram F, Schnabl D (2017) Association between chronic tension-type headache coexistent with chronic temporomandibular disorder pain and limitations in physical and emotional functioning: A case-control study. J Oral Facial Pain Headache 31(1): 55-60.
- Peck CC, Goulet JP, Lobbezoo F (2014) Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J Oral Rehabil 41: 2-23.
- Naeije M, TeVeldhuis AH, TeVeldhuis EC (2013) Disc displacement within the human temporomandibular joint: a systematic review of a noisy annoyance. J Oral Rehabil 40(2): 139-158.
- Cong N, Wang N, Huang S (2021) Diagnostic significance of magnetic resonance imaging in distinguishing temporomandibular disorders: a retrospective chart review. BMC Oral Health 21(1): 481.
- Poluha RL, Canales GD, Costa YM (2019) Temporomandibular joint disc displacement with reduction: a review of mechanisms and clinical presentation. J Appl Oral Sci 27: e20180433.
- Murray GM, Phanachet I, Uchida S (2004) The human lateral pterygoid muscle: a review of some experimental aspects and possible clinical relevance. Aust Dent J 49(1): 2-8.
- Molinari F, Manicone PF, Raffaelli L (2002) Temporomandibular joint soft-tissue pathology, I :Disc abnormailities. Semin Ultrasound CT MR 28(3): 192-204.
- Ferreira NDR, Sanz CK, Raybolt A (2022) Action of hyaluronic acid as a damage-associated molecular pattern molecule and its function on the treatment of temporomandibular disorders. Front Pain Res (Lausanne) 3: 852249.
- Dworkin SF (1994) Perspectives on the interaction of biological, psychological and social factors in TMD. J Am Dent Assoc 125(7): 856-863.
- Ohrbach R, Dworkin SF (1998) Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain 74(2-3): 315-326.
- Yin Y, He S, Xu J (2020) The neuro-pathophysiology of temporomandibular disorders-related pain: a systematic review of structural and functional MRI studies. J Headache Pain 21(1): 78.
- Genc H, Baykan B, Bolay H (2023) Cross-sectional, hospital-based analysis of headache types using ICHD-3 criteria in the Middle East, Asia, and Africa: the Head-MENAA study. J Headache Pain 24(1): 24.
- Kim BS, Moon HS, Sohn JH (2016) Short-term diagnostic stability of probable headache disorders based on the International Classification of Headache Disorders. 3rd edition beta version, in first-visit patients: a multicenter follow-up study. J Headache Pain 17: 13.
- Albers L, Straube A, Landgraf MN (2014) High diagnostic stability of confirmed migraine and confirmed tension-type headache according to the ICHD-3 beta in adolescents. J Headache Pain 15: 36.
- Turner DP, Smitherman TA, Black AK (2015) Are migraine and tension-type headache diagnostic types or points on a severity continuesm? : An exploration of the latent taxometric structure of headache. Pain 156(7): 1200-1207.
- Chantaracherd P, John MT, Hodges JS (2015) Temporomandibular joint disorders’ impact on pain, function, and disability. J Dent Res 94(3 suppl): 79S-86S.
- Mongini F, Italiano M, Raviola F (2000) The McGill Pain Questionnaire in patients with TMJ pain and with facial pain as a somatoform disorder. Cranio 18(4): 249-256.
- Effat KG (2021) A minireview of the anatomical and pathological factors pertaining to Costen’s syndrome symptoms.
- Effat KG (2016) Otological symptoms and audiometric findings in patients with temporomandibular disorders: Costen’s syndrome revisited. J Laryngol Otol 130(12): 1137-1141.
- Effat KG (2019) A comparative clinical and radiographic study on patients with chronic facial pain with and without temporomandibular disorder presenting to an otolaryngology clinic. Cranio 39(1): 1-7.
- Costa YM, Porporatti AL, Stuginski-Barbosa J (2015) Headache attributed to masticatory myofascial pain: clinical features and management outcomes. J Oral Facial Pain Headache 29(4): 323-330.
- Hara K, Shinozaki T, Okada-Ogawa A (2016) Headache attributed to temporomandibular disorders and masticatory myofascial pain. J Oral Sci 58(2): 195-204.
- Svensson P (2007) Muscle pain in the head: overlap between temporomandibular disorders and tension-type headaches. Curr Opin Neurol 20(3): 320-325.
- Molina OF, Marçal R, Hassumi MY (2022) Sensory receptors in the temporomandibular joint: A review. IOSR-J Dent Med Sci 21(8): 63-76.
- Favia G, Maiorano E (1995) Presence of neuroreceptors in normal and diseased temporo-mandibular joints. Boll Soc Ital BiolSper 71(7-8): 205-212.
- Zampino C, Ficacci R, Checcacci M, Francioliniet F, Catacuzzenoal L (2018) Pain control by proprioceptive and exteroceptive stimulation at the trigeminal level. Front Physiol 9: pp 1037
- Murray GM, Peck CC (2007) Orofacial pain and jaw muscle activity: a new model. J Orofac Pain. 21(4): 263-278.
- Poluha RL, Grossmann E (2018) Inflammatory mediators related to arthrogenic temporomandibular dysfunctions. Br J Pain. 1(1): 60-65.
- Jerele C, Avsenik I, Šurlanpopović K (2021) MRI characteristics of the asymptomatic temporomandibular joint in patients with unilateral temporomandibular joint disorder. Oral Radiol 37(3): 469-475.
- Ernberg M (2017) The role of molecular pain biomarkers in temporomandibular joint internal derangement. J Oral Rehabil. 44(6): 481-491.
- Del Palomar AP, Doblaré M (2006) 3D finite element simulation of the opening movement of the mandible in healthy and pathologic situations. J Biomech Eng 128(2): 242-249.
- Ferreira NDR, Sanz CK, Raybolt A, Maria Pereira C , Fabio DosSantos M (2022) Action of hyaluronic acid as a damage-associated molecular pattern molecule and its function on the treatment of temporomandibular disorders. Front Pain Res (Lausanne) 18(3): pp 852249.
- Asakawa-Tanne Y, Su S, Kunimatsu R, Hiroseet N, Mitsuyoshi T, et al. (2015) Effects of enzymatic degradation after loading in temporomandibular joint. J Dent Res 94(2): 337-343.
- Nitzan D W (2003) Friction and adhesive forces’ –possible underlying causes for temporomandibular joint internal derangement. Cells Tissues Organs 174(1-2): 6-16.
- Koolstra J H (2012) Biomechanical analysis of the influence of friction in jaw joint disorders. Osteoarthritis cartilage 20(1): 43-48.
- Muňoz-Guerra MF, Rodriguez-Campo FJ, Escorial-Hernández, Brabyn PJ, Fernández-Domínguez M, et al. (2021) The minimally invasive anterior myotomy in the treatment of internal derangement of the temporomandibular joint. A detailed description of the surgical technique. J Stomatol Oral Maxillofac Surg 122(1): 50-55.
- González-González AM, Herrero AJ (2021) A systematic review of the temporomandibular disorder diagnostic methods. Cranio 29: 1-13
- Shrivastava M, Battaglino R, Ye L (2021) A comprehensive review on biomarkers associated with painful temporomandibular disorders. Int J Oral Sci 13(1): P 23.
- Kothari SF, Ba ad-Hansen L, Hansen LB, Banget N, Hovgaard Sørensen L, et al. (2016) Pain profiling of patients with temporomandibular joint arthralgia and osteoarthritis diagnosed with different imaging techniques. J Headache Pain 17(1): P 61.
- Ferrilo M, Giudice A, Marotta N, Fortunato F, Di Venere D, et al. (2022) Pain management and rehabilitation for central sensitization in temporomandibular disorders: A comprehensive review. Int J Mol Sci 23(20): PP 12164.
- Furquim BD, Flamengui LMSP, Conti PCR (2015) TMD and chronic pain: A current view. Dental Press J Orthod 20(1): 127-133.
- Chen XF, He P, Xu KH, Han Jinet Y, Chen Y, et al. (2022) Disrupted spontaneous neural activity and its interaction with pain and emotion in temporomandibular disorders. Front Neurosci 16: PP 941244
- Latremoliere A, Woolfe CJ (2009) Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain 10(9): 895-926.
- Monaco A, Catteneo R, Marci MC, Pietropaoli D , Ortu E (2017) Central sensitization-based classification for temporomandibular disorders: A pathogenetic hypothesis. Pain Res Manag 2017: PP 5957076.
- Melzack R (2001) Pain and the neuromatrix in the brain. J Dent Educ 65(12): 1378-1382.
- Iannetti GD, Mouraux A (2010) From the neuromatrix to the pain matrix (and back). Exp Brain Res 205(1): 1-12.
- Moseley GL (2003) A pin neuromatrix approach to patients with chronic pain. Man Ther 8(3): 130-140.
- Melzack R (2004) Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: presented at the Third World Congress of World Institute of Pain, Barcelona 2004. Pain Pract 5(2): 85-94.
- Kopp S (2001) Neuroendocrine, immune, and local response related to temporomandibular disorders. J Orofac Pain 15(1): 9-28.
- Khalsa PS (2004) Biomechanics of musculoskeletal pain: dynamics of the neuromatrix. J Electromyogr Kinisiol 14(1): 109-120.
- Bodéré C, Téa SH, Giroux-Metges MA, Woda A, et al. (2005) Activity of masticatory muscles in subjects with different orofacial pain conditions. Pain 116(1-2): 33-41.
- Ge HY, Fernández-de-les-Peňas C, Yue SW (2011) Myofascial trigger points: spontaneous electrical activity and its consequences for pain induction and propagation. Chin Med 6: 13.
- Fernández-de-las-Peňas C, Cuadrado ML, Arendt-Nielsen L (2007) Myofascial trigger points and sensitization: An updated pain model for tension-type headache. Cephalagia 27(5): 383-393.
- Karl H W, Trescot A M (2019) Nerve entrapment headaches at the temple: zygomaticotemporal and/or auriculotemporal nerve? Pain Physician 22(1): E15-E36.
- Blake P, Burstein R (2019) Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain 20(1): 76.
- Hagan RR, Fallucco MA, Janis JE (2016) Supraorbital rim syndrome: definition, surgical treatment, and outcomes for frontal headache. PlastReconstrSurg Global Open 4(7): e795.
- Noseda R, Burstein R (2013) Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 154 (suppl 1): S44-S53.
- Byun SH, Min C, Yoo DM (2020) Increased risk of migraine in patients with temporomandibular disorder: A longitudinal follow-up study using a national health screening cohort. Diagnostics (Basel) 10(9): 724.
- Schueler M, Neuhuber WL, Col RD (2014) Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache 54(6): 996-1009.
- Schueler M, Messlinger K, Dux M (2013) Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain 154(9): 1622-1631.
- Kosaras B, Jakubowski M, Kainz V (2009) Sensory innervation of the calvarial bones of the mouse. J Comp Neurol 515(3): 331-348.
- Zhao J, Levy D (2014) The sensory innervation of the calvarial periosteum is nociceptive and contributes to headache-like behavior. Pain 155(7): 1392-1400.
- Spekker E, Tanaka M, Szabo Á (2021) Neurogenic inflammation: The participant in migraine and recent advancements in tranalational research: Biomedicines 10(1): 76.
- Kunkel R S (2001) Clinical manifestations of migraine. Clinical Cornerstone 4 (3):18-25.
- Gillies GT, Broaddus WC, Stenger JM (1998) A biomechanical model of the craniomandibular complex and cervical spine based on the innervated pendulum. J Med Eng Technol 22(6): 263-269.
- Gillies GT, Christy DW, Stenger M (2009) Equilibrium and non-equilibrium dynamics of the cranio-mandibular complex and cervical spine. J Med EngTenchnol 27(1): 32-40.
- Ciancaglini R, Testa M, Radaelli G (1999) Association of neck pain with symptoms of temporomandibular dysfunction in the general adult population. Scand J Rehabil Med 31(1): 17-22.
- Kim D, Ko S-G, Lee E-K (2019) The relationship between spinal pain and temporomandibular joint disorders in Korea: a nationwide propensity score-matched study. BMC Musculoskeletal Disord 20(1): 631.
- Evcik D, Aksoy O (2000) Correlation of temporomandibular joint pathologies, neck pain and postural differences. J PhysTher Sci 12: 97-100.
- Xiao C-Q, Wan Y-D, Li Y-Q (2023) Do temporomandibular disorder patients with joint pain exhibit forward head posture? A cephalometric study. Pain Res Manag 2023: 7363412.
- Šedy J, Rocabado M, Olate LE (2002) Neural basis of etiopathogenesis and treatment of cervicogenic orofacial pain. Medicina (Kaunas) 58(10): 1324.
- Kobayashi M, Yabushita T, Zeredo JL (2007) Splenius muscle activities induced by temporomandibular joint stimulation in rats. Brain Res Bull 72(1): 44-48.
- Nardone R, Ausserer H, Bratti A (2008) Trigemino-cervical reflex abnormalities in patients with migraine and cluster headache. Headache 48(4): 578-585.
- Nardone R, Tezzon F (2003) The trigemino-cervical reflex in tension-type headache. Eur J Neurol 10(3): 307-312.
- Costen JB (1997) A syndrome of ear and sinus symptoms dependent upon disturbed function of the temporomandibular joint. Ann Otol Rhinol Laryngol 106(10 Pt1): 805-819.
- Riga M, Xenellis J, Peraki E (2010) Aural symptoms in patients with temporomandibular joint disorders: multiple frequency tympanometry provides objective evidence of changes in middle ear impedance. OtolNeurotol 31(9): 1359-1364.
- Kierner AC, Mayer R, Kirschhofer KV (2002) Do the tensor tympani and tensor veli palatine muscles of man form a functional unit? A histochemical investigation of their putative connections. Hearing Res 165(1-2): 48-52.
- Pihut M, Majewski P, Wisniewska G (2011) Auriculovestibularsymptoms related to structural and functional disorders of stomatognathic system. J PhysiolPharmacol 62(2): 251-256.
- Norena AJ (2015) Revisiting the cochlear and central mechanisms of tinnitus and therapeutic approaches. AudiolNeurotol. 20(suppl 1): 53-59.
- Shore SE, Roberts LE, Langguth B (2016) Maladaptive plasticity in tinnitus-triggers, mechanisms and treatment. Nat Rev Neurol 12(3): 150-160.
- Brandt T, Dieterich M (2019) Thalamocortical network: a core structure for integrative multimodal vestibular functions. CurrOpin Neurol 32(1): 154-164.
- De Rossi SS (2013) Orofacial pain: a Primer. Dent Clin N Am 57(3): 389-392.
- Jones NS (2004) Midfacial segment pain: implications for rhinitis and sinusitis. Curr Allergy Asthma Rep 4(3): 187-192.
- West B, Jones NS (2001) Endoscopy-negative, computed tomography- negative facial pain in a nasal clinic. Laryngoscope 111(4 Pt1): 581-586.
- Agius AM, Jones NS, Muscat R (2014) Prospective three-year follow up of a cohort study of 240 patients with chronic facial pain. J Laryngol Otol 128(6): 518-526.
- Wieckiewicz M, Boening K, Wiland P (2015) Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J Headache Pain 16: 106.
- Bindayel NA (2018) Occurrence of malocclusion in patients with orofacial pain and temporomandibular disorders. J Contemp Dent Pract 19(5): 477-482.
- Harper DE, Schrepf A, Clauw DJ (2016) Pain mechanisms and centralized pain in temporomandibular disorders. J Dent Res 95(10): 1102-1108.
- Fillingim RB, Ohrbach R, Greenspan JD (2013) Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 14(120).
- Godoy LD, Rossignoli MT, Delfino-Pereira P (2018) A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Front BehavNeurosci 12.
- Johnston V, Jull G, Darnell R (2008) Alterations in cervical muscle activity in functional and stressful tasks in female office workers with neck pain. Eur J Appl Physiol 103(3): 253-264.
- Cathcart S, Bhullar N, Imminek M (2012) Pain sensitivity mediates the relationship between stress and headache intensity in chronic tension-type headache. Pain Res Manag 17(6): 377-380.
- Maleki N, Becerra L, Borsook D (2012) Migraine: Maladaptive brain responses to stress. Headache 52(suppl 2): 102-106.
- Commisso MS, Martinez-Reina J, Mayo J (2014) A study of the temporomandibular joint during bruxism. Int J Oral Sci 6(2): 116-123.
- Tanaka E, Hirose M, Inunushi T (2007) Effect of hyperactivity of the lateral pterygoid muscle on the temporomandibular joint disk. J Biomech Eng 129(6): 890-897.
- Gameiro GH, Andrade AS, Nouer DF (2006) How may stressful experiences contribute to the development of temporomandibular disorders? Clin Oral Investig 10(4): 261-268.
- Kanehira H, Agariguchi A, Kato H (2008) Association between sress and temporomandibular disorder. J Jpn Prosthodont Soc 52(3): 375-380.






























