Synthesis of Phenanthridine Derivative and It’s use as a Selective Colorimetric Sensing for Cu (II) Ions
Nilesh Kshirsagar1, Ratnamala Sonawane1*, Prashant Patil3, Jitendra Nandre3, Girdhar Pal Singh4, Yogesh Kshirsagar2, and Sultan Pathan4*
1Department of Chemistry, The Institute of Science Mumbai, India
2Department of Chemistry, PVP Collage Loni, India
3S. S. V. P. S. L. K. Dr. PR Ghogrey Science College, India
4Department of Chemistry, Bhupal Nobles University, India
Submission: August 16, 2022;Published: September 07, 2022
*Corresponding author: Dr. Sultan Pathan, Department of Chemistry, Bhupal Nobles’University-313001 Udaipur, Rajasthan, India
Dr. Ratnamala Sonawane, Department of Chemistry, The Institute of Science Mumbai, Maharashtra, India
How to cite this article: Nilesh K, Ratnamala S, Yogesh K, Prashant P, Jitendra N, et al. Synthesis of Phenanthridine Derivative and It’s use as a Selective Colorimetric Sensing for Cu (II) Ions. Organic & Medicinal Chem IJ. 2022; 11(5): 555824. DOI: 10.19080/OMCIJ.2022.11.555824
Abstract
6-(thiophen-2-yl) phenanthridine (6TP), a new phenanthridine-based chemosensor, was created and described using HRMS and NMR spectroscopic techniques. The ability of 6TP to detect metal ions was tested using UV visible absorption and naked-eye methods. Cu (II) was added to the colorless solution of 6TP, turning it green and causing the appearance of a new absorption band between 500 and 600nm. The Job’s plot data supports the creation of a novel complex species in a 1:1 binding stoichiometry between 6TP and Cu (II). Without any notable interference from the other investigated metal cations, the sensor 6TP enabled the detection and quantification of Cu (II) down to 104μM and 316μM.
Keywords: Phenanthridine; Spectroscopic techniques; Metal ions; Enzyme
Introduction
Transition metals play an important role in various chemical and biological processes in the body and environment [1-4]. As an essential element of life, copper play’s crucial role in biological, enzyme catalyzed and redox reactions also physiological process like altering the central nervous system, energy generation and signal transduction [5-8]. The abnormal levels of Cu (II) ions can lead some negative health effects such as increasing blood pressure and respiratory rates, hematological manifestation, diarrhea, liver damage, stomach cramps, pelvic inflammations, neurotoxicity and neurodegenerative disease [9-13], also the higher concentration of copper ions could cause the severe disease like Alzheimer’s, Parkinson’s and Menke`s diseases [14-17] and environmental pollution.
The other applications of copper are in industry such as electrical wires, machine parts, batteries, pharmaceutical and fertilizers [18-21].
Due to diversified function of copper ion has led to a strong interest in the discovery of novel and selective colorimetric Cu (II) probes for biological and environmental applications. Existing techniques for detection of metal ions such as electrochemical, inductively coupled plasma, atomic absorption, atomic emission and piezoelectric quartz crystals [22-28], exhibits some limitations to their use due to the requisite of expensive equipment, laboratories and the procedures are time consuming. Due to its capacity for ‘naked-eye’ detection and provision of qualitative and quantitative information without the restrictions, emission and absorption spectrophotometric methods have grown in popularity for detecting cations.
Many literary evident sensors for these metal cations are often structurally complicated and require tedious synthetic procedures. Therefore, the development of simple and easy-to-make chemosensors is strongly demanded. Since our main goal is to design and synthesis of colorimetric chemo-sensors containing phenanthridine moiety for cation detection.
Materials and Methods
All of the analytical-grade chemical reagents, metal nitrates, and solvents utilized in the research were obtained from commercial vendors, including Sigma Aldrich, TCI Chemical, and RANKEM. Thin layer chromatography (TLC) was used to monitor reactions. All 1H-NMR and 13C-NMR spectra were recorded using Bruker 400MHz in DMSO(d6) solvent, with internal standard TMS used to express chemical shifts in ppm downfield and maXis impact used to record the HRMS. On an Agilent Technologies, all UV spectra were examined.
Synthesis of 6TP
The receptor 6TP was efficiently synthesized by TFA mediated single step process for the reaction of [1,1’-biphenyl]-2-amine and thiophene-2-carbaldehyde (Scheme 1).
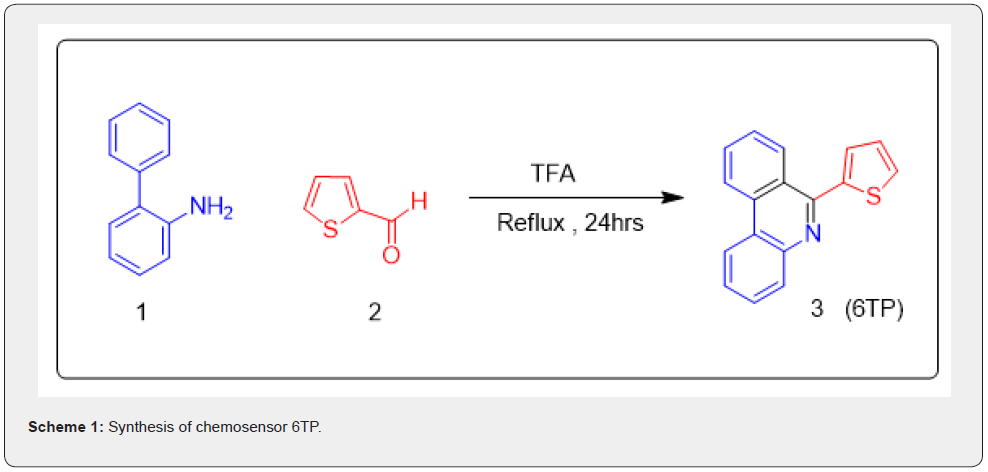
By utilizing phenyl boronic acid and 2-bromoaniline in the presence of PdCl2(PPh3)2 and potassium carbonate in DMF, the Suzuki coupling reaction was employed to create the intermediate molecule [1,1’-biphenyl]-2-amine.
Synthesis of 2[1,1’-biphenyl]-2-amine (1):
Phenylboronic acid (1.83g, 15.11mmol) was added to a 100ml 2-necked flask equipped with a reflux condenser, magnetic stirrer, and an inert atmosphere. Next, 50mL DMF, 9mL water, 2g, 11.62mmol, and potassium carbonate (3.21g, 23.25mmol) were added. PdCl2(PPh3)2 (816mg, 1.16mmol) was then added to the reaction mixture, which was then agitated for 24 hours at 80°C after being bubbled through nitrogen for 10 minutes. The reaction mixture was then added 50mL of saturated sodium bicarbonate solution after cooling. The reaction mixture was then extracted with 2X50mL of ethyl acetate, and the combined organic layer washed with 50mL of saturated brine solution before being dried over anhydrous sodium sulphate and concentrate to get crude [1,1’-biphenyl]-2-amine. Pure [1,1’-biphenyl]-2-amine (1.3g, 7.68mmol) was obtained in a yield of 61.9 percent after the crude was purified using silica gel column chromatography with ethyl acetate in hexane (5:1, v/v). Melting point 51.4-52.3 °C
Synthesis of 6TP (3):
A 10ml seal tube was used to conduct the reaction. [1,1’-biphenyl] -2-amine (1.0g, 5.90mmol) and Thiophene-2- carbaldehyde (1.324g, 11.81mmol) was added to in TFA (0.1M) in a sealed tube. For 24 hours, the seal tube was agitated at 140 °C with a tight cap on top. Following the completion of the reaction, the excess TFA was blown away with air, and the reaction mixture was basified with a saturated sodium bicarbonate solution, extracted with ethyl acetate, and washed with brine. After drying over Na2SO4, the ethyl acetate layer was concentrated under reduced pressure. Using ethyl acetate in hexane (4:1, v/v) and silica gel column chromatography, the crude product is refined to produce 6TP. 80 percent of the yield. The structure of 6TP was confirmed by various techniques like NMR and Mass. 1H NMR White solid, Yield 80%, m.p. 274.2-276.5 °C, .1H NMR (400 MHz, DMSO-d6) δ 8.95-8.93 (d, J =8.0 Hz, 1H ), 8.82-8.81 (d, J = 4.0 Hz, 1H ), 8.61-8.59 (d, J = 8.0 Hz, 1H ), 8.08-8.06(d, J = 8.0 Hz, 1H ), 8.01-7.98 (m, 1H ), 7.86-7.78 (m, 4H ), 7.73-7.11 (m, 1H ), 7.32-7.30 (m, 1H ) 13C NMR (101 MHz, DMSO-d6) δ 153.07, 142.99, 142.21, 133.08, 131.29, 129.79, 129.46, 129.32, 129.01, 128.33, 127.95, 127.55, 127.39, 123.60, 123.06, 122.76, 116.78; ppm, HRMS (ESI) Calcd. for C17H11NS [M+H]+ 261.3411 Found 262.0363.
The cation detection ability of 6TP towards different metal ions was studied by experimental (naked-eye, UV-visible,) and theoretical methods.
Spectroscopic study
The stock solution of 6TP was created in CH3OH since the receptor 6TP is not soluble in water. Water was used to prepare all cation solutions (1.0 ×10-3 M). . After the proper dilution, these solutions are utilized for a various spectroscopic study.
Using a micropipette, the required quantity of the diluted receptor 6TP (2mL, 2×10-5 M, in CH3OH) was added directly to the cuvette for the UV titration. The spectra were then recorded after each addition of Cu(II) (1×10-3 M, H2O), in aliquots of (20μL). The obtained UV titration data were used to plot a calibration curve between the absorbance at 560nm and the added concentration of Cu (II). In order to determine the limit of detection (LOD) for the receptor 6TP, the IUPAC approved formula was used: LOD = (3 x standard deviation)/slope of the calibration curve.
Results and Discussion
Preliminary selectivity study of 6TP
By examining Cu(II) colour changes with the naked eye (colorless to greenish blue) under day and UV light, the ability of 6TP (2ml, 1 x 10-3 M, in CH3OH) to be recognised was tested after adding different metal ions (1mL, 1×10-2 M, in H2O), such as Al(III), Ag(I), Ca(II), Cd(II), Co(II), Cu(II), Fe(II), Hg(II), Mg(II), Mn(II), Ni(II),and Zn(II).
The absence of any noticeable colour shift of 6TP in the presence of other cations suggests that 6TP’s colorimetric reaction to Cu (II). Spectrophotometric techniques were used to assess the quantitative and qualitative metal ion sensing capabilities of 6TP.
Photophysical properties of sensor 6TP
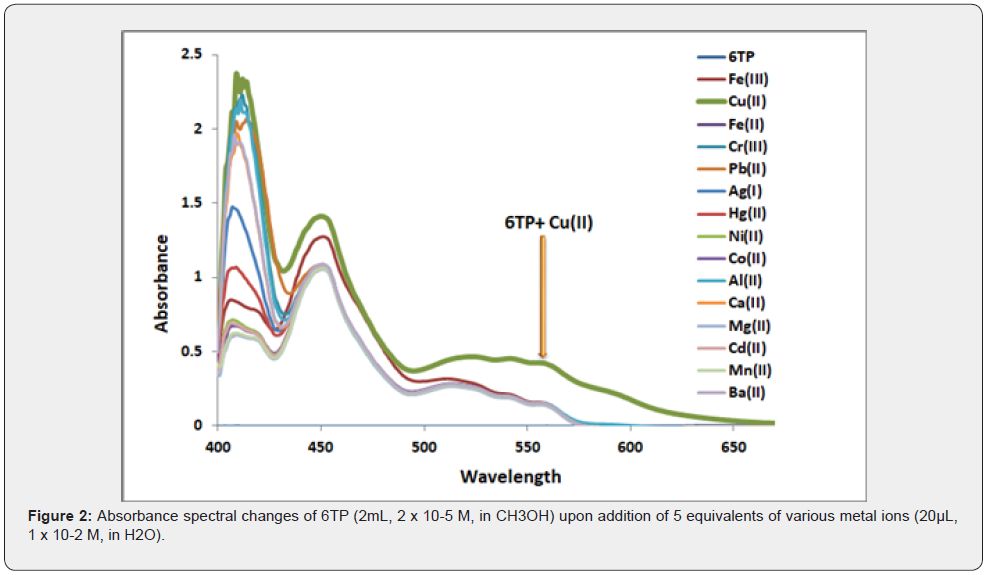
The receptor 6TP (2 x 10-5 M, in CH3OH) was subjected to a UV-Vis absorption spectrum investigation in the absence and presence of five equivalents of various metal ions, including Fe(III), Cu(II), Fe(II), Cr(III), Pb(II), Ag(I), Hg(II), Ni(II), Co(II), Al(III), Ca(II), Mg(II), Cd(II (II), Mn(II) and Ba(II) (1 х 10-2M, in H2O). One absorption band at 560nm was detected in receptor 6TP. A new broad charge transfer band between 500 and 600nm appeared after the addition of Cu(II) ions to the solution of 6TP. A hypochromic shift was seen at 500nm and a blue shift was seen at 560 nm. In the presence of the Cu(II) ion, the charge transfer band of reduced intensity was also seen (Figure 1). The intermolecular charge transfer (ICT) process, which occurs as a result of the delocalization of electrons from the imine nitrogen (C=N) of phenanthridine to the metal ions during complexation, is responsible for the spectrum shifts associated with 6TP. The selectivity towards Cu was revealed by the lack of noticeable changes in the 6TP’s absorption spectrum when other metal ions were investigated (II). The capacity of Cu(II)ion to recognise 6TP was next tested using absorbance titrations with Cu(II)metal ions.

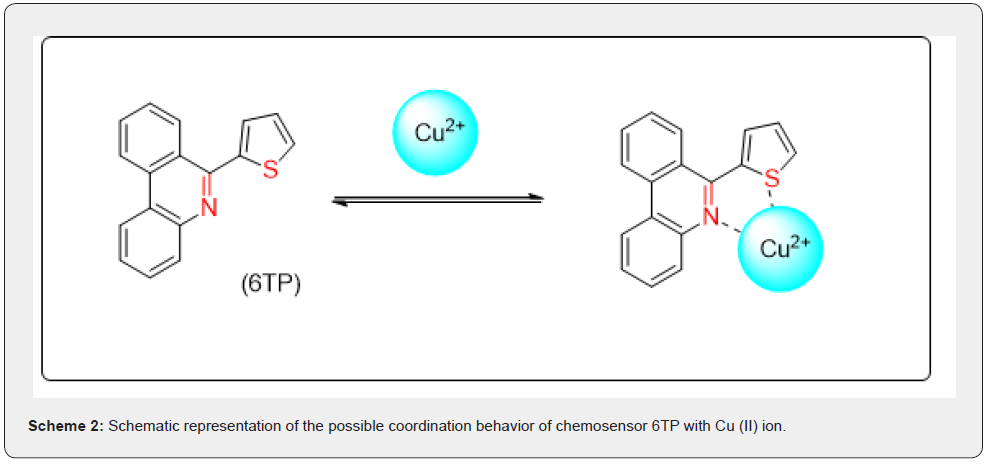
The potential binding process of 6TP with Cu (II) ion is depicted in scheme-2 based on the findings from the UV–vis and naked eye tests. The Cu (II) ion may coordinate with the N- and S atoms of phenanthridine during complex formation.
Interference study
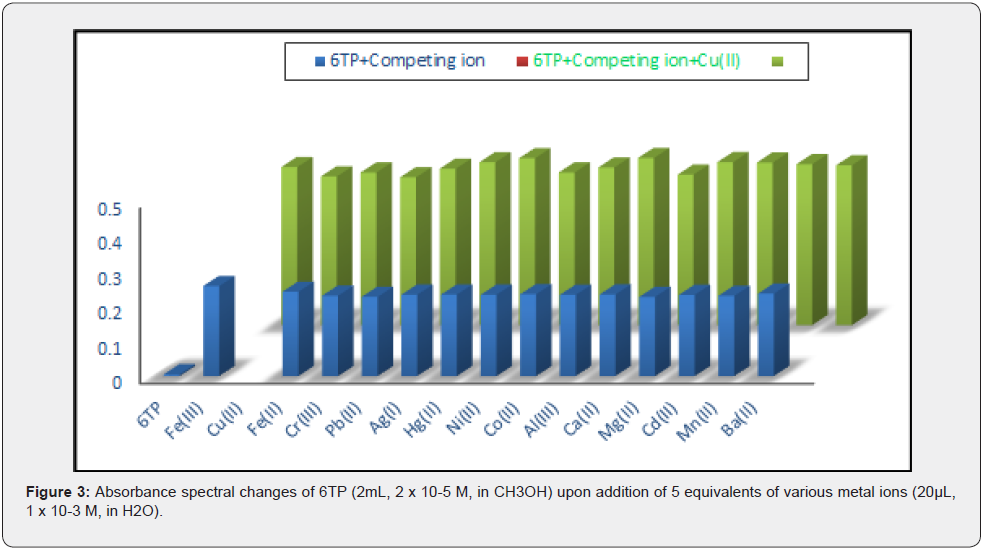
By conducting competitive experiments, where the absorption spectra of 6TP (2mL, 2X10-5 M, in CH3OH) were recorded in the presence of Cu(II) (20μL, 1×10-3 M, in H2O) and equimolar amounts of other interfering metal ions (20μL, 1×10-3 M, in H2O), it was determined whether coexisting metal ions interfere with the detection of Cu(II) by 6TP. The detection of Cu(II) is not impeded by the coexistence of the tested interfering metal ions, according to the bar representation of the change in absorption intensity of 6TP at 560nm (Figure 2). Consequently, the highly selective towards Cu receptor 6TP can be studied (II).
Spectrophotometric titration
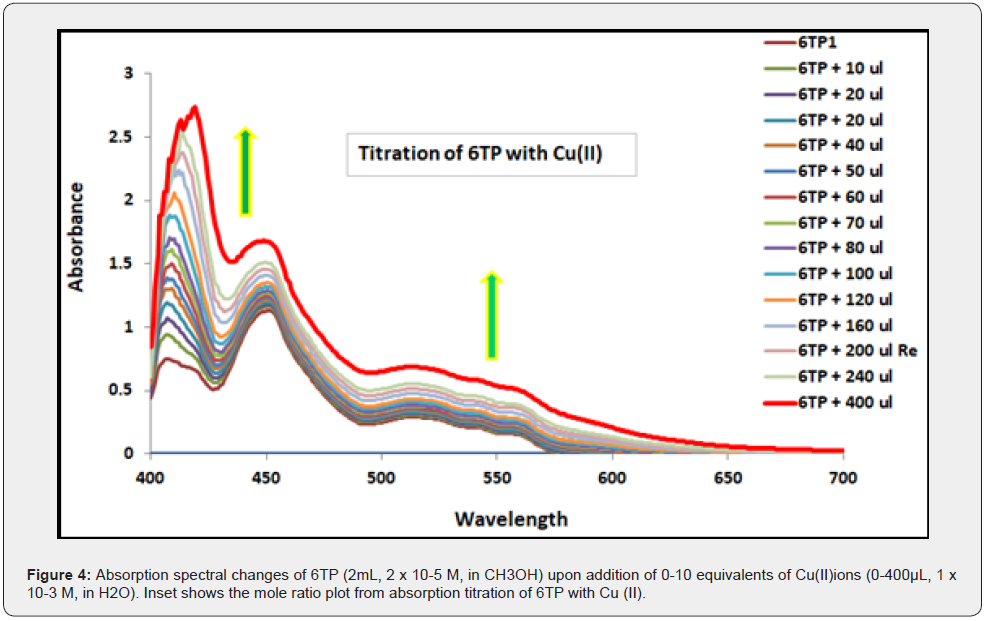
The absorption spectra of 6TP (2ml, 2×10-5 M, in CH3OH) were recorded after each aliquot (10μL) injection of Cu(II) (0-400μL, 1×10-3, in H2O) in the spectrophotometric titration experiment, which was performed to ascertain the sensitivity of the receptor 6TP. Cu(II) was added in small amounts at a time to 6TP, increasing the absorption intensity at 560nm (Figure 3). Plotting 6TP’s increased 560 nm absorption intensity against the increased Cu concentration (II).
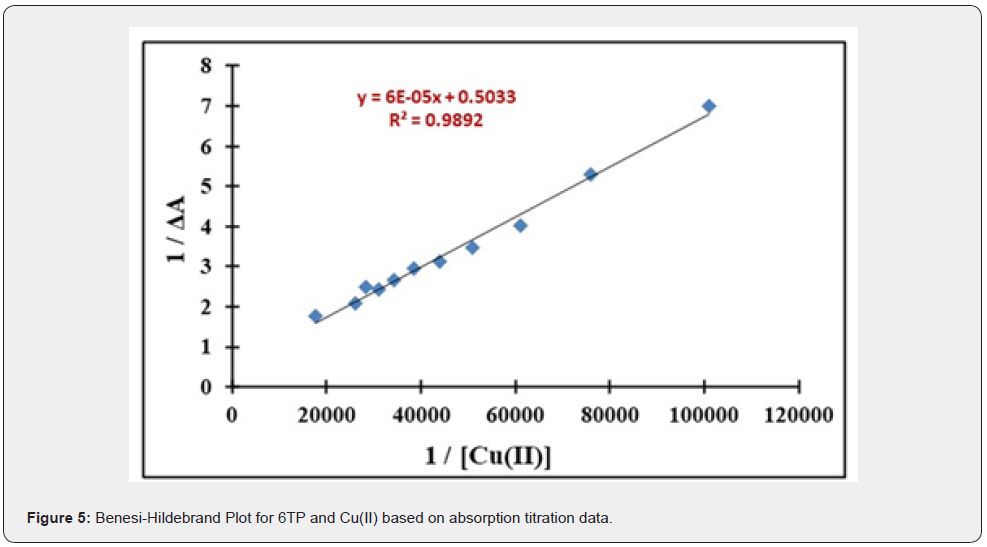
As seen in Figure 4, the receptor bands were gradually shifted with the appearance of a new charge transfer band at 500 to 600nm due to the change in colour of the solution from colorless to greenish blue on successive addition of 0 to 10 equivalents of incremental amounts of Cu(II) ion to the 6TP solution (2mL, 2 x 10-5 M, in MeOH). The Benesi-Hildebrand Plot was used to compute the binding constant for Cu(II) using the absorption titration data, which came out to be 8.3X10-3 M (Figure 5). According to estimates, the detection and quantification limits for Cu(II) are 104 M and 316 M, respectively.
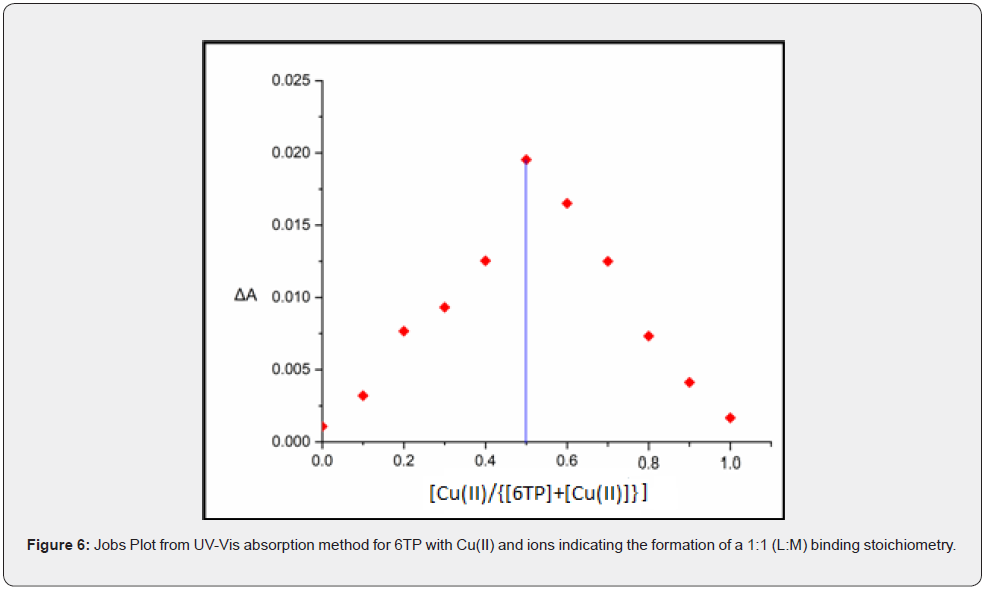
The Job’s plot between the mole fractions of Cu(II) and the variations in absorbance at 560nm was used to calculate the binding stoichiometry for Cu(II) complexes (Figure 6). The maximum was reached at a molar fraction of 0.5, which amply demonstrated that a complicated stoichiometry of 1:1 had formed.
Practical application
Test strips with sensor 6TP put on them were created to examine the practical application of receptor 6TP to detect Cu(II) ions in an aqueous environment. The teeny-tiny cellulose paper strips (Whatman no. 42) were made by soaking them in a solution of 6TP (1X10-3 M) in methanol and letting them air-dry. When the boring strips were dipped into an aqueous solution of Cu(II) (1X10-2 M) and allowed to dry for 15 minutes in the sun, they abruptly changed colour to a vivid shade of green (Figure 7, TLC plate (silica supported aluminium plate)). The practical use of 6TP is plainly seen in the colour shift of test strips in solutions.

Conclusion
The library of Cu(II) selective chemosensors has now grown to include a new colorimetric chemosensor. At the adsorption band, the receptor 6TP and Cu(II) produced complex species in a 1:1 binding stoichiometry (560nm). Therefore, utilizing a silica TLC plate and sensor 6TP, Cu(II) ions may be calorimetrically detected in aqueous media without any discernible interference effects ( silica support method).
References
- de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, et al. (1997) Signaling Recognition Events with Fluorescent Sensors and Switches. Chemical Reviews 97(5): 1515-1566.
- Chundawat NS, Pathan S, Singh GP, Arup, Narendra, et al. (2021) Synthesis and characterization of chitosan pyridyl imine palladium (CPIP) complex as green catalyst for organic transformations. Chem Pap 75: 2835-2850.
- Shi S-M, Li Q, Hu SL (2019) A new hydrazone-based colorimetric chemosensor for naked-eye detection of copper ion in aqueous medium. Journal of Chemical Research 43(9-10): 426-430.
- Qiao R, Xiong WZ, Bai CB, Zhang L, Zhu MM, et al. (2018) A highly selective fluorescent probe for Zn2+ based on a rhodamine-6G dye derivative modified by a furan unit. Journal of Chemical Research 42(4): 194-197.
- Harris ED (2001) Copper and Iron: A landmark connection of two essential metals. The Journal of Trace Elements in Experimental Medicine 14(2): 207-210.
- Xu J, Wang Z, Liu C, Zhu B, Kun W, et al. (2018) A Colorimetric and Fluorescent Probe for the Detection of Cu2+ in a Complete Aqueous Solution. Analytical Sciences 34(4) 453-457.
- Tang L, Zheng Z, Bian Y (2016) AN-(2-hydroxyethyl)piperazine dangled 2,5-diphenyl-1,3,4-oxadiazole-based fluorescent sensor for selective relay recognition of Cu2+and sulfide in water. Luminescence 31(8): 1456-1460.
- Malvankar PL, Shinde VM (1991) Ion-pair extraction and determination of copper(II) and zinc(II) in environmental and pharmaceutical samples. The Analyst 116(10): 1081.
- Parsaee Z, Haratipour P, Lariche MJ, Vojood A (2018) A novel high performance nano chemosensor for copper (II) ion based on an ultrasound-assisted synthesized diphenylamine-based Schiff base: Design, fabrication and density functional theory calculations. Ultrasonics Sonochemistry 41: 337-349.
- Yun D, Chae JB, Kim C (2019) A novel benzophenone-based colorimetric chemosensor for detecting Cu2+ and F−. Journal of Chemical Sciences 131(2).
- Maity D, Govindaraju T (2011) Highly Selective Visible and Near-IR Sensing of Cu2+ Based on Thiourea-Salicylaldehyde Coordination in Aqueous Media. Chemistry - A European Journal 17(5): 1410-1414.
- Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J (1993) Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper–transporting ATPase. Nature Genetics 3(1): 7-13.
- Bull P, Thomas G, Rommens J, Forbes JR, Cox DW (1993) The Wilson disease gene is a putative copper transporting P–type ATPase similar to the Menkes gene. Nat Genet 5: 327-337.
- Ghule NV, Bhosale RS, Puyad AL, Bhosale SV, Bhosale SV (2016) Naphthalenediimide amphiphile based colorimetric probe for recognition of Cu2+ and Fe3+ Sensors and Actuators B: Chemical 227: 17-23.
- Pathan S, Singh GP (2021) Synthesis of novel tetrazole tetrahydrobenzo[b]thiophene via Ugi-MCR: As new antileishmanial prototype. Journal of Saudi Chemical Society 25(8): 101295.
- Jung JM, Lee SY, Nam E, Lim MH, Kim C (2017) A highly selective turn-on chemosensor for Zn2+ in aqueous media and living cells. Sensors and Actuators B: Chemical 244: 1045-1053.
- Barceloux DG, Barceloux D (1999) Copper. Journal of Toxicology: Clinical Toxicology 37(2): 217-230.
- Peralta-Domínguez D, Rodriguez M, Ramos-Ortiz G, Maldonado JL, Luna-Moreno D, et al. (2016) A Schiff base derivative used as sensor of copper through colorimetric and surface plasmon resonance techniques. Sensors and Actuators B: Chemical 225: 221-227.
- Jo TG, Na YJ, Lee JJ, Lee MM, Lee SY, et al. (2015) A diaminomaleonitrile based selective colorimetric chemosensor for copper(ii) and fluoride ions. New Journal of Chemistry 39(4): 2580-2587.
- Butler OT, Cook JM, Harrington CF, Hill SJ, Rieuwerts J, et al. (2006) Atomic spectrometry update. Environmental analysis. J Anal At Spectrom 21(2): 217-243.
- Li Y, Chen C, Li B, Sun J, Wang J, Gao Y, et al. (2006) Elimination efficiency of different reagents for the memory effect of mercury using ICP-MS. J. Anal. At. Spectrom 21(1): 94-96.
- Kshirsagar, Nilesh Sonawane, Ratnamala Patil, Prashant Nandre, Jitendra Sultan, et al. (2020) Fluorescent chemosensor for Al(III) based on chelation-induced fluorescence enhancement and its application in live cells imaging. Inorganica Chimica Acta 511: 119805.
- Zheng Y, Orbulescu J, Ji X, Andreopoulos FM, Pham SM, et al. (2003) Development of Fluorescent Film Sensors for the Detection of Divalent Copper. Journal of the American Chemical Society 125(9): 2680-2686.
- Xiang Y, Tong A (2008) Ratiometric and selective fluorescent chemodosimeter for Cu(II) by Cu(II)-induced oxidation. Luminescence 23(1): 28-31.
- Pathan Sultan Ismail, Chundawat Narendra Singh, Narendra Pal Singh, Girdhar Pal (2020) A review on synthetic approaches of heterocycles viainsertion-cyclization reaction. Synthetic Communications 1-35.
- Honglei Mu, Rui Gong, Qiao Ma, Yimin Sun, Enqin Fu (2007) A novel colorimetric and fluorescent chemosensor: synthesis and selective detection for Cu2+ and Hg2+. Tetrahedron Letters 48(31): 5525-5529.
- Kaur P, Sareen D, Singh K (2011) Selective colorimetric sensing of Cu2+ using triazolyl monoazo derivative. Talanta 83(5): 1695-1700.
- Takamatsu K, Hirano K, Satoh T, Miura M (2014) Synthesis of Carbazoles by Copper-Catalyzed Intramolecular C–H/N–H Coupling . Organic Letters 16(11): 2892-2895.






























