Lead Tetraacetate in Organic Synthesis
Ajoy K Banerjee1*, Betzabeth Briceño1, Alexis Maldonado1, Liadis Bedoya1, Elvia V Cabrera2 and Dioni Arrieche3
1Chemistry Center, Venezuelan Institute of Scientific Research (IVIC), Caracas, Venezuela
2Faculty of Chemical Engineering, Central University of Ecuador, Quito, Ecuador
3Department of Chemistry, Technical University Federico Santa María, Valparaíso, Chile
Submission: December 13, 2020;Published: January 04, 2021
*Corresponding author: Ajoy K Banerjee, Centro de Química, IVIC, Apartado- 21827, Caracas-1020A, Venezuela
How to cite this article: Ajoy K B, Betzabeth B, Alexis M, Liadis B, Elvia V C, et al. Lead Tetraacetate in Organic Synthesis. Organic & Medicinal Chem IJ. 2021; 10(3): 555788. DOI: 10.19080/OMCIJ.2021.10.555788
Abstract
Lead tetraacetate (LTA),a versatile oxidizing agent for different functional groups, has been widely used for oxidative decarboxylation of carboxylic acid, cleavage of 1,2-diol, formation of the cyclic ether, acetoxylation, methylation, dehydrogenation etc. The present micro review describes the utility of LTA in 1,2-glycol-cleavage and decarboxylation of carboxylic acid.
Keywords: Lead tetraacetate (LTA); Oxidative decarboxylation; Glycol cleavage; Bromination
Introduction
The commercially available LTA is hygroscopic and turns brown due to the formation of lead oxide. Therefore, LTA should be stored in absence of moisture, kept tightly sealed and stored under 10oC in the dark and in the presence of about 5% glacial acetic acid. LTA is very toxic and may be absorbed through the skin. Due to the high toxicity the reagent should be handled with care in chemical fume hood. It is soluble in hot acetic acid, benzene, cyclohexane, chloroform, and carbon tetrachloride.
Use of LTA in Organic Synthesis
1,2-glycol-cleavage
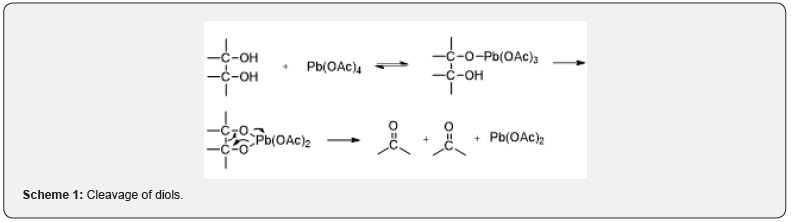
LTA is one of the most frequently used reagents for the cleavage of glycols and for the preparation’s carbonyl compounds. The reactions are performed either in aprotic solvents (benzene, nitrobenzene,1,2-dichloroethane) or in protic solvents such as acetic acid [1]. The role of LTA in glycol cleavage is highly dependent on the structure and stereochemistry of the substrate. The cleavage of diols proceeds via a cyclic intermediate [2]. as shown in Scheme 1. The cleavage of cis diol occurs more easily than the trans-diol which does not permit the easy formation of
the cyclic intermediate. Some examples are given in Scheme 2.
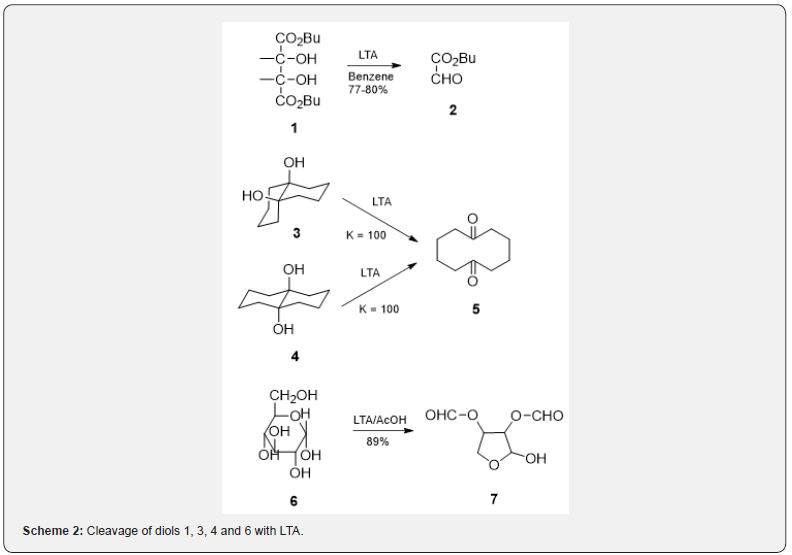
The diol 1 on treatment [3] with LTA in benzene affords aldehyde 2 but the diols 3 and 4 with LTA suffer4 cleavage and yield ketone 5. 1,2-Glycol cleavage [4] by LTA has been widely applied for the oxidation of carbohydrates and sugars [5]. The diol 6 on oxidation with LTA in acetic acid yields the aldehyde 7. The reactivity of individual glycol units in sugar molecules is often different and thus the LTA reaction is helpful tool for structural determination and for degradation studies in carbohydrate chemistry [6]. It has been observed that trans-1,2-diols which are cleaved slowly with LTA in acetic acid are readily cleaved if pyridine is used as reaction solvent [7].
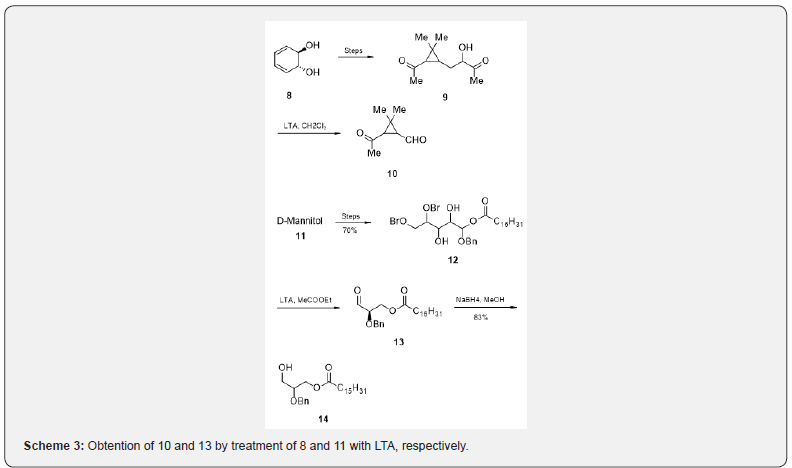
The cyclopropene ester 9, prepared from the bromo derivative of cis-1,2-hydrocatechol 8, on treatment with LTA produces [8] the cyclopropane aldehyde 10 which is a potential intermediate for the cis-pyrethroid class of insecticides. The trans-diol 12, obtained from D-mannitol 11, with LTA affords ketone [9] 13 in unspecified yield. Reduction of 13 with sodium borohydride produces the alcohol 14 which is utilized for the synthesis of mixed-acid phospholipids polyunsaturated fatty acid as shown in Scheme 3.
Decarboxylation of carboxylic acid
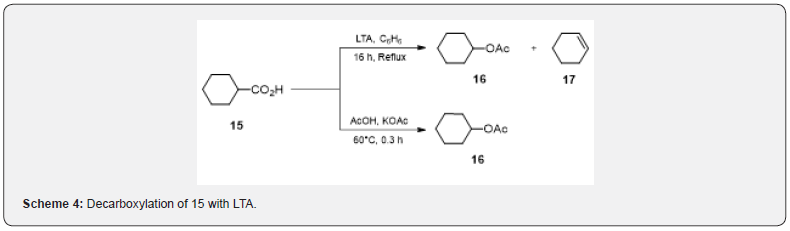
Oxidative decarboxylation of carboxylic acids by LTA has been frequently used in the synthesis of terpenoid compounds. Oxidative decarboxylation by LTA depends on the conditions of reaction, core agents and structure of acids and hence a variety of products such as acetate esters, alkanes, alkenes, and alkyl halides can be obtained [10]. The reactions are performed [10] in nonpolar solvents (benzene, carbon tetrachloride) or polar solvents (acetic acid, pyridine, HMPA). Decarboxylation of primary and secondary carboxylic acids usually affords acetate esters as major products. If a mixture of acetate and olefin is formed, it is recommended to perform the reaction in presence of potassium acetate [10]. The cyclohexane carboxylic acid 15 if heated under reflux with LTA in benzene furnishes a mixture of acetate 16 and the olefin 17 but only the acetate 16 is produced in high yield when heated with potassium acetate in acetic acid (Scheme 4).
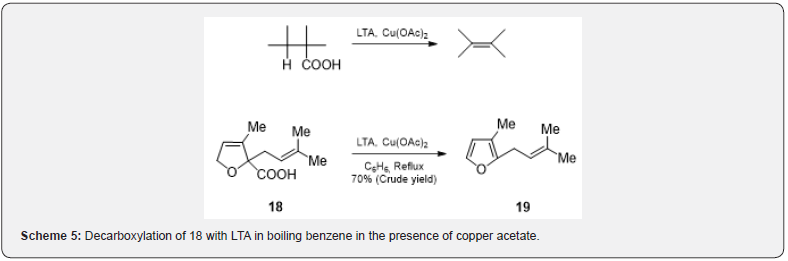
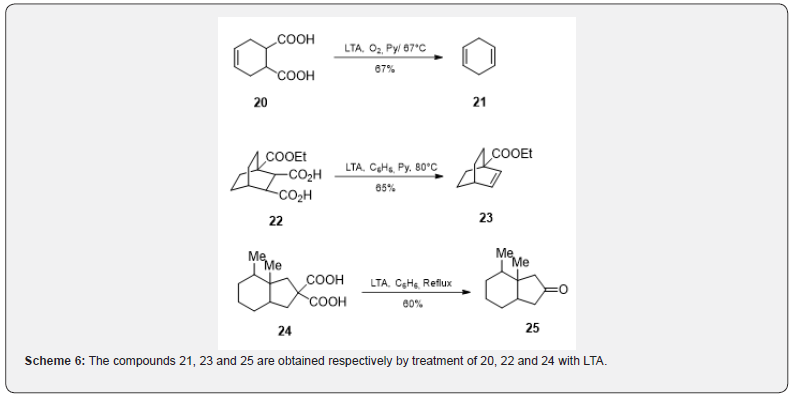
The monocarboxylic acid on oxidation with LTA in presence of copper (II) salts gives mainly alkenes (Scheme 5). The free radical mechanism is generally accepted [11]. Rosefuran 19 has been obtained in crude form (70%) by the oxidative decarboxylation of 3-methyl-2-furoic acid 18 with LTA in boiling benzene in the presence of copper acetate [12]. Bisdecarboxylation [13] of compounds containing carboxyl groups on adjacent carbons can be achieved with LTA in the presence of oxygen and pyridine. Thus, the dicarboxylic acid [14] 20 on decarboxylation affords the tetrahydrobenzene 21. Similarly, the acid [15] 22 if subjected to bisdecarboxylation can yield compound 23 (Scheme 6). The compounds containing germinal carboxyl groups (malonic acid derivatives) 24 are decarboxylated with LTA to give gem diacetate which can easily be hydrolyzed to ketone [16] 25.
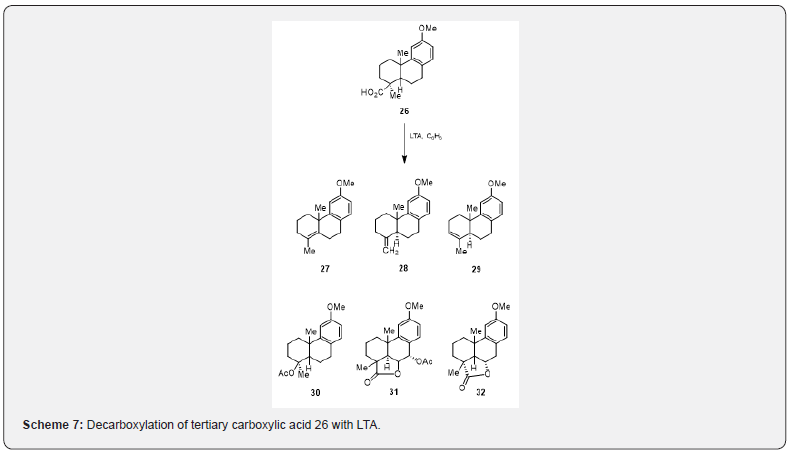
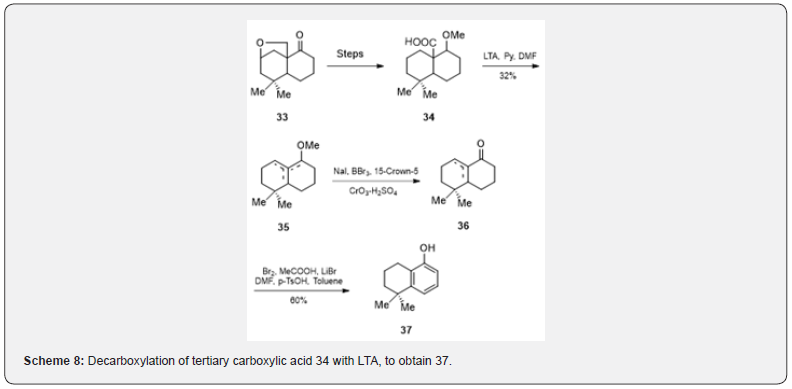
The LTA decarboxylation of tertiary carboxylic acids gives a mixture of alkenes and acetate esters. O-methylpodocarpic acid 26 on heating with LTA yields a mixture of olefins [17] 27, 28, 29 and acetates 30 and 31. In addition a lactone 32 is obtained (Scheme 7). Banerjee and collaborators [18] have observed that the decarboxylation of the acid 34, prepared from the cyclic ether 33, with LTA, pyridine and DMF [19] affords a mixture of olefins 35 (scheme 8). The transformation of 35 into the ketone 36 is affected in two steps: (a) demethoxylation [19] with sodium iodide, boron tribromide, 15-crown ether-5, (b) oxidation [20] with Jones Reagent. Bromination of 36 followed by dehydrobromination and aromatization respectively yield tetraol 37 in 60% Yield. The tetraol 37 is a potential intermediate [21] for the synthesis of diterpenoid quinones cryptotanshinone and tanshinone IIA.
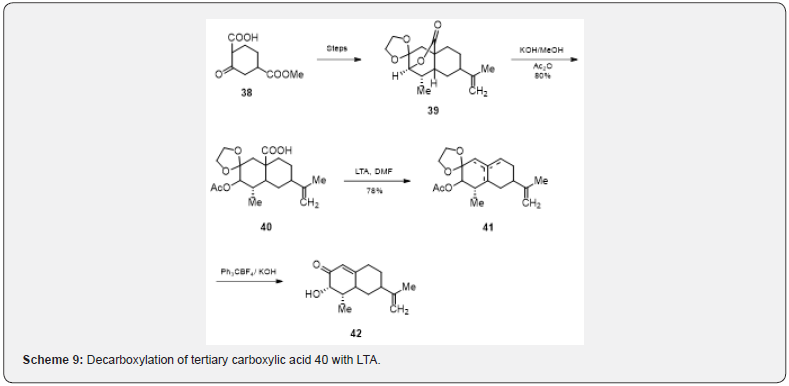
Masamune and collaborators [19] have also studied the decarboxylation of tertiary carboxylic acid with LTA in relation of the studies on the synthesis of the terpene glutinosone. The lactone 39, prepared from the ketoester 38, on alkaline hydrolysis and acetylation respectively is converted to the acid 40 (Scheme 9). The acid 40 on being heated with LTA and DMF undergoes decarboxylation and produces a mixture of olefins 41. Treatment of 41 with methanolic potassium hydroxide (5%) followed by the addition of triphenyl methyl fluoborite afford glutinosone 42.
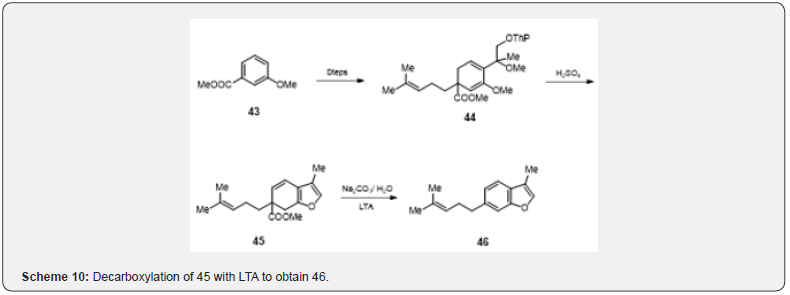
Oxidative decarboxylation reaction has proved usefull in the synthesis [22] of sesquiterpene furoventalene 46 as depicted in the scheme 10. m-Anisic ester 43 is converted into the ester 44 in three steps (reductive alkylation, metalation, and alkylation). Acidic hydrolysis and cyclization of the ester 44 yield dihydro benzofuran 45. Alkaline hydrolysis and oxidative decarboxylation with LTA lead the formation of furoventalene 46. Banerjee and collaborators [23] have utilized oxidative decarboxylation for the synthesis of sesquiterpene (±) frullanalide the details are described in scheme 11.

The ketone acid 48, prepared from the cyclic ether 47, on heating with LTA and copper (II) acetate undergoes oxidative decarboxylation yielding enone 49. Alkylation of 49 with ethyl bromoacetate produces ketoester 50 which on reduction with sodium borohydride in methanol followed by stirring with hydrochloric acid afford the lactone 51. As the lactone 51, has already converted [24] into (±) frullanalide 52 the present synthesis of 51 constitutes a formal total synthesis [25] of frullanalide 52.
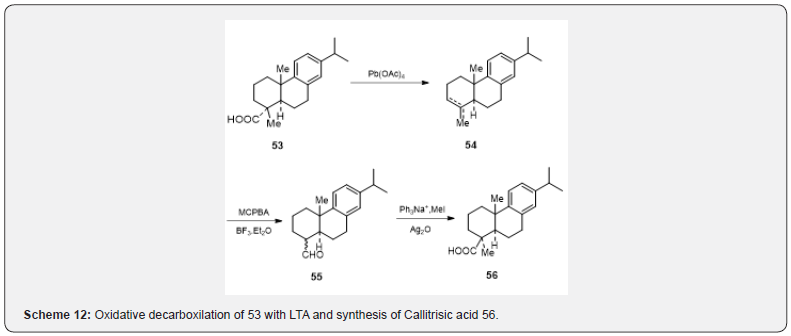
Another interesting use of oxidative decarboxylation [26] with LTA is shown in scheme 12. The acid 53 on decarboxylation with LTA gives a mixture of olefins 54. Epoxidation followed by hydrolysis with Lewis acid produces aldehyde 55 which on methylation and oxidation respectively afford Callitrisic acid 56. The above-mentioned examples exhibit the importance of LTA in the synthesis of terpenoid compounds.
Other Applications
LTA has also been utilized for acetoxylation [27,28] of ketones in enol form, nuclear methylation [29], oxidation [30] of phenols. Alkyl sulfides [31], alkyl hydroperoxides [32] and organometallic compounds are also oxidized33 by LTA. Several cyclic ethers have been synthesized by LTA oxidation [34].
Conclusion
The present review describes application of LTA in cleavage of diols and decarboxylation of carboxylic acids. In near future we expect to see more new applications of LTA in organic synthesis.
Acknowledgement
The authors gratefully acknowledge the collaborations of Ana Caldera, chief of the library Biblioteca Marcel Roche, José Gregorio Rodríguez, and Luis Mujica during the preparation this manuscript.
References
- Bishop CT (1962) Methods Carbohydrate Chemistry.J Chem Educ6: 350-354.
- Tufariello JJ, Kissel WJ (1966) A new method for the oxidative decarboxylation of disubstituted malonic acids using lead tetraacetate. Tetrahedron letters 7(49): 6145 -6150.
- Wolf FJ, Weijlard J (1963) n-Butyl Glioxylate. Org Synth Coll 4: 124.
- Mihailovic ML, Cekuvic Z (1995) Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, LA paquete (Eds.), New York, USA 5: 2949.
- Bunton CA (1995) Oxidation in Organic Chemistry. In:Wiberg K(Edt.),Academic, New York, USA, pp. 398-405.
- Gorin PAJ, Perlin AS (1958) D-Apiose. Canad J Chem 36(3): 480-485.
- Goldschmid HR, Perlin AS (1960) Scission of Stericaixy hindered vic-Diols. Canad J Chem 38(11): 2280-2284.
- Banwell MG, Forman GS (1996) Chemoenzymatic routes to chiral (non-racemic) cyclopropanes. Preparation of a key intermediate for the synthesis of (1R)-cis- pyrethroids. J Chem Soc Perkin Trans 21: 2565-2566.
- Xia J, Hui YZ (1997) The stereospecific synthesis of mixed -acid phospholipids with polyunsaturated fatty acid from D-mannitol. Tetrahedron: Asymmetry 8(3): 451-458.
- Sheldon RA (1972) Oxidative decarboxylation of acids by lead tetraacetate. Organic Reactions 19: 279-421.
- Anderson RJ, Henrick CA (1975) Stereochemical control in witting olefin synthesis. Preparation of the bollworm sex pheromone mixture, gossyplure. J Am Chem Soc 97(15): 4327-4334.
- Birch AJ, Slobbe J (1976) Oxidative decarboxylation of dihydroaromatic acids with lead tetraacetate: a synthesis of olivetol dimethyl ether and of rosefuran. Tetrahedron Letters 17(24): 2079-2082.
- Grob CA, Ohta M, Renk E, Weiss A (1958)Synthese undReaktionen 1-substituerter Bicyclo-[2,2]-octane. Untersuchungen in der Bicyclo – [2,2,2]-octan-Reihe, 2. Mitteilung. Helvetica Chimica Acta 41(5): 1191-1197.
- Kochi JK (1965) The mechanism of oxidative decarboxylation with Lead (IV) acetate. J Am Chem Soc87(8): 1811-1812.
- Van Tamelen EE, Pappas SP (1962) Chemistry of Dewar Benzene. 1,2,5-Tri-t-Butilbicyclo[2.2.0]Hexa-2,5-Diene. J Am Chem Soc 84(19): 3789-3791.
- Meinwald J, Tuffariello JJ, Hurst JJ (1964) Fused Small-Ring compounds.I. Synthesis of Some trans -Bicyclo[3.2.0]heptanes and trans -Bicyclo[4.2.0]octanes1. J Am Chem Soc 29(10): 2914-2919.
- Bennett CR, Cambie RC (1967) Chemistry of the Podocarpaceae-XII: Oxidation of O-methylpodocarpic acid with lead tetra-acetate. Tetrahedron 23(2): 927-941.
- Banerjee AK, Azocar JA, Sulbarán de Carrasco MC, LayaMimó M (2001) Potential intermediates for diterpenoid quinones.: Cryptotanshinone, Tanshinone IIA and Miltirone. Synthetic Communications 31(16): 2471-2478.
- Murai A, Taketsuru H, Masamune T (1980) The Synthesis of (±)-Glutinosone.Bulle Chem Soc Jap53: 1049-1056.
- Bowers A, Halsall TG, Jones ERH, Lemin AJ (1953) The Chemistry of the Triterpenes and related compounds. Part. XVIII.* Elucidation of the Structure of Polyporenic acid C. J Chem Soc Perkin Transactions 1: 2548-2560.
- Danheiser RL, Casebier DS, Firooznia F (1995) Aromatic Annulation Strategy for the synthesis of Angularly Fused Diterpenoid Quinones. Total synthesis of (+)-Neocryptotanshinone, (-)-Cryptotanshinone, Tanshinone IIA, and (±)-Royleanone. J Org Chem 60(26): 8341-8350.
- Castanedo R, Zetina CB, Maldonado LA (1987) An alternate synthesis of Furoventalene using an extension of Hagiwara´s construction of the 3-Methylfuran moiety. Heterocycles 25:175-178.
- Banerjee AK, CanudasGonzález N, Hernández SB, Fuentes SP, PeñaMatheud CA (1990) A formal total synthesis of (±) frullanolide. Journal of Chemical Research – Part S, pp. 158-159.
- Kido F, Tsutsumi K, Maruta R, Yoshikoshi A (1979) Total synthesis of racemic Frullanolide. Chemistry Letters, pp. 311-312.
- Still WC, Schneider MJ (1997) A convergent route to α-substituted acrylic esters and application to the total synthesis of (±)-prullanolide. J Am Chem Soc 99: 948-950.
- Sharma M, Ghatak UR, Dutta PC (1963) Synthetic studies on resin acidsV: Synthesis of three isomers of dehydroabietic acid. Tetrahedron 19: 985-994.
- Fieser LF, Stevenson R (1964) Cholesterol and Companions. IX. Oxidation of Δ5-Cholestene-3-one with Lead Tetraacetate. J Am Chem Soc 76(7): 1728-1733.
- Fieser LF, Romero MA (1953) Rearrangement of 6β-Bromo- Δ4-cholestene-3-one to 2α-Acetoxy- Δ4-3-one-cholestene-3-one1. J Am Chem Soc 75(19): 4716-4719.
- Fieser LF, Chang FC (1942) Alkylation of α-Naphtoquinones with Esters of Tetravalent Lead.J Am Chem Soc 64(9): 2043-2052.
- Mihalovic MLJ, Ceekovic LJ (1971) The chemistry of the hydroxyl group, TAI, s. Ed. Wily. New York, USA, Part 1,pp. 505-592.
- Field L, Lawson JE (1958) Organic Disulfides and Related Substances. I. Oxidation of Thiols to Disulfides with Lead Tetraacetate. J Am Chem Soc 80(4): 838-841.
- Kropf H, Von Wallis H (1981) 1,2-Dioxane and 1,2-Dioxepane durch Barton-Typ-ReaktionausHydroperoxiden. Synthesis 8: 633-635.
- Corey EJ, Wollenberg RH (1974) Nucleophilic ethynyl group equivalent and its use in conjugate addition to alpha.,.beta.-enones. J Am Chem Soc 96(17):5581-5583.
- Banerjee AK (1996) Synthesis of Cyclic Ethers and their Utility in the Synthesis of Terpenoid Compounds. Journal of Scientific & Industrial Research 55: 915-930.






























