Extraction of Nickel from Spent Catalyst of Primary Reformer
Mohamed Ahmed Fouad Mohamed Gaber*
Department of Chemical Engineering, Alexandria University, Egypt
Submission: March 15, 2019; Published: March 28, 2019
*Corresponding author: Mohamed Ahmed Fouad Mohamed Gaber, Chemical Engineering Department, Alexandria University, Egypt
How to cite this article: Mohamed Ahmed Fouad Mohamed Gaber. Extraction of Nickel from Spent Catalyst of Primary Reformer. Recent Adv Petrochem Sci. 2018; 6(2): 555690. DOI: 10.19080/RAPSCI.2019.06.555690
Abstract
Large quantities of spent catalysts are available from petrochemical industry and fertilizer industry. Disposal of spent catalyst is a problem as it falls under the category of hazardous industrial waste due to its concentration. Most of these catalysts are usually supported on alumina containing a variable percentage of elements such as nickel. Hence these catalysts contain environmentally critical and economically valuable metals. In this paper, a spent primary reformer catalyst was treated with Ethylenedi-aminetetracetic acid (EDTA). Parameters such as time, temperature, EDTA concentration, solid-liquid ratio and particle size are studied to investigate leaching rate of nickel. The results indicated that the increase in the leaching Extraction of 92% of nickel was achieved form of NiAlO4 catalystat optimum reaction conditions.
Keywords: Spent Nickel Catalyst, Leaching Process, EDTA as leaching agent
Abbrevations: EDTA: Ethylenedi-aminetetracetic acid; AAS: Atomic Absorption Spectrophotometer
Introduction
Increasing demand for nickel requires further intensive studies of its extraction methods from low-concentration ores and secondary resources. Extraction of nickel can be effectively performed from spent catalysts. Recycling of spent catalysts has become an unavoidable task not only to lower their costs but also to reduce the catalysts waste in order to prevent the environmental pollution. Nickel is widely used as a catalyst in several technological processes: in hydrogenation, hydro-desulphurization, and hydrorefin [1,2].
Heterogeneous supported nickel catalysts are commonly used for various industrial processes such as hydrogenation reactions, hydro-treating, steam reforming, carbon mono-oxide conversion methanation and ammonia synthesis [3]. Nickel is cheap and sufficiently active, and allows suitable catalysts to be economically produced. These catalysts deactivate over time, and when the activity of a catalyst declines, the catalyst has to be discharged. Fresh catalyst is required to be loaded in the reactors and regeneration of the catalyst to reuse is difficult especially in the case of primary and secondary reformer because of harsh conditions. Also spent catalysts contribute a significant amount of the solid wastes generated in the chemicals, petrochemicals and refineries plants. The dumping of catalysts in landfills is unacceptable environmentally, as the metals present in the catalysts can be leached into the groundwater, resulting in an environmental catastrophe. Also, the recovery of these metals can save the depletion of raw materialsworldwide and it can also promote sustainable development. The metal recovery from nickel catalyst is also extremely important from an economic point of view, as these metals command a significant price in the market. Market price of nickel metal equal to 40,000$/ton, so recovery and resale of nickel from spent catalyst is profitable. Finally, in many countries all over the world, companies are being forced to process their waste, side products, residues and spent catalyst [4,5].
The main goals of this research are to achieve the optimum metal value recovery, to recycle metals into ingots which can be directly sold, and to achieve a zero waste solution. Leaching is the removal of materials by dissolving them away from solids. It is a chemical process used by various industries and the process is usually called extraction, when organic solvents are often used. The overall recovery of the metal and the difficulty of separating it from impurity metals are generally governed by the efficiency and selectivity of the leaching process [6-8.
Deactivation of Nickel Catalystse
In general, the mechanisms of solid catalyst deactivation can be grouped into six intrinsic mechanisms of catalyst decay:
a) poisoning
b) fouling
c) thermal degradation
d) vapor compound formation and/or leaching accompanied by transport from the catalyst surface or particle
e) vapor-solid and/or solid-solid reactions
f) attrition/crushing. As mechanisms 1, 4, and 5 are chemical in nature while 2 and 5 are mechanical, the causes of deactivation are basically threefold: chemical, mechanical, and thermal [9].
The steam-reforming process for reforming natural gas and to produce hydrogen for ammonia or methanol production require that the catalysts be replaced after every 5 years in service, although the life of catalysts has extended to 10 years according to plant operation conditions and plant capacity. The quantity of spent catalysts discharged from different processing units depends largely on the amount of fresh catalysts used, their
Leaching
Leaching require the maximum solubilization of the sample in an appropriate medium for the other central step, the separationof solubilized elements. For this purpose, it is necessary to pretreat the sample in order to remove coke and other volatile species present. This step “cleans” the catalyst surface, thus reducing losses of recoverable metals by physical blocking. Care must be taken to avoid catalyst ignition during pre-treatment, thus forming refractory oxides that are difficult to solubilize in the leaching medium [9].
Material and methodology
Material
The tested spent catalyst is a steam reforming catalyst consisting in (30% Ni, 68% Al2O3, and 2% CaO). The catalyst has been sampled from an industrial ammonia plant after about 6 years in operation. EDTA used as chelation agent at different concentration and sulfuric acid used as de-chelation agent. The catalyst average sample was grounded, milled and sorted, the granules having the range of 90-105μ. Chelation temperature had adjusted from 90 0C to 95 0C. Speed of the mixer had adjusted from 900 to 1100rpm as shown in Figure 1. Concentration of the EDTA was ranged from 0.2M to 1M.
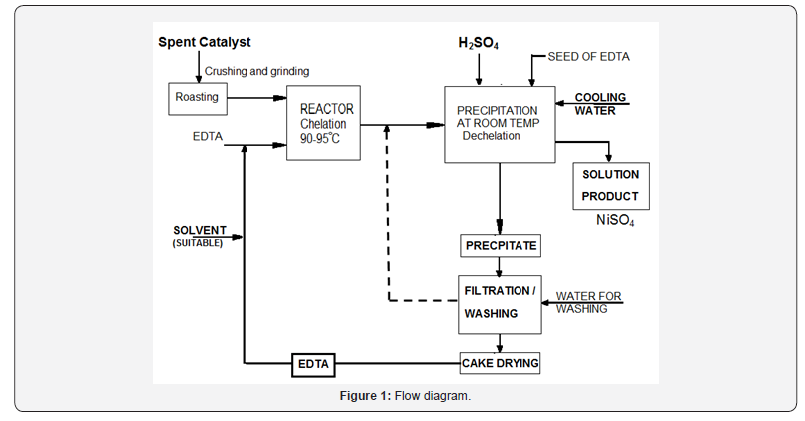
Method
The samples were ground and sieved using ASTM standard sieves. The solids were characterized using XRF, and XRD. The solutions were analyzed using atomic absorption spectrophotometer (AAS) for nickel.The dissolution process was carried out in a 10 liter reactor and the slurry was agitated by mixer. The reaction temperature was maintained constant during the dissolution process throughout electrical heater while the pH was continually controlled in the range between 8 and 10. The effect of EDTA concentration, time of digestion, particle size, solid–liquid ratio, stirring speedand temperature, multi stage chelation on the dissolution kinetics were investigated.
Acid Leaching Reaction Between Nickel Spent Catalyst and Sulfuric Acid
Material
In general, diffusion of reactants from the interface to the bulk of the second phase takes place. Further, chemical reactions between the reactants in phase one and those in phase two occur. Finally, the products diffuse within the second phase and/or out of phase two into the bulk of phase one. There has been report on the recovery of nickel from a spent catalyst used in an ammonia ormethanol plant by leaching in sulphuric acid. The main reactions taking place is as follow:

The side reaction taking placing is as follows:
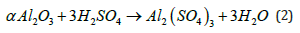
Method
The nickel was recovered as NiSO4. The rate of the side reaction is very slow since α-Al2O3 is inert towards acids [12,13].
Results and Discussion
Effect of concentration
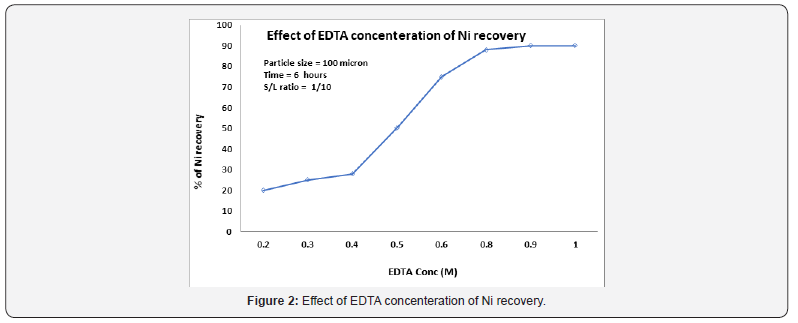
The effect of (EDTA) concentration on leaching of spent reformer catalyst was studied using different concentrations in the range 0.2M to 0.8M at a constant temperature equal to 25 °C; the results are shown in Figure 2. Figure 2 reveals that the recovery rate of nickel increases as the concentration of EDTA is increased from 0.2 to 0.8M. With 0.2M of EDTA the recovery of nickel was 25% compared with 92% which was achieved with 0.8M. An increasein EDTA concentration beyond 0.8M did not result in any further change in dissolution. For the experimental conditions tested, a maximum extraction of 92% was achieved with 0.8M EDTA. Due to at high (EDTA) concentrations cause a greater probability of collision between the molecules, thus 0.8M EDTA addition is considered to be the optimum.
Effect of particle size
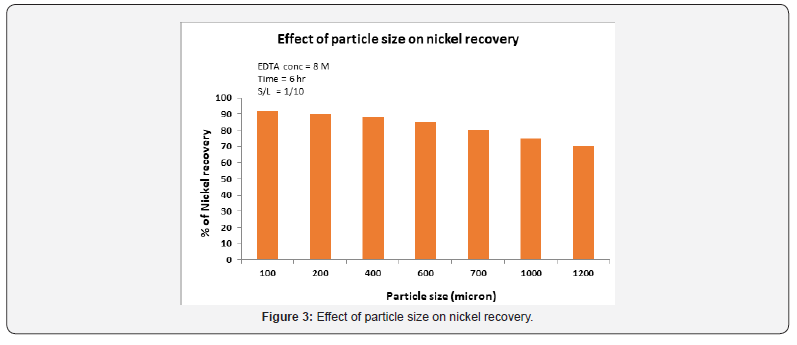
The effect of particle size on leaching of spent reformer catalyst was studied using different concentrations in the range of particle size from 100μm to 1200μm. Figure 3 shows that the nickel recovery rate is also dependent on particle size. The smaller the particle size, the greater the recovery rate of nickel. The increase in recovery rate with reduction in particle size is due to the increased surface area of the particles exposed to the leaching solution collectively. The finer the particle size was the greater geometric surface area and degree of contact surface between the EDTA- solution and the particles. Results show that an average of 92% of nickel was extracted from the size fraction which is an increase of 22% compare to the dissolution at 1200μm.
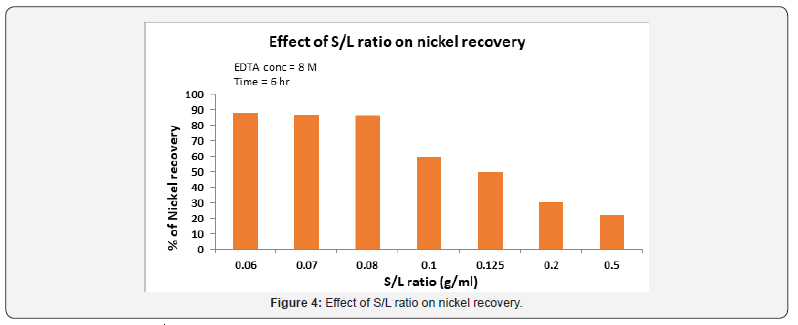
Effect of solid/liquid ratio
Figure 4 illustrates the influence of the pulp density on nickel extraction at 90 °C in 0.8M EDTA concentration after 6 hour leaching. The effect of solid/liquid ratio on the recovery rate is shownin Figure 4. For the ranges tested nickel recovery increases with increase in the amount of liquid in the same amount of the catalyst from 20% to 87% in the S/L ratio from 10/20(g/ml) to 10/160(g/ ml). Considering the process performance the results indicate S/ L=10/120 0r 0.08 as the optimum solid percent.
Effect of stirring speed
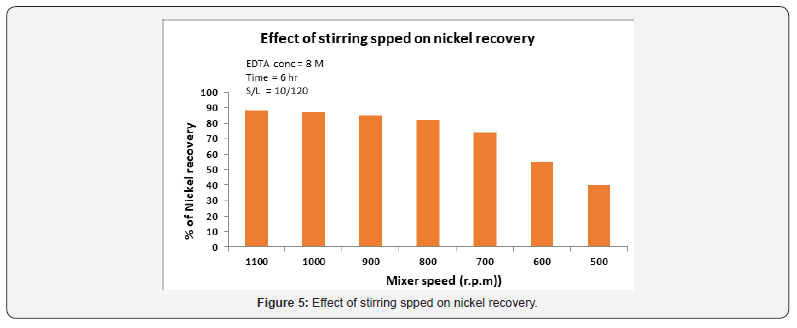
Figure 5 illustrates the influence of the stirring speed on the nickel extraction from the spent catalysts was investigated in the solution containing 0.8M EDTA concentration at 90 °C. The variation of the stirring speed within the range of 100-500rev/min had no effect on the reaction rate. This indicates that the reactant diffusion from the solution to ward the surface of particles, as well as the reaction products away from the particles to the solution occurs quickly and hence does not control the leaching rate within the range of the stirring speed tested. All the subsequent experiments were carried out at a stirring speed of 600-1100rev/min to assure invariance of this parameter. The rate of nickel recovery increases with an increase in stirring speed. Recovery increased from 40% to 88% as the stirring speed was increased from 600 to 1100rpm. Any further increase in speed of stirring beyond1100rpm did not have an pivotal influence. This indicates that at 1100rpm stirring speed, there is an adequate suspension of the solid particles in the solution; hence, rate of 900rpm was used in all experiments.
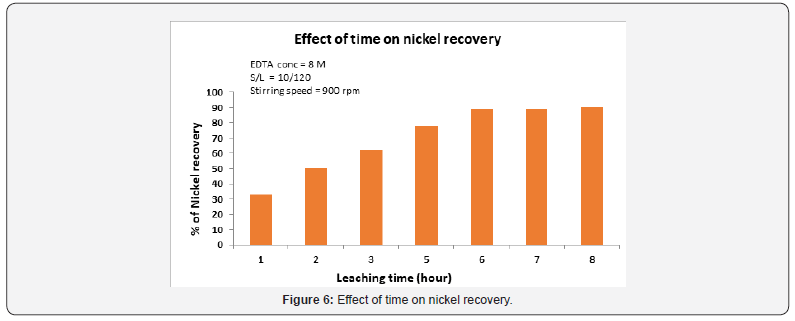
Effect of time of digestion on nickel recovery
A spent catalyst with 0.8M EDTA concentration, during a period of time from 1 to 6 hour, at a constant temperature of 90 °C was carried out as shown in Figure 6. The time has a significant effect on the dissolution of nickel. After 5 hours of leaching time, 70% of nickel was extracted. A further increase of time had no remarkable influence on nickel recovery rate which may be attributed to the gradual decrease of surface area of the reactant
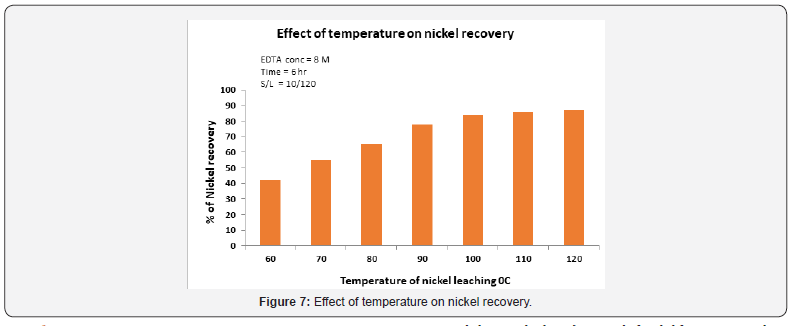
Effect of temperature on the nickel leaching
Temperature plays apivotal role in the recovery rate of nickel. Temperatures ranged from 60 to 110 °C as shown in Figure 7. The nickel recovery increased from 40% at 60 °C to 92% at 95 °C afterleaching for 6 hours. The major reason of this is that increases in the reaction velocity, with increases of the reaction temperature. The leaching at 90 °C could provide better recovery of nickel if the process was critically controlled as the experiment was happening under atmospheric pressure.
Conclusion
The dissolution of nickel increases with increase in temperature, leaching time, particle size and EDTA concentration. The decrease in the particle size and solid to liquid ratio increased nickel recovery. The extraction rate increased with increasing stirring speed and it was the highest at 1100rpm. The maximum leaching degree was 92% at 95 °C after 6 hour.
References
- Flue gas Flow Patterns in Top-fired Steam Reforming Furnaces” Barry Fisher, Johnson Matthey Catalysts reference.
- Richardson J, Drucker R (1997) High Temperature Operation of Hydro desulphurisation Catalyst. AIChE Ammonia Safety Symposium, San Francisco, CA, USA.
- Nitrogen (1997) Ammonia, Methanol, Hydrogen, Carbon Monoxide: Modern production, Technologies. M Appl, p. 261.
- Oza R, Shah N, Patel S (2011) Removal of Nickel from Spent Catalysts using Ultrasonication Assisted Leaching. J Chem Technol Biotechnol 86(10): 1276-1281.
- Singh B (2009) Treatment of spent catalyst from the nitrogenous fertilizer industry - A review of the available methods of regeneration, recovery and disposal. J Hazard Mater 167(1-3): 24-37.
- Kolosnitsyn VS, Kosternova SP, Yapryntseva OA, Ivashchenko AA, Alekseev SV, et al. (2006) Recovery of nickel with sulfuric acid solutions from spent catalysts for steam conversion of methane. Russian J Appl Chem 79(4): 539-543.
- Ivascan S, Roman O (1975) Nickel recovery from spent catalyst. I Solvation process, Bu1 Inst Politeh Iasi Sect 22: 47-51.
- Chaudhary AJ, Donaldson JD, Boddington SC, Grimes SG (1993) Heavy metals in environment: part II. A hydrochloric acid
leaching process for the recovery of nickel value from a spent catalyst. Hydrometall 34(2): 137-150.
- Bosio V, Vierra M, Donati E (2008) Integrated bacterial process for the treatment of a spent nickel catalyst. J Hazard Mater 154(1-3): 804-810.
- LaRue T (1988) AMAX Port Nickel -A New Dimension in Reclaiming Spent Catalysts. Spring AIChE National Meeting, mar.
- Sinka G, Vigvari M, Koracsi G, Legal T, Gyalasi I, et al. (1988) Recovery of Ni from spent catalyst. Hung Teljes HU 46: 556.
- Al-Mansi NM, Abdel Monem NM (2002) Recovery of nickel oxide from spent catalyst. Waste Manage 22(1): 85-90.
- Gavrila L, Asaftei S, Ivascan S (1995) Capitalization possibilities of spent catalysts with low nickel content, Conferinta de ChimiesiInginerie Chimica, “Politehnica”. University Bucharest 1(I): 230-235.






























