Partial Nitrification to Nitrite using Activated Sludge Entrapped in Polymerized Gel: Continuous and Batch Operation in an Airlifting Reactor
Lina Chi1, Xiaofeng Ye2*, Jun Li3, Zhirong Li1, Zheng Jiang4 and Zhenjia Zhang1*
1School of Environmental Science and Engineering, Shanghai Jiao Tong University, China
2Novozymes (China) Investment Co., Ltd., China
3School of Municipal and Environmental Engineering, Shenyang Jianzhu University, China
4Faculty of Engineering and the Environment, University of Southampton, UK
Submission: October 02, 2017; Published: November 30, 2017
*Corresponding author: Zhengjia Zhang, School of Environmental Science and Engineering, Shanghai Jiaotong University, China. Email:zjzhang@ sjtu.edu.cn; Xiaofeng Ye, Novozymes (China) Investment Co., Ltd., Email: xiaofengyip@gmail.com
How to cite this article: Lina C, Xiaofeng Y, Jun L, Zhirong L, Zheng J , et al. Partial Nitrification to Nitrite using Activated Sludge Entrapped in Polymerized Gel: Continuous and Batch Operation in an Airlifting Reactor. Recent Adv Petrochem Sci. 2017;4(1): 555626. DOI:10.19080/RAPSCI.2017.04.555626
Abstract
Partial nitrification to nitrite using activated sludge entrapped in polymerized gel was investigated in an air-lifting reactor in both continuous and batch operations. High-rate partial nitrification to nitrite was achieved under non-limiting dissolved oxygen conditions during the start-up of the reactor. After the stable operation was established, HRT was optimized to achieve the effective ammonium conversion to nitrite and provide the effluent suitable potential for anaerobic ammonium oxidization (ANAMOX). The influences of process parameters, including dissolved oxygen concentrations, temperatures, and initial NH4+-N conditions, on partial nitrification kinetics were studied in batch operation. Data of the batch experiments indicate that it is feasible to achieve 95% ammonium removal with dominant nitrite accumulation under non-limiting DO conditions at the temperature of 24 °C or higher Reaction time affected the nitrite accumulation and the NH4+-N/NO2--N ratio in batch operation. These data demonstrate the potential of using immobilized activated sludge for partial nitrification in continuous and sequencing batch reactors and its applicability as the pretreatment for anaerobic ammonium oxidization process.
Keywords: Partial nitrification; Nitrite; Activated sludge entrapped in polymerized gel; HRT; Dissolved oxygen
Highlights
A. High rates oxidization of ammonium to nitrite under non-limiting bulk DO conditions
B. Optimization of operational parameters for effective partial nitrification
C. Dominant nitrite accumulation when 95% of ammonium was removed
D. Effective nitrification under low temperature of 8 °C
E. Effluent suitable for the ANAMOX by controlling the HRT and the batch reaction time
Introduction
Due to increasingly stringent effluent discharge requirement, population growth, and the growing desire to reuse wastewater, wastewater treatment plants are expanding all over the world. However, in many countries, especially in metropolitan areas, limited space is a major challenge for the expansion projects [1]. Various immobilized cell technologies have been developed to address this challenge with minimal additional footprint such as moving bed bioreactors and integrated fixed film in activated sludge process [2-4]. The gel-immobilized activated sludge process is one of the newly developed immobilized cell technologies that has been previously demonstrated to achieve high rates degradation of COD and nitrification in over 11 wastewater treatment plants in Japan and the USA [5-7]. Gel-immobilized biomass can be directly added to aeration basins with additional screens to keep the gel pellets inside the tank [5]. A number of advantages are associated with immobilized-cell treatment systems, including small footprint, high biomass concentration, reduction of excess sludge production, and dissociation of solid and liquid retention times [5,6].
Immobilized activated sludge has potential in nitrification processes due to the retention of biomass, which can overcome the relatively slower growth rates for nitrifying autotrophs [8]. Biological ammonia oxidation involves two steps: ammonia conversion to nitrite by ammonium oxidizing bacteria (AOB) and the subsequent nitrite conversion to nitrate by nitrite oxidizing bacteria (NOB) [9]. In most cases nitrite seldom accumulates in the environment due to a low Smin . (minimum substrate concentration capable of supporting steady-state biomass) value and relatively higher substrate utilization rates of nitrite oxidizers [9,10]. Partial nitrification to nitrite has been attracting increasing attention, not only due to its potential saving of oxygen consumption and electron donor requirement in subsequent denitrifying process, but also because it is the prerequisite for anaerobic ammonium oxidization processes [11-14]. Partial nitrification has been suggested to be the rate limiting step for the combined partial nitrification-ANAMOX process to remove nitrogen via nitrite [15].
Partial nitrification to nitrite can be achieved by optimizing process parameters, including HRT, dissolved oxygen, temperature and influent ammonium-nitrogen concentration [10,16]. Limiting the dissolved oxygen concentration has been reported to be effective in accumulating nitrite, since the oxygen saturation coefficient of Monod kinetics was significantly lower for ammonium oxidization than that for nitrite oxidization [16-18]. However, DO limitations could potentially sacrifice the overall kinetics of nitrification [19]. Partial nitrification to nitrite has been previously reported at relatively higher temperatures, since AOB have higher growth rate than NOB at elevated temperature (>15 0C) (Sinha & Annachhatre [20]); however, limited information is available on the influence of temperature on the nitrite accumulation of immobilized activated sludge system.
In this study, we investigated the partial nitrification performance of immobilized activated sludge in the air-lifting fluidized reactor in both continuous and batch operations. Highrate ammonium conversion to nitrite under non-DO limiting conditions was achieved during the start-up of the reactor. The influence of HRT on the ammonium oxidization rates and the nitrite accumulation was studied in continuous operation after the stable operation was established. Other factors including dissolved oxygen concentrations, temperatures and initial NH4+-N concentrations were investigated in batch operation to determine appropriate conditions for partial nitrification of immobilized activated sludge. The potential of using partial nitrification immobilized activated sludge process as the pretreatment of anaerobic ammonium oxidization (ANAMOX) is also discussed.
Materials and Methods
Experimental set-up
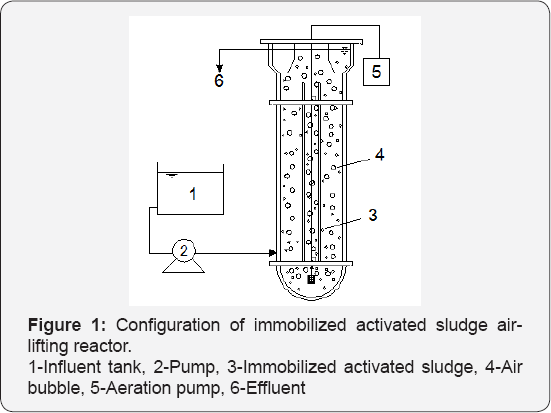
A schematic diagram of the experimental set-up is presented in Figure 1. The air-lifting reactor had a working volume of 18L with 8-10% (v/v) immobilized activated sludge. The air was supplied from the bottom through an air sparger to create inner circulation. Temperature within the reactor was measured on-line and maintained at different levels by a water jacket.
Waste water composition
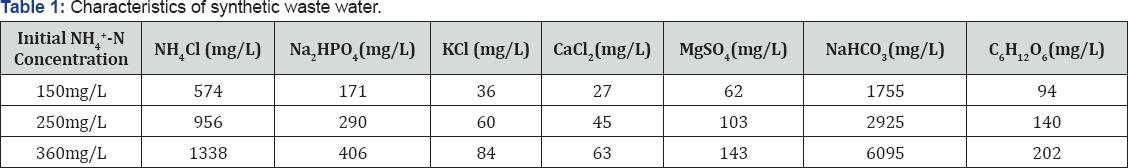
Synthetic wastewater was composed of NH4Cl as the nitrogen source and glucose as the carbon source. Other chemicals such as KCl, Na2HPO4, CaCl2, and MgSO4 were added as nutrients. NaHCO3 was also added as a buffering agent. The specific compositions of wastewater at different initial NH4+-N concentrations are shown in Table 1.
Immobilized activated sludge
Immobilized activated sludge was provided by Hitachi Plant Engineering and Construction Co. Ltd, Japan. Gel pellets were composed of 10% (w/v) PEG (Polyethylene glycerol), 0.5% (w/v) promoter (N,N,N',N'-tetramethylenediamine), 0.25% initiator (potassium per sulfate) and around 107-109 cells/ml- pellets (Hashimoto & Sumino [5]). The immobilization process started with dissolving the PEG polymer and the promoter in water, followed by adding activated sludge and the initiator The polymerized gel was then cut into 3*3*3mm cubes.
Acclimation and start-up of the reactor
Temperature was controlled at 28 ± 1 0C and DO was kept at 6.0 ± 0.5mg/L. Influent NH4+-N concentrations were increased step-wise from 100 to 300mg/L within 5 weeks. After the acclimation process, the reactor was then operated in continuous mode to develop nitrite accumulation. DO was kept at 5-6mg/L in the first stage of continuous operation and then lowered to 1-2mg/L to increase the nitrite accumulation in the second stage. After stable nitrite accumulation was established, dissolved oxygen was then increased to 3-4mg/L at the third stage. The influent NH4+-N concentration was maintained around 300mg/L; pH and temperature were controlled at 7.8 and 28 oC, respectively, during continuous operation.
Continuous experiments
HRT was controlled by adjusting the flow rates of the feed to the reactor. At each HRT, the reactor was operated for a minimum of two weeks to ensure that steady state was achieved; this was also demonstrated by a standard deviation of three consecutive samples of less than 5%.
Batch experiments
Batch experiments were conducted in the fluidized reactor after over one month of stable continuous operation. Experiments utilized synthetic wastewater with an initial NH4+-N concentration of about 250mg/L. DO concentrations and temperatures were varied to study their influences on ammonium oxidization kinetics and nitrite accumulation in the batch reactor. The influence of different initial NH4+-N concentrations on nitrification kinetics and nitrite accumulation was investigated by operating the reactor at pH=8.0, DO=4mg/L, and temperature at 24 oC pH variations in the ammonia oxidation process were minimal due to abundant buffering capacity in the influent.
Analytical methods
NH4+-N, NO2--N and NO3--N concentrations were measured daily by standard methods (APHA [21]). DO levels were measured twice per day with an oxygen electrode (ORION 830A, Thermo Orion company) pH and flow rates were monitored on a daily basis.
Results and Discussion
Acclimation and start-up of partial nitrification continuous reactor
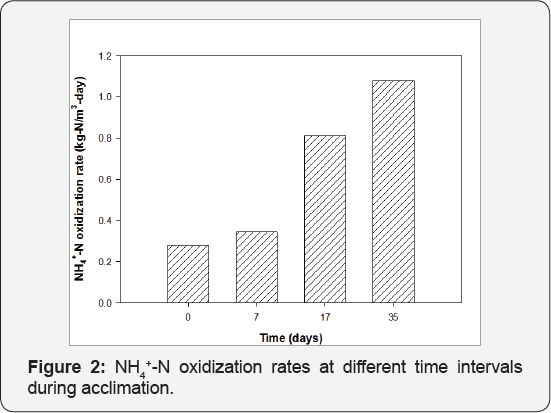
Acclimation of immobilized activated sludge: Immobilized activated sludge was stored under anaerobic conditions at 4 °C with little nutrients for 4 months due to international transportation. NH4+-N oxidization rates was 0.28kg-N/m3-dayafter long term anaerobic storage and increased to 1.08kg-N/m3-day at 35 days, 3.9 times the original rates prior to acclimation (Figure 2). These results suggest that the activity of immobilized activated sludge can be recovered within five weeks after long time storage under minimal nutrient and low temperature conditions.
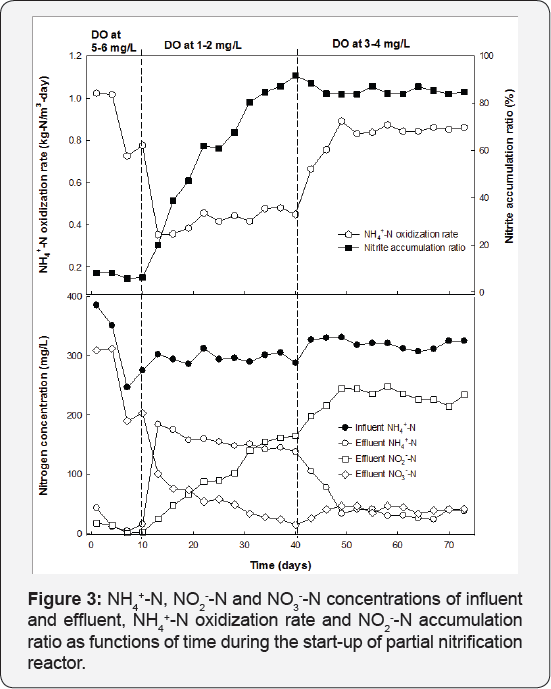
Start-up of partial nitrification continuous reactor: Figure 3 shows the NH4+-N concentrations in the influent and effluent and NO2--N accumulation ratio at different stages of the startup process. Immobilized activated sludge effectively converted NH4+-N to NO3_-N during stage one of continuous operation at dissolved oxygen concentration of 5-6mg/L. Between 5-7% of oxidization products were accumulated in the form of NO2--N at this time. The dissolved oxygen concentration was then decreased to 1-2mg/L during stage 2 to develop nitrite accumulation. NH4+-N oxidization rates dropped from 0.8 to 0.35-0.5kg-N/m3-day. Nitrite concentrations in the effluent concurrently increased to 160mg/L; the NO2--N accumulation ratio increased from below 10% at 10 days to over 90% at 40 days. Despite high nitrite accumulation, NH4+-N concentration stayed above 100mg/L in the effluent due to inefficient NH4+-N oxidization rates under oxygen- limited conditions. Thus, at the third stage, dissolved oxygen concentrations were further increased to 3-4mg/L to stimulate ammonium removal. NH4+-N oxidization rates were significantly improved from 0.3-0.5 to above 0.8kg-N/m3-day. Nitrite accumulation ratio decreased slightly but could be maintained above 80%.
Effective oxidization of NH4+-N to NO2--N was achieved in continuous operation with NH4+-N oxidization rates of 0.8-0.9kg- N/m3-day and over 80% of all products accumulated in the form of nitrite, even though the activated sludge immobilized inside the gel had not been pre-selected for ammonium oxidizing bacteria. Limiting DO concentrations has been previously demonstrated to be an effective strategy in accumulating nitrite in the nitrification process [10,18,22], which was successfully applied in the immobilized activated sludge system to achieve partial nitrification to nitrite in this study; however, the ammonium oxidization kinetics were also inhibited under DO-limitation conditions. Increasing dissolved oxygen concentrations from 0-1.5mg/L to 3-4mg/L improved the ammonium oxidization rates by approximately 60-70%, while only decreased NO2--N accumulation ratio by around 10% during stage 3. These data suggested that partial nitrification to nitrite was feasible in the immobilized activated sludge reactor under non-limiting dissolved oxygen conditions, which could be important to achieve dominant nitrite accumulation in the effluent while maintaining efficient oxidization of NH4+-N.
Influence of HRT on NH4+-N removal and NO2--N accumulation
NH4+-N removal efficiency was improved significantly, but the nitrite accumulation ratio declined as the HRT increased from 4 to 15 hours and from 6 to 19 hours for influent NH4+-N concentrations of 220-250mg/L (Figure 4a) and 320-360mg/L (Figure 4b), respectively. For influent NH4+-N concentrations of 220-250mg/L, only 50-80% NH4+-N removal was achieved when the HRT was less than 8 hours. Nitrite composed 80% of the oxidization products. Increasing the HRT to 8 hours improved NH4+-N removal to 85% without negatively influencing the NO2--N accumulation (83%). NH4+-N oxidation rates remained almost constant at 0.86-0.83kg- N/m3-day when the HRT was maintained at 4, 6 and 8 hours. Increasing HRT to 10 hours continuously improved the NH4+-N removal to over 98% but decreased the nitrite accumulation ratio to about 50%. Additional HRT increases did not change the NH4+-N removal yet significantly decreased the NO2--N accumulation ratio in the effluent and NH4+-N oxidation rates of the reactor. Similar trends were observed with the influent NH4+-N concentration of 320-360mg/L, except that it required around 2-4 hours longer of HRT to reach similar levels of NH4+-N removal and nitrite accumulation.
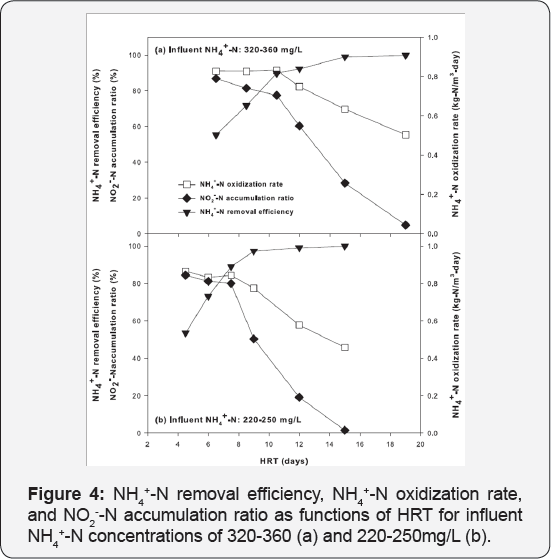
These results demonstrate the importance of HRT in achieving effective ammonium oxidization to nitrite using immobilized activated sludge. Running the reactor with a short hydraulic retention time could not effectively remove NH4+-N in the effluent despite high NH4+-N oxidization rates. When the HRT was too long, NH4+-N oxidization rates and nitrite accumulation were sacrificed; at full-scale, longer hydraulic residence times translate into larger effective volumes and larger footprints. In this study, the data showed that the HRT could be controlled at around 8 hours for influent NH4+-N of 220-250mg/L, and 10 hours for influent NH4+-N of 320-360mg/L to achieve high rates of ammonium removal (NH4+-N oxidization rates exceeded 0.8kg-N/m3-day) with about 80% oxidation products accumulated in the form of nitrite. Meanwhile, controlling the HRT in the partial nitrification immobilized activated sludge reactor could also potentially provide an effluent suitable for anaerobic ammonium oxidization processes. An NH4+-N/NO2--N ratio close to 1:1 was previously reported for optimal anaerobic ammonium oxidization process [6,15]. HRT can be controlled around 9 and 13 hours for influent NH4+-N concentrations of 220-250 and 320-360mg/L, respectively, to keep NH4+-N/NO2--N ratio close to 1:1 in the effluent.
Factors affecting the NH4+-N removal and NO2-- N accumulation
The influences of dissolved oxygen, temperature, and initial NH4+-N concentration on NH4+-N oxidization kinetics and NO2--N accumulation were investigated in batch operation and discussed as below.
Influence of dissolved oxygen on NH4+-N removal and NO2"-N accumulation: Figure 5 presents the concentrations of NH4+-N, NO2-- N and NO3-- N and the nitrite accumulation ratio over time under different DO conditions. When DO was controlled at 2mg/L, it took 13 hours until NH4+-N concentration reached below 10mg/L. Nitrite was the major oxidization product (levels exceeded 80%) until 13 hours, and was then oxidized to nitrate. Increasing DO to 4mg/L shortened the time from 13 hours to 6-7 hours for NH4+-N concentrations to decrease below 10mg/L. NO2--N composed around 60-80% of the oxidization products at 6-7 hours, and then was oxidized to NO3--N after the depletion of NH4+-N. When DO was controlled at 6mg/L, it took around 6-7 hours for NH4+-N concentration to reach below 10mg/L; however, the NO2-- N accumulation ratio was significantly lower than those at DO concentrations of 2 and 4mg/L.
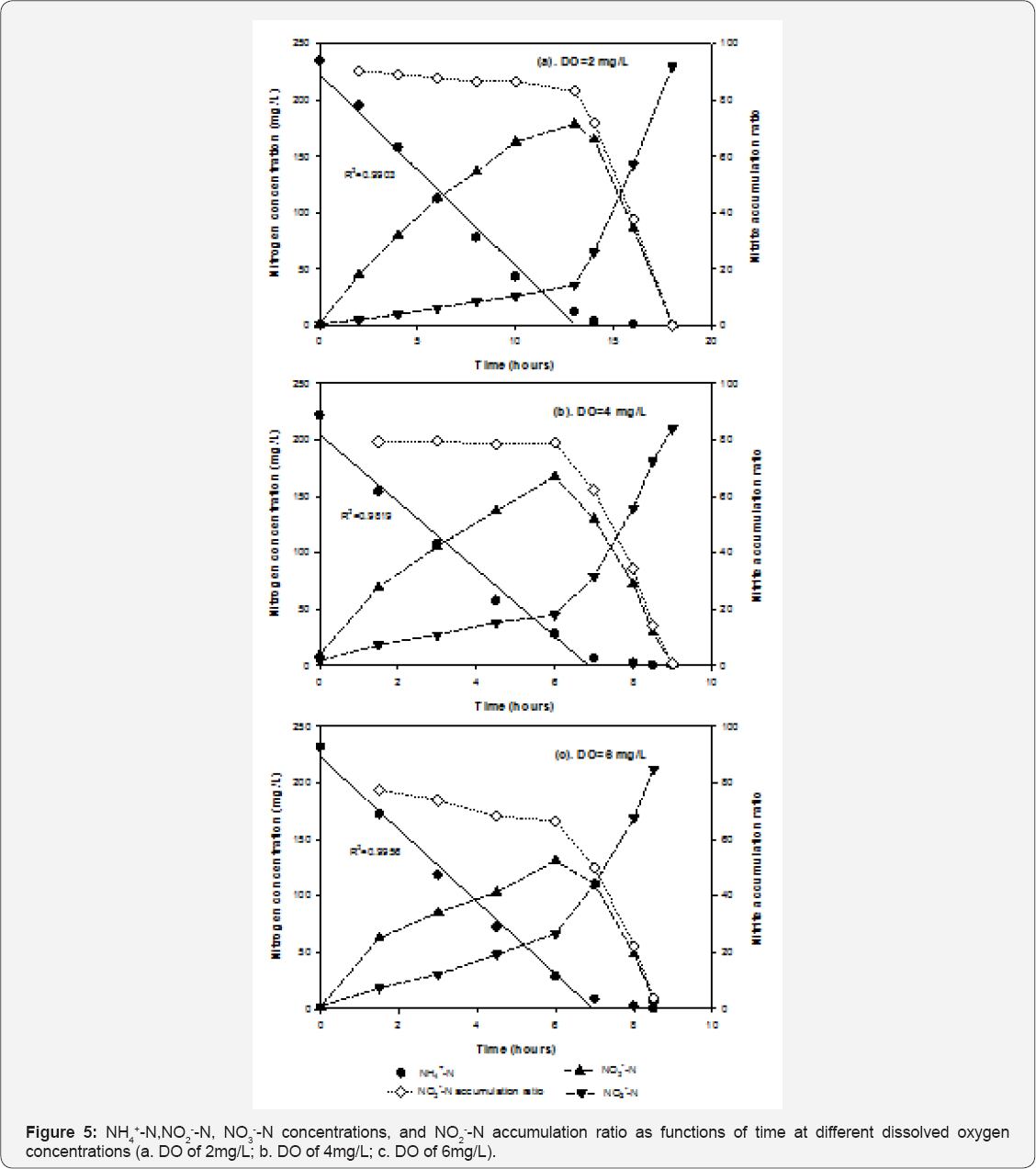
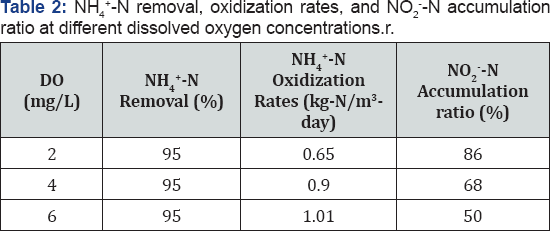
NH4+-N oxidation rates was obtained using a linear regression method to fit the first 6 data points of NH4+-N concentration. Since the values of coefficient determination (R2) in the regression were all above 0.98, the nitrification process of immobilized activated sludge virtually followed zero order kinetics. Table 2 summarizes the NH4+-N oxidization rates and nitrite accumulation ratios when NH4+-N removal reached above 95% (NH4+-N concentration<15mg/L). Increasing DO concentration from 2 to 4mg/L significantly improved the NH4+-N oxidization rates by 38%, suggesting that partial nitrification was limited by dissolved oxygen as a co-substrate. Even though the dissolved oxygen profile inside the PEG gel is not sufficiently understood, it is possible that increasing the bulk dissolved oxygen concentrations from 2 to 4mg/L was beneficial for bacteria encapsulated inside the gel to uptake more oxygen, which led to increased NH4+-N oxidization rates. The NO2--N accumulation ratio decreased from 87% to 80% and then to 66% when dissolved oxygen increased from 2 to 4 and then to 6mg/L, respectively. These results are consistent with several published results of bio film partial nitrification reactors (Okabe et al. [15]). Okabe et al. demonstrated that both nitrite and nitrate production rates increased with increased air flow rates, and nitrate production was not significant when air flow rate/ Ammonium loading rate was kept below 0.15 [(m3/day)/(kg-N/ m3-day)] (Okabe et al. [15]).
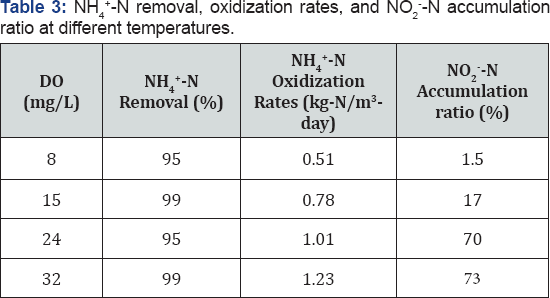
Influence of temperature on NH4+-N removal and NO2- -N accumulation: Figure 6 shows the concentrations of NH4+-N, NO2--N and NO3--N and the nitrite accumulation ratios over time under different temperature conditions. When temperature was controlled at 8 °C, 45% of the maximum oxidization products accumulated as nitrite for the first 5.5 hours with 50% removal of NH4+-N. Nitrite and ammonium were simultaneously oxidized to nitrate after 5.5 hours, and little nitrite accumulation was observed at the end of the batch experiment. Similar trends were observed at 15 °C. 42% of maximum oxidization products stayed in the form of nitrite at 7 hours with 81% NH4+-N removal. Increasing the temperature to 24 and 32 °C increased nitrite production. NO2- -N composed 75% of the oxidization products, with above 95% NH4+-N removal at 24 and 32 °C.
Table 3 summarizes the NH4+-N oxidization rates and NO2--N accumulation ratios when NH4+-N removal exceeded 95% (NH4+-N concentration<15mg/L) at different temperatures. Increasing temperature from 8 to 32 °C improved the NH4+-N oxidization rates by 139%. When temperatures were maintained at 8 and 15 °C, nitrite accumulation was not significant; less than 20% of oxidization products were in the form of nitrite when over 95% of NH4+-N removal was achieved. Due to slower growth rates of Nitrosomonas than Nitrobactor at relatively lower temperatures, it is difficult to achieve 95% ammonium oxidization to nitrite under lower temperatures [13,23]; however, at 8 °C immobilized activated sludge can oxidize NH4+-N at the rate of 0.5kgN/m3-day, which is higher than a number of reported results [10,24]. It is also possible to combine this process with an aerobic ammonium oxidization at 15 °C by controlling the batch reaction time at 6 hours, which gave a NH4+-N/NO2--N ratio of 1:1 in the reactor (Figure 6a) [6,15].
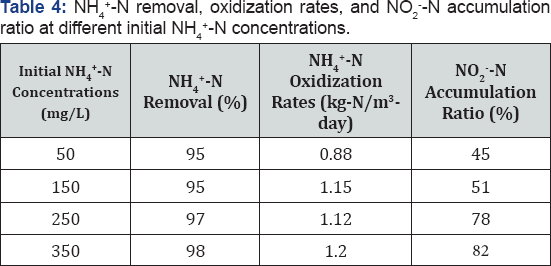
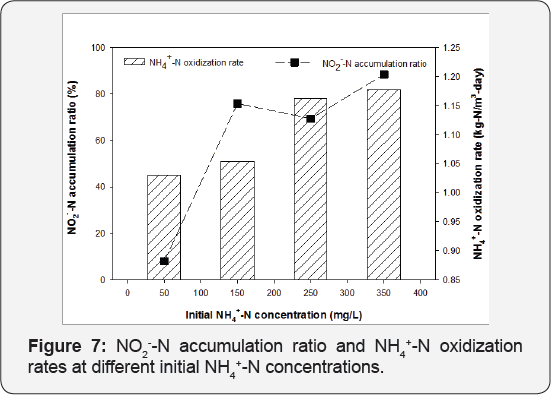
Influence of initial NH4+-N concentration on removal and NO2-- N accumulation: Table 4 presents the NH4+- Noxidization rates and NO2"-N accumulation ratios when NH4+- Nconcentration reached below 15mg/L for different influent NH4+-N concentrations, ranging from 50 to 350mg/L. Increasing the initial NH4+-N concentration from 50mg/L to 150mg/L increased the NH4+-N oxidization rates from 0.88 to 1.15kg- N/m3-day, which remained relatively constant as the initial NH4+-N concentration increased from 150 to 350mg/L. Nitrite accumulation was more significant at higher initial NH4+-N concentrations. When over 95% of ammonium removal was achieved, 78-82% of oxidization products stayed in the form of nitrite at initial NH4+-N concentrations of 250 and 350mg/L; while only 45-51% products accumulated as nitrite when initial NH4+-N concentrations were 50 and 150mg/L. These results suggested that effective partial nitrification to nitrite can be achieved with initial NH4+-N concentration exceeding 150mg/L in the immobilized activated sludge air-lifting reactor; this is consistent with previous findings that nitrite accumulation was more significant at higher initial NH4+-N conditions in suspended activated sludge system [10]. Increased NH4+-N concentrations resulted in higher free the activity of nitrite oxidizing bacteria more severely than that of ammonium concentrations, which have been reported to inhibit ammonium oxidizing bacteria (Antoniou et al. [25]).
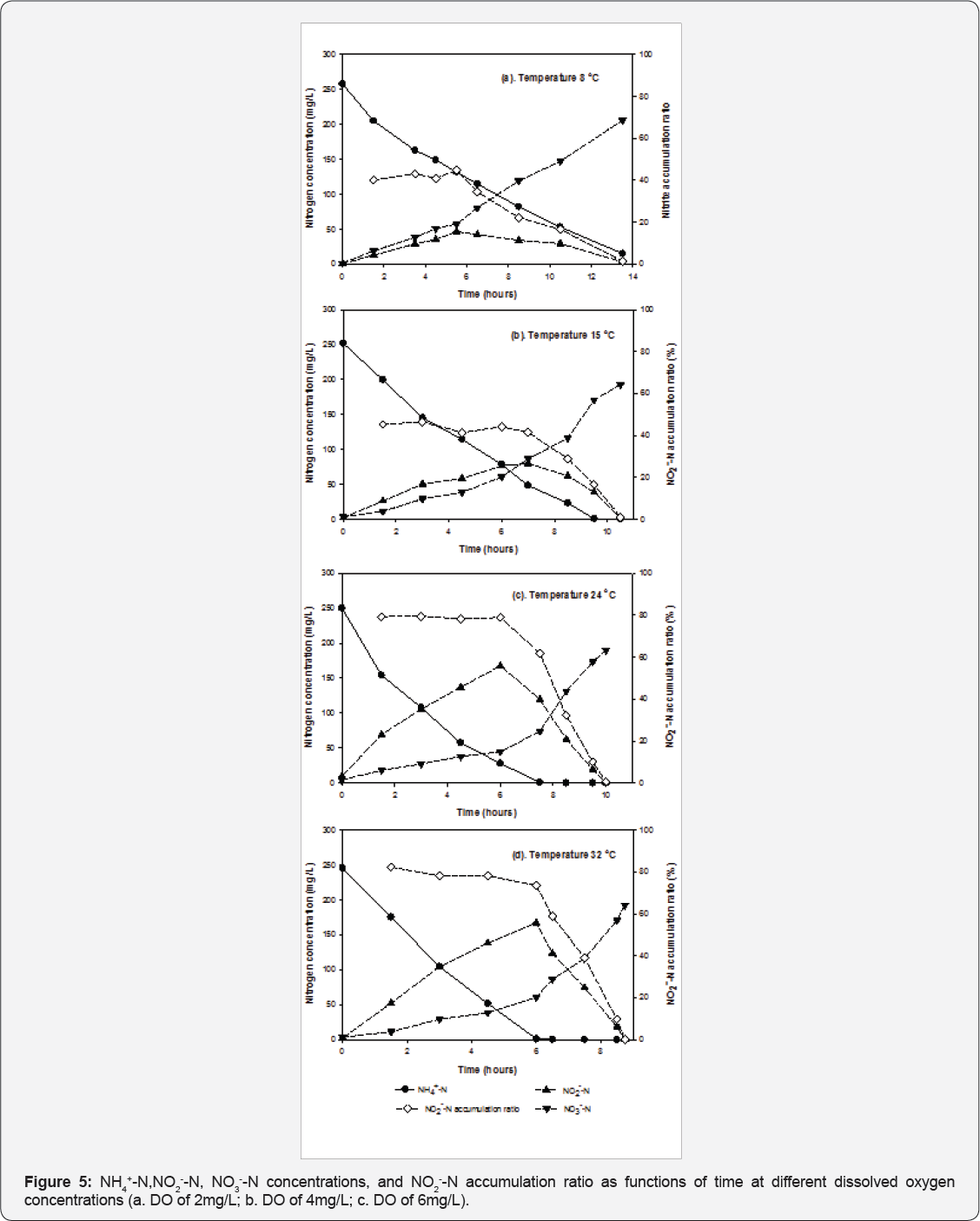
Practical implication of batch experiments: Batch experiments investigated could potentially provide relevant information for future utilization of immobilized activated sludge in sequencing batch reactors (SBR). It has been shown in this study that it is possible to keep effluent NH4+-N concentrations low (<15mg/L) with most oxidization products present as NO2--N by controlling the batch reaction time in SBR operations to treat high strength ammonium wastewater (initial NH4+-N concentration >150mg/L) when the temperature was above 15 °C. Dissolved oxygen concentrations maintained at 4mg/L maximized NH4+-N oxidization rates without affecting nitrite accumulation. For example, a batch aeration time of 7 hours with an influent NH4+- Nconcentration of 250mg/L, DO of 4mg/L, and temperature of 24 °C effectively removed ammonium to below 1mg/L with over 65% oxidization products accumulated in the form of NO2--N (Figure 6b).
In addition, it is promising to connect the immobilized activated sludge partial nitrification reactor with anaerobic ammonium oxidization processes. Effluent NH4+-N/NO2--N ratios can be maintained at 1:1 by optimizing the batch reaction time for different operating conditions. For example, the batch process can be operated for 3 hours to treat influent NH4+-N concentrations of 250mg/L at 24 °C to maintain a NH4+-N/NO2--N ratio of 1:1 with only 10% of total nitrogen in the form of nitrate in the effluent (Figure 6c). Even at low temperatures of 15 °C, it is possible to reach the ideal NH4+-N/NO2--N ratio of 1:1 in the effluent by extending the batch reaction time to 6 hours (Figure 7).
Conclusion
The potential of using immobilized activated sludge for partial nitrification to nitrite was investigated in an air-lifting reactor operated in both continuous and batch modes. We have successfully achieved partial nitrification to nitrite under non- DO limiting conditions, while maintaining high oxidization rates. Even though only 80% of oxidization products stayed in the form of nitrite when 95% of ammonium removal was achieved, the nitrification rates of close to 1kg-N/m3-day were achieved under non bulk dissolved oxygen limiting conditions. We also demonstrate that the immobilized activated sludge partial nitrification process could be potentially linked to anaerobic ammonium oxidization operated either continuously or as a batch reactor Since the ammonium conversion to nitrite was considered as the rate-limiting step for the Partial nitrification-ANAMOX process, the high rates conversion of ammonium to nitrite using immobilized activated sludge was of special interest to potentially debottleneck the kinetic limitation of partial nitrification process and promote the full-scale application of Partial nitrification- ANAMOX for nitrogen removal.
Acknowledgement
We acknowledge financial support from Shanghai Jiao Tong University, China and the Newton Research Collaboration Award from Royal Academy of Engineering, UK (Reference: NRCP/1415/261).
References
- Parker DS (2010) Introduction of New Process Technology into the Wastewater Treatment Sector. Proceedings of the Water Environment Federation pp. 1-30.
- Phattaranawik J, Leiknes T (2011) Extractive biofilm membrane bioreactor with energy recovery from excess aeration and new membrane fouling control. Bioresource Technology 102(3): 23012307.
- Regmi P, Thomas W, Schafran G, Bott C, Rutherford B, et al. (2011) Nitrogen removal assessment through nitrification rates and media biofilm accumulation in an IFAS process demonstration study. Water Research 45(20): 6699-6708.
- Thalla AK, Bhargava R, Kumar P (2010) Nitrification kinetics of activated sludge-biofilm system: A mathematical model. Bioresource Technology 101(15): 5827-5835.
- Hashimoto N, Sumino T (1998) Wastewater treatment using activated sludge entrapped in polyethylene glycol prepolymer. Journal of Fermentation and Bioengineering 86(4): 424-426.
- Isaka K, Date Y, Sumino T, Tsuneda S (2007) Ammonium removal performance of anaerobic ammonium-oxidizing bacteria immobilized in polyethylene glycol gel carrier. Appl Microbiol Biotechnol 76(6): 1457-1465.
- Tanaka K, Sumino T, Nakamura H, Ogasawara T, Emori H, et al. (1996) Application of nitrification by cells immobilized in polyethylene glycol. In: Wijffels RH, Tramper J (Eds.), In Progress in Biotechnology, RMBCB Elsevier, pp. 622-632.
- Qiao X, Zhang Z, Chen Q, Chen Y (2008) Nitrification characteristics of PEG immobilized activated sludge at high ammonia and COD loading rates. Desalination 222(1-3): 340-347.
- Rittmann BE, Mc Carty PL (2011) Environmental biotechnology: principles and applications, McGraw-Hill, New York, USA.
- Wang J, Yang N (2004) Partial nitrification under limited dissolved oxygen conditions. Process Biochemistry 39(10): 1223-1229.
- Ganigué R, Gabarró J, Sanchez-Melsió A, Ruscalleda M, López H, et al. (2009) Long-term operation of a partial nitritation pilot plant treating leachate with extremely high ammonium concentration prior to an anammox process. Bioresource Technology 100(23): 5624-5632.
- Hao X, Heijnen JJ, Loosdrecht VMCM (2002) Model-based evaluation of temperature and inflow variations on a partial nitrification-ANAMMOX biofilm process. Water Research 36(19): 4839-4849.
- Hellinga C, Schellen AAJC, Mulder JW, Loosdrecht VMCM, Heijnen JJ, et al. (1998) The SHARON process: An innovative method for nitrogen removal from ammonium-rich waste water. Water Science and Technology 37(9): 135-142.
- Yamamoto T, Takaki K, Koyama T, Furukawa K (2008) Long-term stability of partial nitritation of swine wastewater digester liquor and its subsequent treatment by Anammox. Bioresource Technology 99(14): 6419-6425.
- Okabe S, Oshiki M, Takahashi Y, Satoh H (2011) Development of longterm stable partial nitrification and subsequent anammox process. Bioresource Technology 102(13): 6801-6807.
- Brockmann D, Morgenroth E (2010) Evaluating operating conditions for outcompeting nitrite oxidizers and maintaining partial nitrification in biofilm system using biofilm modeling and Monte Carlo filtering. Water Research 44(6): 1995-2009.
- Bernet N, Dangcong P, Delgenes JP, Moletta R (2001) Nitrification at low oxygen concentration in bio film reactor. Journal of Environmental Engineering-Asce 127(3): 266-271.
- Wyffels S, Hulle VSWH, Boeckx P, Volcke EIP, Cleemput VO, et al. (2004) Modeling and simulation of oxygen-limited partial nitritation in a membrane-assisted bioreactor (MBR). Biotechnol Bioeng 86(5): 531542.
- Kuai LP, Verstraete W (1998) Ammonium removal by the oxygen- limited autotrophic nitrification-denitrification system. Appl Environ Microbiol 64(11): 4500-4506.
- Sinha B, Annachhatre A (2007) Partial nitrification-operational parameters and microorganisms involved. Reviews in Environmental Science and Biotechnology 6(4): 285-313.
- APHA (1995) Standard methods for the examination of water and wastewater, American Public Health Association, Washington DC, USA.
- Blackburne R, Yuan Z, Keller J (2008) Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 19(2): 303-312.
- van Dongen U, Jetten MS, van Loosdrecht MC (2001) The SHARON- Anammox process for treatment of ammonium rich waste water. Water science and technology: a journal of the International Association on Water Pollution Research 44(1): 153-160.
- Hoilijoki TH, Kettunen RH, Rintala JA (2000) Nitrification of anaerobically pretreated municipal landfill leachate at low temperature. Water Research 34(5): 1435-1446.
- Antoniou P, Hamilton J, Koopman B, Jain R, Holloway B, et al. (1990) Effect of temperature and ph on the effective maximum specific growth rate of nitrifying bacteria. Water Research 24(1): 97-101.






























