Proteomics and Biomarkers Based Diagnosis of Brain Tumor: A Review
Noreen Samad*, Wajiha Jahangir, Fatima Ashraf and Saba Qamar
Department of Biochemistry, Bahauddin Zakariya University, Pakistan
Submission: December 17, 2017;;Published: May 11, 2018
*Corresponding author: Noreen Samad, Assistant Professor, Department of Biochemistry, Bahauddin Zakariya University, Multan, Pakistan, Email: noreen.samad@bzu.edu.pk
How to cite this article: Noreen S,Wajiha J,Fatima A,Saba Q. Proteomics and Biomarkers Based Diagnosis of Brain Tumor: A Review. Theranostics Brain Spine Neuro Disord. 2018; 3(1): 555605. DOI: 10.19080/TBSND.2018.03.555605
Abstract
Quantitative proteomics represents a powerful approach for the comprehensive analysis of proteins expressed under defined conditions of brain tumor. It has become a major subject of studies to apply proteomics for biomarker. In the last decades, technical advances in mass spectrometry have increased the capacity of protein identification and quantification. To improve the accuracy of the discoveries made using proteomics in human tumors, it is necessary to combine 2DP-GE and matrix assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) for more accurate results. This review presents different diagnostic techniques outline for brain tumor. Particular attention has been given to the inclusion of proteomic investigations of tumor tissue.
Keywords: Brain tumor; Proteomics ; Biomarkers; MALDI; Metastasis
Introduction
Brain tumor is a collection or Cancer is a debilitating disease whose trend is continuously increasing. The most studied molecular deviations in cancer are genetic alteration. Metabolism of tumor cell is different to some extent as compared to normal cell. In order to defeat cancer, mechanism of disease must be well understood [1]. Tumor growth is associated with angiogenesis. For the proper growth of tumor new vessels supplying the tumor with oxygen and nutrients is necessary and it allows cancerous cell to invade adjacent tissue [2]. Intracellular hypoxia and transcription factors which responds to the change in oxygen concentration are created by fast growing tumors [3,4]. Brain tumor is a collection or mass of abnormal cells in brain(AboutTM).Brain tumor represent one of the largest public health problem [5]. The significant health burden represented by cancer has lead to the pursuit of a more comprehensive understanding of the changes driving tumor initiation, progression and metastasis. Broad categories of brain tumor have been studied like glioma, astrocytoma, glioblastoma, ependymoma, oligodendroglioma, and meningioma. The WHO classification of brain tumor is based on histology and classified from grade 1 to 4 where grade 4 is most undifferentiated one. The more the undifferentiated the more poor prognosis and more fatal it is. Tumors which are confined within the skull cavity is mainly the brain tumor. They mainly originate from the supportive cell population in brain tissue, neuro-epithelial cells and meninges which protect the brain. Brain tumor can be benign or malignant and it originates in different anatomical location of brain and CNS. Glooms constitute about 2% of all adult tumor it is fourth largest cause of cancer death due to its aggressive nature. This type of tumor is a broad category of brain and spinal cord tumor that arises from the supportive cells of the brain called neuroepithelial or glial cells. Based on their cellular origin and the degree of malignancy these gliomas are classified histologically and immune histochemically.
The degree of malignancy which is graded from 1-4 according to WHO(world health organization) grading system [6]. Astrocytoma are most abundant glial cell and these are most common gliomas tumor. Low grade of these types of tumor are uncommon. These are generally slow growing tumor. Anaplastic astrocytoma or astrocytoma 3 grows rapidly and invasively. Survival time varies from high to low grade tumor [7]. Glioblastoma also known as grade 4 astrocytomas constitute about 50% of allgliomas and are the most common intracranial malignancy [8]. Oligodendrogliomas are composed of cells that morphologically resemble oligodendroglia. These tumor of WHO grade 2 are slow growing and well differentiated tumors that show tendencies to diffusely infilterate the surrounding brain. Anaplastic oligodendrogliomas WHO grade 3 show more malignant histological feature [6]. The cause of brain tumors is yet to be elucidated.,/
However some proposed mechanism for etiology is radiations and inheritance [9]. The symptoms mainly depends upon the size and localization of the tumor within the skull cavity. The most common symptoms are Headache, diplopia (double vision), raised ICP (intra cranial pressure), seizures, compression of vital centers, and personality disorders [10]. Genome encoded set of proteins comprises proteome. The proteome is dynamic and it alters in response to physiological status of the organism. The field which encompasses studying such sets is known as proteomics [11]. Proteomics has leveraged multiple sample preparation, fractionation and MS techniques over the past two decades to gain an understanding of tumor-specific changes in protein abundance and modification state. Fortuitously, biomarker studies also benefit from using tumor tissue in the discovery phase, as the tissue should have the highest concentration of any tumor-specific markers [5].
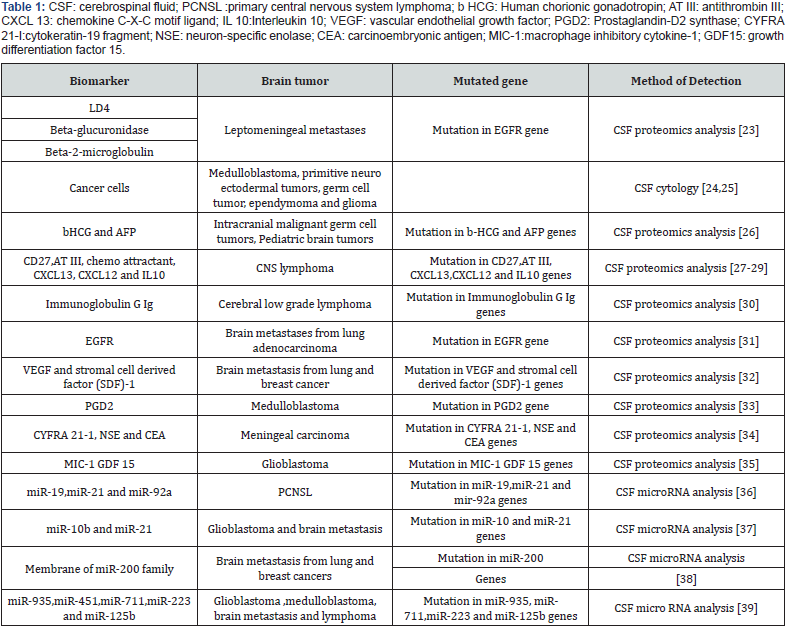
The source allows rapid screening, low sample consumption and accurate identification by proteomic technology. Almost all proteins normally present in cerebrospinal fluid (CSF) are derived from serum. The normal protein content of CSF is 100- to 400-fold lower compared to serum and depends upon the relative exclusion of macromolecules by the BBB (blood brain barrier) [12]. Under pathological conditions one may find that some of the proteins are usually not present in CSF. The presence of such proteins either results from disruption of the intrathecal production or BBB (blood brain barrier). An example of the latter is the increased CSF concentration of the tumor markers like LD5, beta-glucuronidase, and beta-2-microglobuline in cases of lepto-meningeal metastases [12,13].
The identification of proteins in the CSF that are intrathecally secreted or shed by the tumor or its microenvironment may reveal cellular mechanisms relevant to cancer biology. Also, it may result in the development of new tumor markers and may ultimately target new therapies. Recently, protein expression profiling has become a valuable tool in obtaining information about the state of protein circuits inside tumor cells and at the tumor host interface [14]. Proteomic techniques are applicable to serum but also to CSF for the detection of peptides and proteins resulting from diseases, including cancer. A major advantage of the search for disease-related proteins in CSF over serum is the lower protein concentration in the former.
Proteomics
Brain tumors have universally fatal outcomes; new therapeutics is desperately needed and will come from improved understandings of glioma biology. Exosmic are endosomally derived 30–100 nm membranous vesicles released from many cell types into the extracellular milieu; surprisingly, exosomes are virtually unstudied in neuron-oncology. These micro vesicles were used as vaccines in other tumor settings, but their immunological significance is unevaluated in brain tumors.Findings show that these vesicles have biophysical characteristics and proteomic profiles similar to exosomes from other cell types but that brain tumor exosomes have unique features (e.g., very basic isoelectric points, expressing the mutated tumor antigen EGFRvIII and the putatively immunosuppressive cytokine TGF-β). Administration of such exosomes into syngeneic animals produced both humoral and cellular immune responses in immunized hosts capable of rejecting subsequent tumor challenges but failed to prolong survival in established orthotopic models.
Control animals received saline or cell lysate vaccines and showed no antitumor responses. Exosmic and micro vesicles isolated from sera of patients with brain tumors also possess EGFR, EGFRvIII, and TGF-β. We conclude that exosomes released from brain tumor cells are biochemically/biophysically like other exosomes and have immune-modulating properties. They can escape the blood-brain barrier, with potential systemic and distal signaling and immune consequences. Biomarkers are measurable indicators of some biological state or conditions [15]. In this review article we describe some biomarkers which are used in brain tumor. Such as in case of leptomeningeal metastases we can use LD4, Beta-glucuronidase and Beta-2-microglobulin [12,16]. Urinary biomarkers also predict brain tumor presence and response to therapy. Recent studies confirmed and support the premise that tumor stage and progression correlate with urinary levels of MMPs [17]. Urinary levels of MMP-2 (gelatinase A) and MMP-9 (gelatinase B), and their complexes are elevated in patients with a variety of cancers, both organ confined and metastatic, both within and outside the urogenital tract.
These studies were the first to suggest that the measurement MMPs and related biomarkers in the urine of affected patients might represent a novel, noninvasive method of detecting disease status, progression, and therapeutic efficacy [17]. Given that [1]. MMPs are present in brain tumors and [2]. that urinary MMPs have shown utility as noninvasive biomarkers for non-central nervous system cancers, we initiated this study to determine whether urinary MMPs might have potential as noninvasive biomarkers to detect the presence of brain tumors. Glioblastoma tumor cells release micro vesicles (exosomes) containing mRNA, micro RNA and angiogenic proteins. These micro vesicles are taken up by normal host cells, such as brain microvascular endothelial cells. By incorporating an mRNA for a reporter protein into these micro vesicles, we demonstrate that messages delivered by micro vesicles are translated by recipient cells.
These micro vesicles are also enriched in angiogenic proteins and stimulate tubule formation by endothelial cells. Tumorderived micro vesicles therefore serve as a means of delivering genetic information and proteins to recipient cells in the tumor environment. Glioblastoma microvesicles also stimulated proliferation of a human glioma cell line, indicating a self-promoting aspect. Messenger RNA mutant/variants and miRNAs characteristic of gliomas could be detected in serum microvesicles of glioblastoma patients. The tumour-specific (epidermal growth factor receptor) EGFRvIII was detected in serum microvesicles from 7 out of 25 glioblastoma patients. Thus, tumour-derived microvesicles may provide diagnostic information and aid in therapeutic decisions for cancer patients through a blood test [18] (Table 1).
Techniques
Two-dimensional polyacrylamide gel electrophoresis (2D PAGE)
It is a widely used to study protein expression profiles with a high resolution for the separation of complex protein mixtures [12]. The identification of proteins separated by 2D PAGE has improved over the last decade due to advances in matrix-assisted laser desorption/ionization time-of-flight-mass spectrometry (MALDI-TOF-MS) [13,14]. The combination of the 2 techniques has become a powerful tool in protein expression analysis in all fields of life science [19]. Although 2D PAGE and subsequent analysis by MALDI-TOF-MS have successfully been used for the identification of proteins in CSF [20] and in CSF of patients with neurological diseases [21], these techniques have not yet been applied to search for brain tumor-related proteins in CSF. In the present study, the CSF protein profiles of 12 patients with primary brain tumors (i.e. 2 oligodendrogliomas, 3 anaplastic oligodendrogliomas, 2 anaplastic oligoastrocytomas, 2 pilocyticastrocytomas, 1 glioblastoma, and 2 medulloblastomas) were compared with those of normal controls. The presence of the identified proteins was further confirmed by immunohistochemistry on tissue sections from the tumors of the patients from whom the CSF samples were obtained.
MALDI-TOF-MS
Matrix Assisted Laser Desorption/Ionisation is a soft ionization technique used in spectrometry, allowing to analysis the biomolecules like DNA, protein, peptides. Biomolecules and synthetic polymers have low volatility and are thermally unstable, which has limited the use of MS as a means of characterization. These problems have been minimized through the development of MALDI-TOF MS, which allows for the mass determination of biomolecules by ionization and vaporization without degradation, a Laser beam used to ionize the sample. Protein sample have been characterized by HPLC or SDS PAGE by generating peptide maps. These peptide maps have been used as fingerprints of protein or as a tool to know the purity of a known protein in a known sample. Mass spectrometry gives a peptide map when proteins are digested with proteolytic enzymes like trypsin. This peptide map can be used to search a sequence database to find a good match from the existing database [22].
Conclusion
The growth in proteomics over the past decade has been driven by the pursuit of proteome-based information from the vast FFPE archives which exist around the world. This is largely a result of the possibility for combining proteomics with mining of the patient meta-data associated with the archived samples, which can include disease course and patient outcome. These kinds of studies would be hugely beneficial to the field, as it would no longer be difficult to assemble large retrospective cohorts. Quantitative proteomics still requires standardization to overcome variability in protein extraction and fractionation results. Quantitative proteomic studies have shown a greater level of success, most likely due to the combination with other modern techniques. It is this success which has led to other quantitative studies. As discussed in this review, researchers identified potential prognostic tumour markers using quantitative proteomics. As evidenced by this review, about proteomics and other diagnostic techniques for brain tumor archives are an incredibly valuable resource and will invariably form a significant component of large-scale retrospective studies in future [23-45] (Table 1).
References
- Levine AJ, Puzio-Kuter AM (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330(6009): 1340-1344.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5): 646-674.
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. (1998) Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394(6692): 485-490.
- Vaupel P (2004) The role of hypoxia-induced factors in tumor progression. Oncologist 9(5): 10-17.
- Gustafsson OJ, Arentz G, Hoffmann P (2015) Proteomic developments in the analysis of formalin-fixed tissue. Biochim Biophys Acta 1854(6): 559-580.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2): 97-109.
- Ohgaki H, Kleihues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64(6): 479-489.
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, et al. (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21(21): 2683-2710.
- Sanai N, Chang S, Berger MS (2011) Low-grade gliomas in adults: a review. J Neurosurg 115(5): 1-18.
- Gzell C, Wheeler H, Guo L, Kastelan M, Back M (2014) Employment following chemoradiotherapy in glioblastoma: A prospective case series. J Cancer Surviv 8(1): 108-113.
- Barbosa EB, Vidotto A, Polachini GM, Henrique T, de Marqui ABT, et al. (2012) Proteomics: methodologies and applications to the study of human diseases. Rev Assoc Med Bras 58(3): 366-375.
- Klose J (1975) Protein mapping by combined isoelectric focusing and electrophoresis of mouse tissues. Humangenetik 26(3): 231-243.
- Mann M, Talbo G (1996) Developments in matrix-assisted laser desorption/ionization peptide mass spectrometry. Curr Opin Biotechnol 7(1): 11-19.
- Siuzdak G (1994) The emergence of mass spectrometry in biochemical research. Proceedings of the National Academy of Sciences 91(24): 11290-11297.
- Yang Y, Zhao S, Zhao C, Lei C (2015) A Bio-network based pathway extension approach for cancer prognosis.
- Patterson SD (2000) Mass spectrometry and proteomics. Physiological Genomics 2(2): 59-65.
- Smith ER, Zurakowski D, Saad A, Scott RM, Moses MA (2008) Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res 14(8): 2378-2386.
- Skog J, Würdinger T, Van Rijn S, Meijer DH, Gainche L, et al. (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10(12): 1470-1476.
- Bonk T, Humeny A (2001) MALDI-TOF-MS analysis of protein and DNA. Neuroscientist 7(1): 6-12.
- Woitalla D, Kuhn W, Meyer HE (2000) Identification of proteins from human cerebrospinal fluid, separated by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 21(13): 2721- 2728.
- Rohlff C (2000) Proteomics in molecular medicine: applications in central nervous systems disorders. Electrophoresis 21(6): 1227- 1234.
- Wu KJ, Shaler TA, Becker CH (1994) Time-of-flight mass spectrometry of underivatized single-stranded DNA oligomers by matrix-assisted laser desorption. Anal Chem 66(10): 1637-1645.
- Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, et al. (2016) Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol 11(11): 1962-1969.
- Gajjar A, Fouladi M, Walter AW, Thompson SJ, Reardon DA, et al. (1999) Comparison of lumbar and shunt cerebrospinal fluid specimens for cytologic detection of leptomeningeal disease in pediatric patients with brain tumors. J Clin Oncol 17(6): 1825-1825.
- Weston CL, Glantz MJ, Connor JR (2011) Detection of cancer cells in the cerebrospinal fluid: current methods and future directions. Fluids Barriers CNS 8(1): 14.
- Samuel N, Remke M, Rutka JT, Raught B, Malkin D (2014) Proteomic analyses of CSF aimed at biomarker development for pediatric brain tumors. J Neurooncol 118(2): 225-238.
- Roy S, Josephson SA, Fridlyand J, Karch J, Kadoch C, et al. (2008) Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol 26(1): 96-105.
- Zetterberg H, Andreasson U, Blennow K (2009) CSF antithrombin III and disruption of the blood-brain barrier. J Clin Oncol 27(13): 2302- 2303.
- Murase S, Saio M, Andoh H, Takenaka K, Shinodaa J, et al. (2000) Diagnostic utility of CSF soluble CD27 for primary central nervous system lymphoma in immunocompetent patient. Neurol Res 22(5): 434-442.
- Pantazis G, Psaras T, Krope K, von Coelln R, Fend F, et al. (2009) Cerebral low-grade lymphoma and light chain deposition disease: exceedingly high IgG levels in the cerebrospinal fluid as a diagnostic clue. Clin Neuropathol 29(6): 378-383.
- Yang H, Cai L, Zhang Y, Tan H, Deng Q, et al. (2014) Sensitive detection of EGFR mutations in cerebrospinal fluid from lung adenocarcinoma patients with brain metastases. J Mol Diagn 16(5): 558-563.
- Groves MD, Hess KR, Puduvalli VK, Colman H, Conrad CA, et al. (2009) Biomarkers of disease: cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J Neurooncol 94(2): 229-234.
- Rajagopal MU, Hathout Y, MacDonald TJ, Kieran MW, Gururangan S, et al. (2011) Proteomic profiling of cerebrospinal fluid identifies prostaglandin D2 synthase as a putative biomarker for pediatric medulloblastoma: A pediatric brain tumor consortium study. Proteomics11(5): 935-943.
- Wang P, Piao Y, Zhang X, Li W, Hao X (2013) The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer Biomark 13(2): 123-130.
- Shnaper S, Desbaillets I, Brown DA, Murat A, Migliavacca E, et al. (2009) Elevated levels of MIC‐1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int J Cancer 125(11): 2624-2630.
- Baraniskin A, Kuhnhenn J, Schlegel U, Maghnouj A, Zöllner H, et al. (2011) Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol 14(1): 29-33.
- Teplyuk NM, Mollenhauer B, Gabriely G, Giese A, Kim E, et al. (2012) MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol 14(6): 689-700.
- Shi R, Wang PY, Li XY, Chen JX, Li Y, et al. (2015) Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget 6(29): 26971- 26981.
- Shalaby T, Achini F, Grotzer MA (2016) Targeting cerebrospinal fluid for discovery of brain cancer biomarkers. Journal of Cancer Metastasis and Treatment 2: 176-187.
- Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, et al. (2012) Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol 30(21): 2641-2647.
- Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, et al. (2009) Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 23(5): 1541-1557.
- Rasheed BA, Stenzel TT, McLendon RE, Parsons R, Friedman AH, et al. (1997) PTEN gene mutations are seen in high-grade but not in lowgrade gliomas. Cancer Res 57(19): 4187-4190.
- Rubenstein JL, Wong VS, Kadoch C, Gao HX, Barajas R, et al. (2013) CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 121(23): 4740-4748.
- Ty AU, See SJ, Rao JP, Khoo JB, Wong MC (2006) Oligodendroglial tumor chemotherapy using decreased-dose-intensity PCV: a Singapore experience. Neurology 66(2): 247-249.
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, et al. (2009) IDH1 and IDH2 Mutations in Gliomas. New England Journal of Medicine 360(8): 765-773.






























