Zeolites: The Aluminosilicate Framework with Promising Scenarios
Ashu Chaudhary* and Anshul Singh
Department of Chemistry, Kurukshetra University, India
Submission: July 25, 2017; Published: August 16, 2017
*Corresponding author: Ashu Chaudhary, Assistant Professor, Department of Chemistry, Kurukshetra University, Kurukshetra, Haryana, Pin: 136119, Tel: +91-9729864551; Email: ashuchaudhary21@gmail.com
How to cite this article: Ashu C, Anshul S. Zeolites: The Aluminosilicate Framework with Promising Scenarios. Recent Adv Petrochem Sci. 2017; 2(5): 555598. DOI:10.19080/RAPSCI.2017.02.555598
Abstract
Zeolites are micro porous crystalline materials playing imperious roles in catalysis, ion exchange, and adsorption sciences, along with many industrial applications. These multifarious applications of zeolites arise due to their unique shape selectivity properties and decisive features such as high surface area, uniform porosity, inter-connected pore/channel system, accessible pore volume, high adsorption ability, ion-exchange capability and enriched catalytic activity. Zeolites have so far been widely used in petrochemical industries and possibly will play an imperative role in the major challenges ahead of us. This review illustrates a brief overview of applicative potential of zeolites in diverse fields (Figure 1).
Keywords: Zeolites; Petroleum refining; Eutrophization; Parex process; Sorption
Introduction
Zeolites have been clinched to be minerals merely displayed in museums, although laying back a commercial and scientific success-story owing to their large-scale utilization in industry [1]. The history of zeolites was started by the discovery of the first zeolites mineral stilbit in 1756 by Cronsted. The enormous development in the knowledge of structure, synthesis, modification, characterization, and very broad usage of unique characteristics of zeolites and zeolites like materials was reached in the period of about 250 years from the discovery of the first zeolitic material and especially in the period of the last 50-60 years. The development started from aluminosilicate zeolites to new zeolites and zeolites-like materials as aluminophosphates, metallosilicates, and metallophosphates and from micro porous to mesoporous molecular sieves [2].
Zeolites are micro porous crystalline materials that play imperative roles in catalysis, ion exchange, and adsorption sciences, and the molecular-sieving properties of zeolites are exploited in many industrial applications. The reason for such wide range of zeolites applications is commonly ascribed to their unique shape selectivity properties. Today, this zeolites property is extensively exploited in oil refining processes such as catalytic cracking, hydro cracking, hydro isomerization of short and long paraffin's, and isomerization of n-butenes [3]. Likewise, zeolites catalyze processes namely transformation of aromatics: isomerization of C8 aromatics, disproportionate of toluene/ transalkylation toluene- xylenes aromatics and alkylation of benzene by short and long olefins. Due to their shape selectivity, zeolites are widely practiced in the petrochemical sector. The commercially substantial zeolites are synthetic and manufactured hydrothermally. With boundless demand for commercial applications, zeolites are produced in large quantities as detergent builders, petroleum refining and petrochemical processing catalysts and a variety of adsorbents or molecular sieves in treatment of nuclear waste and extraction of ammonium ions from municipal waste water [4]. The present article illustrates a brief overview of all the prospective applications of zeolites.
Elementary Features of Zeolites
The atomic structure of zeolites is based on three-dimensional framework of silica and alumina tetrahedra, that is, silicon or aluminum ions surrounded by four oxygen ions in
a tetrahedral configuration. Each oxygen is bonded to two adjacent silicon or aluminum ions, linking them together. Clusters of tetrahedra form boxlike polyhedral units that are further linked to build up the entire framework. The polyhedral units in different zeolites may be equidimensional, sheet like, or chainlike. The aluminosilicate framework of zeolites has a negative charge, which is balanced by the cations housed in the cage like cavities. Zeolites have much more open, less dense structures than other silicates; between 20 and 50 percent of the volume of a zeolites structure is voids. Silicates such as zeolites that have three-dimensional frameworks of tetrahedra are termed tectosilicates. Besides the zeolites, other tectosilicates include quartz and feldspars [5].
Dimensions shape, and linkage of zeolites pores and voids are characteristic of zeolites materials. The pores and interconnected voids are occupied with cations and water molecules. Cations can be changed by ion exchange and water can be removed reversibly by application of heat.
The basic types of zeolites structures consist of internal pore system with interconnected cage-like voids or system of uniform one-, two- or three-dimensional channels. By pore size or diameter of pores zeolites include [6]:
1. small-pore zeolites (eight-ring pores) with free diameter of pores 0.3-0.45nm (eg zeolite A)
2. medium-pore zeolites (ten-ring pores) with free pore diameter 0.45-0.6nm (eg zeolite ZSM-5)
3. large-pore zeolites (twelve-ring pores) with free pore diameter 0.6-0.8nm (eg Y Beta & Mordenite zeolites)
4. Extra-large-pore zeolites (fourteen-ring pores) with free pore diameter 0.8-1.0 nm (eg UTD-1)
Abundant work on zeolites, predominantly the synthesis of new zeolites comes from the former Union Carbide and Mobil companies. Undoubtedly one of the most significant paradigms is the launch of using quaternary ammonium hydroxides in the synthesis of zeolites [7]. This steered to the discovery of zeolite ZSM-5 in 1963 by former Mobil. Often in order to attain industrial applications and commercialization, external aspects (market push) are prerequisite. Chen designates how the US government's decision to eliminate lead from gasoline, provided the needed economic incentive to commercialize the expensive ZSM-5 zeolite. Even with such external stimuli, it takes often several years before delivering commercially practical applications. For instance, the MSTDP (Mobil Selective Toluene Disproportionate Process) was commercialized in 1988, seventeen years after the discovery of the ZSM-5 synthesis [8]. These discoveries of the early sixties have headed to a new discipline in material science, catalysis, separation science and petrochemical or organic chemistry. These disciplines have since then developed at an incessant pace.
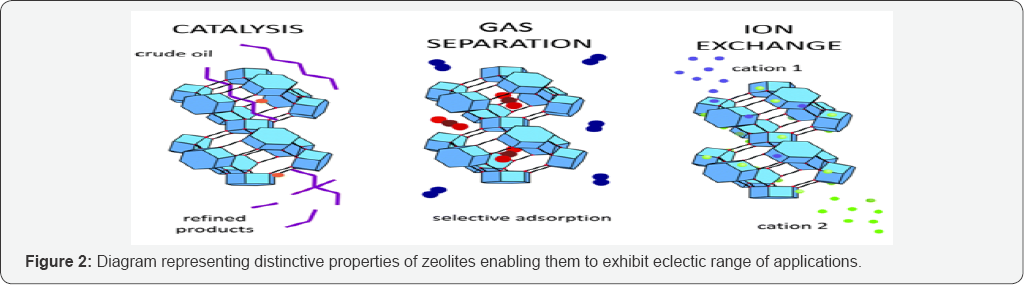
Applications of Zeolites
Zeolites are extremely useful having an eclectic range of applications which arises due to their distinctive properties (Figure 2):
a. They can hold large molecules and help them break into smaller pieces (catalytic cracking),
b. They can selectively absorb ions that fit the cavities in their structures (molecular sieves) and
c. Their power to interact with water so as to absorb or release ions (ion exchange)
In all, out of outsized number of zeolitic structures, only about a dozen are used in industrial or pre-industrial applications [9]; these are the following:
A. A detergents, desiccation and separation
B. FAU X (desiccation, purification, separation) and Y (separation, catalysis)
C. MOR adsorption and catalysis
D. LTL KL-type zeolite (catalysis: aromatization)
E. MFI silicalite and ZSM-5 (adsorption and catalysis)
F. BEA beta-type zeolite (catalysis: cumene)
G. MTW zeolite MCM-22 (catalysis: ethylbenzene, probably
cumene?)H. CHA SAPO-34 (methanol to olefins or MTO process demonstration unit)
I. FER ferrierite (skeletal isomerization of n-butenes demonstration unit)
J. AEL and SAPO-11 and possibly ZSM-22 (improvement of /or TON pour point for petroleum
cuts by straight long paraffin isomerization) [10].
Zeolites in petroleum refining
The first catalytic utilization of zeolites in petroleum refining industry dated back 40 years ago with the introduction of zeolite X as cracking catalyst by MOBIL Company. This zeolite was substituted later by zeolite Y, which was also utilized as isomerization catalyst. Y zeolite has the most important utilization till now (95% of the world market of zeolites in catalysis) mainly in refining processes (fluid catalytic cracking, hydro cracking). In the 70's of the last century were started some processes of aromatic hydrocarbon transformations over natural and later over synthetic mordenites (dis-proportionation and transalkylation of alkyl aromatics and mainly transformation of toluene and isomerization of C8 aromatics). At the end of the 60's MOBIL Company introduced newfangled and significant synthetic zeolites (ZSM-5 and Beta). These zeolites caught hold of wide-ranging utilization in refining and petrochemical processes. ZSM-5 has unique structure and dimensions of pores suitable for aromatic hydrocarbons transformations and this zeolite reached the pronounced industrial importance. Zeolite Beta turned out to be very important synthetic zeolite also with the utilization in alkylation of aromatic hydrocarbons. A number of other zeolites which play roles at different transformations of hydrocarbons are important: L, ferrierite, MCM-22, and molecular sieves SAPO-11 and SAPO-34 [11]. In the refining industries zeolites are used for these core processes:
a. Catalytic cracking
b. Fluid Catalytic Cracking (FCC) Y, ZSM-5
c. Deep Catalytic Cracking (DCC) ZSM-5
d. Shape-selective cracking ZSM-5
e. Hydro cracking Y, ZSM-5
f. Catalytic dew axing ZSM-5
g. Isomerization of C4 olefins Ferrierite
h. Hydroisomerization of low Pt/Mordeniteparaffins
i. Isomerization of xylenes ZSM-5, Mordenite
j. Aromatization of C3/C4 ZSM-5
k. Methanol to Gasoline process ZSM-5 (MTG)
l. Methanol to Olefin process ZSM-5 (MTO)
Zeolites in detergents
Zeolite type LTA (NaA) is used in modern detergents to replace sodium tripolyphosphate with the aim to soften water. Eutrophization of water arises due to higher concentration of polyphosphates in rivers and lakes. The world consumption of zeolites to detergents is approximately 800 000 t/y of zeolite type A. This zeolite is more effective for exchange of calcium than magnesium. It can be combined with zeolite X which is better for removal of magnesium. However current studies displayed that P zeolite is much more effective to detergents than zeolite A [12].Zeolites in water treatment
The filtering abilities of Zeolites offer a versatile and environmentally friendly option to capture most contaminants found in water systems. Natural Zeolites can perform these functions due to their high ion exchange capacity, adsorption- desorption energies and ability for modification. Zeolites have an open, regular crystalline framework that generates an electric field that interacts, attracts and binds various cations and, after modification, anions.
Zeolites have a particularly high selectivity for ammonium (NH4+) and can reduce the ammonium content in waste-water by up to 97%. NH4+ has serious environmental consequences because of its toxicity to aquatic life, contribution to algal eutrophication, reduction in dissolved oxygen and detrimental effects on disinfection of water. Modifications such as charge change from (-) to (+) provide Zeolite with the flexibility to absorb anions as well as cations and also some non-polar organics such as benzene, toluene and xylene. Zeolites can be charged with 'antibiotic' cations of Ag, Cu, Zn to provide antimicrobial properties. Zeolite filter beds can remove contaminants to purify air (Ammonia NH3, H2S, CO2, CO, SO2) [13].
The hard, durable nature of Zeolites enables them to perform a range of filter functions to produce improved water quality more efficiently than both the conventional slow or rapid sand filter systems. Robust, insoluble Zeolites have improved attrition qualities and are adaptable to re-use through regeneration and recycling.
Utilization of zeolites in adsorption and separation processes
Zeolites, due to their innate ability to absorb polar compounds; have been deliberated as superb candidate materials for separation and purification of gases. Moreover, certain zeolites are known to occur profusely in nature. These attributes notwithstanding, the use of natural zeolites has been small in comparison to that of synthetic zeolites and other adsorbents in commercial gas separations utilizing adsorption [14].
The distinctive pore structure of zeolites and outsized inner volume are the two most essential factors contributing towards their applications in catalysis and adsorption processes. The strictly defined and constant dimensions of pores have consumption in selective separation of molecules with diverse dimensions and shapes [15]. In 1959 the Union Carbide Company introduced the process ISOSIV (CaA zeolite) for separation of linear and branched alkanes. It was the first big industrial process with the usage of "molecular-sieving effect” of zeolites. Parex process uses the molecular-sieving effect for separation of p-xylene from the mixture of C8 aromatics. Tuning of effective diameters of pores can be reached by ion exchange of basic zeolites by different cations. It can be used in separation processes, e.g. at A and X zeolites for separation of gases, hydrocarbons and for drying of gases and liquids. Hydrophobic character of zeolites with high content of silica (eg silicalite) can be used for selective separation of organic molecules from aqueous solutions. Zeolites with hydrophilic character with low content of silica (e.g. NaA and KA) are usable for drying of gases and liquids. The chemical equilibrium of different organic reactions, eg esterification, transesterification, can be moved by sorption of small molecules (water, methanol) as reaction by-products on zeolites. Sorption properties of zeolites are utilized in industrial processes, agriculture, and environmental engineering.
Zeolite use in agriculture
The unique ion exchange, dehydration-rehydration, and adsorption properties of zeolite materials promise to contribute significantly to agricultural and aqua cultural technology. Zeolites mended physical and chemical properties of soils. It is typical for dry soils as zeolites improve water regime(storage and better usage of water).Zeolites in the soils change sodium and potassium cations for NH4+(at simultaneous fertilization with natural and artificial fertilizers). After the 2nd and 3rd year of zeolite action in the soil, zeolite is effective nitrogenous fertilizer. It was shown that very effective is combination of zeolite and natural fertilizer for improving of crop [16]. The application of zeolites to the soil contaminated with heavy metals or radionuclide's can be effective to lower their input by vegetables [17]. Similar to their synthetic counterparts, the high adsorption capacities in the dehydrated state and the high ion-exchange capacities of many natural zeolites make them effective carriers of herbicides, fungicides, and pesticides. Clinoptilolite can be an excellent substrate for benzyl phosphorothioate to control stem blasting in rice.
Conclusion
It is quite evident that zeolites, the micro porous crystalline materials have laid down a success story with their large scale utilization in petrochemical industries. The utilization of zeolites is based on their unique pore structures consisting of pores with molecular dimensions. Zeolites whether natural or synthetic have left out a mark of its significance in various processes such as waste water treatment, agriculture, aquaculture, as catalysts, adsorbents etc. Due to their varied properties they have been used in different industries for decades and hence it is predictable that their role in petrochemicals as well as other industries will prolong to be robust and everlasting.
Acknowledgement
The authors (Ashu Chaudhary and Anshul Singh) wish to express gratitude to the Council of Scientific and Industrial Research (CSIR), New Delhi, India for financial assistance in the form of SRF vide letter no. 09/105(0221)/2015-EMR-I.
References
- Corma A, Martinez A (2005) Zeolites in refining and petro chemistry. Studies in Surface Science and Catalysis 157: 337-366.
- Mravec D, Hudec J, Janotka I (2005) Some possibilities of catalytic and no catalytic utilization of zeolites. Chemical Papers 59(1): 62-69.
- Schuth F, Sing KSW, Weitkamp J (2002) Handbook of Porous Solids. (3rd edn), John wiley and sons, Wiley-VCH, Weinheim, Germany, pp. 18281863.
- Von Farrauto RJ, Bartholomew CH (1997) Fundamentals of Industrial Catalytic Processes. In: Chapman & Hall (Eds.), (1st edn), Blackie Academic and Professional, London.
- Klein C (2001) Manual of Mineral Science. (22nd edn), John wiley and sons, New York, USA, pp. 656.
- Flanigen EM (2001) Zeolites and molecular sieves: An historical perspective. Studies in Surface Science and Catalysis 137: 11-35.
- Kerr GT (1966) Chemistry of Crystalline Alumino silicates. II. The Synthesis and Properties of Zeolite ZK-4. Inorganic Chemistry 5(9): 1537-1539.
- Chen NY (2001) Personal Perspective of the Development of Para Selective ZSM-5 Catalysts. Industrial and Engineering Chemical Research 40(20): 4157-4161.
- Schoonover MW, Cohn MJ (2000) New materials discovery for industrial applications. Topics in Catalysis 13(4): 367-372.?
- Marcilly CR (2000) Where and how shape selectivity of molecular sieves operates in refining and petro chemistry catalytic processes. Topics in Catalysis 13(4): 357-366.
- Marcilly C (2001) Evolution of refining and petrochemicals. What is the place of zeolites. Studies in Surface Science and Catalysis 135: 37-60.
- Townsend RP, Coker EN (2001) Ion exchange in Zeolites. Chapter 11, Studies in Surface Science and Catalysis 137: 467-524.
- Erwe T, Mavrov V, Chmiel H (2003) Characterization of a Synthetic Zeolite P as a Heavy Metal Bonding Agent. Chemical Papers 57(1): 4549.
- Ackley MW, Rege SU, Saxena H (2003) Application of natural zeolites in the purification and separation of gases. Micro porous and Mesoporous Materials 61(1-3): 25-42.
- Hoelderich WF, van Bekkum H (2001) Zeolites and related materials in organic syntheses. Bronsted and Lewis Catalysis. Studies in Surface Science and Catalysis137: 821-910.
- Mumpton FA, Fishman PH (1977) The Application of Natural Zeolites in Animal Science and Aquiculture. Journal of Animal Science 45: 11881203.
- Szabova T, Mitro A (1993) Effect of zeolit on sorption, desorption and distribution coefficient of radio strontium and radio cesium in different soil. Agriculture 39(1): 1-6.






























