Quinoline-Substituted 1,2,3-Triazole-Based Molecules, As Promising Conjugated Hybrids in Biomedical Research
Luz Karime Luna Parada1, Leonor Yamile Vargas Méndez2 and Vladimir V Kouznetsov1*
1Laboratorio de Química Orgánica y Biomolecular, CMN, Universidad Industrial de Santander, Parque Tecnológico Guatiguara, Piedecuesta, Colombia
2Grupo de Investigaciones Ambientales para el Desarrollo Sostenible, Facultad de Química Ambiental, Universidad Santo Tomás, Bucaramanga, Colombia
Submission: May 29, 2018; Published: June 07, 2018
*Corresponding author: Vladimir V Kouznetsov, Laboratorio de Química Orgánica y Biomolecular, CMN, Universidad Industrial de Santander, Parque Tecnológico Guatiguara, Km 2 vía refugio, Piedecuesta, A.A. 681011, Colombia, Email: vkuznechnik@gmail.com
How to cite this article: Luna Parada LK, Vargas Méndez LY, Kouznetsov VV. Quinoline-Substituted 1,2,3-Triazole-Based Molecules, As Promising Conjugated Hybrids in Biomedical Research. Organic & Medicinal Chem IJ. 2018; 7(2): 555708. DOI: 10.19080/OMCIJ.2018.07.555708
Abstract
Building a single molecular framework containing quinoline and triazoles rings of promising biological activity through molecular hybridization strategy offers the viewpoint of better drugs for the treatment of a number of diseases including cancer, malaria, tuberculosis and other illnesses. Presenting modified selectivity profile, different and/or dual modes of action and reduced undesired side effects, these molecular hybrids are gaining momentum worldwide showing their promise in drug discovery. Such hybrid molecules are usually made efficiently through click chemistry technique using multicomponent reactions. In this paper, we discuss chemistry and biology of some quinoline-substituted 1,2,3-triazole hybrids aiming at a better understanding of physicochemical properties of quinoline-triazole hybrids for designing novel bioactive chemical entities. So, principal Lipinski’ parameters and in silico study (lipophilicity and molecular polar surface area) are also briefly mentioned and applied to selected quinoline-1,2,3-triazole hybrids. The mini-review is divided in two general parts: synthetic aspects of the preparation of these hybrids and their pharmacological aspects paying attention to the widespread dangerous diseases such as malaria, cancer, and human fungal infections, especially systemic mycoses.
Keywords: Molecular Hybridization; Quinoline-1,2,3-triazole Hybrids; Lipinski’ Parameters and in silico Study; Azide-Alkyne Cycloaddition Reactions; Cancer; Malaria; Candidiasis
Abbrevations: CQ: Cloroquinine; Qu: Quinoline; TA: Triazoles; PhM: Pharmacophoric Molecule; CuAAC: Copper-Catalyzed Azide-Alkyne Cycloaddition Reactions; NaAsc: Sodium Ascorbate; CRT: Chloroquine Resistance Transporter; PfCRT: P. falciparum Chloroquine Resistance Transporter; TPSA: Molecular Polar Surface Area; GPCR: G Protein–Coupled Receptor
Introduction
The molecular hybridization of biologically active molecules is a powerful strategy for rational design of new ligands or prototypes based on the recognition of pharmacophoric sub-unities in the molecular structure of two or more known bioactive derivatives which, through the adequate fusion of these sub-unities, lead to the drawing of new hybrid architectures that maintain pre-selected characteristics of the original templates [1,2]. Nowadays, it is well recognized that the main goals for exploiting the hybridization techniques for drug design are; 1) to achieve improved activity, 2) to gain higher selectivity, 3) to lower toxicity of drugs. Some of the pharmacological properties exerted by molecular hybrids of N-heterocycles comprise remarkable applications for drug treatment of serious life-threatening conditions such as cancer [3], bacterial or protozoan infections [4-6] and others. Additionally, such combination of two structurally different heterocycles in a single molecule to form molecular hybrids, e.g. heterobifuctional molecules, with improved biological profile could be the suitable and practical solution to stop or slow down the spread of drug resistance [7]. As result, so-called molecules with “two heads” have become very popular [8]. Generally, these molecules are classified in three types: merged, fused and conjugated molecular hybrids. Merged hybrids are those contain underlying two pharmacophores. When these pharmacophores exist as unique parts without superposition, molecules are called fused hybrids. Finally, conjugated hybrids (molecular conjugates) are usually separated by a discrete linker (or space) group that is not found in either of the selective sub-unit [9,10] (Figure 1). These latter hybrids are in the center of our mini-review.
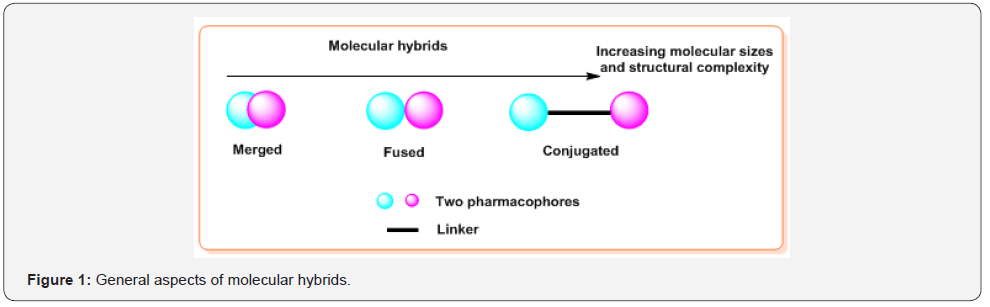
Among numerous N-heterocycles, quinolines and 1,2,3-triazoles are two important classes of small heterocyclic molecules, which have a wide range of chemotherapeutic uses. Remarkably, while quinoline molecules are present commonly in living organisms as important secondary metabolites [11], the 1,2,3-triazoles, chemically inert compounds, have not been detected in naturally occurring products [12]. Nevertheless, both quinoline and 1,2,3-triazole skeletons form part in diverse and numerous conjugated hybrids, valuable biomolecules for drug development. This molecular diversity comes from the multiple possibilities of the combination between quinoline (Qu) and 1,2,3-triazole (TA) nucleus. First, there are three possibilities to connect TA ring with Qu skeleton: 1-(quinolinyl)-1,2,3-triazoles, 4-(quinolinyl)-1,2,3-triazoles and 5-(quinolinyl)-1,2,3-triazoles. Moreover, 1,2,3-TA can act as pharmacophores (Figure 2A) and linkers between quinoline and other pharmacophoric molecules (PhM) (Figure 2B) of interest in molecular hybridization approaches [13,14] (Figure 2).

In this work, we discuss chemistry and biology of some quinoline-substituted 1,2,3-triazole hybrids reported as potent biological agents. Principal Lipinski’ parameters and in silico study are also briefly mentioned and applied to selected quinoline- 1,2,3-triazole hybrids. Therefore, this mini-review aims at a better understanding of physicochemical properties of quinoline-triazole hybrids for designing novel bioactive chemical entities. The minireview is divided in two general parts: synthetic aspects of the synthesis of these hybrids and their pharmacological aspects paying attention to the widespread dangerous diseases such as malaria, cancer, and human fungal infections, especially systemic mycoses.
Synthetic Aspects
One of the most important and practical approaches to the 4-(quinolinyl)-1,2,3-triazoles 3 is the modified Huisgen reaction between quinoline 1 with terminal alkyne moiety and an organoazide 2 through a catalytic 1,3-dipolar cycloaddition process [15] (Figure 3A). Noteworthy, that the Huisgen reaction, which occurs without any catalysts, gives poorly only a regioisomeric mixture of products that reduced its practical application [16], while Meldal and Fokin-Sharpless modified protocols [17,18] that use copper catalysts (CuI, CuBr, CuSO4, Cu2O), allow obtaining exclusively 1,4-disubstituted 1,2,3-triazoles, e.g. 4-(quinolinyl)- 1,2,3-triazoles 3 in very high yields. Another approach to obtain similar 1,4-disubstituted 1,2,3-triazoles, e.g. 1-(quinolinyl)- 1,2,3-triazoles 7 consists in cycloaddition reactions between terminal alkynes such as alkynes 5 and quinoline-azides 6 under the same condition reactions (Figure 3B). In general, sub-product, 1,5-disubstituted 1,2,3-triazoles 4 and 8 could be regioselectively obtained using ruthenium cyclopentadienyl complex (Cp*RuCl(PPh3)2) as catalyst [19]. However, this version of Huisgen cycloaddition reaction has not yet been applied widely and these Qu-TA hybrids were not obtained (Figure 3).
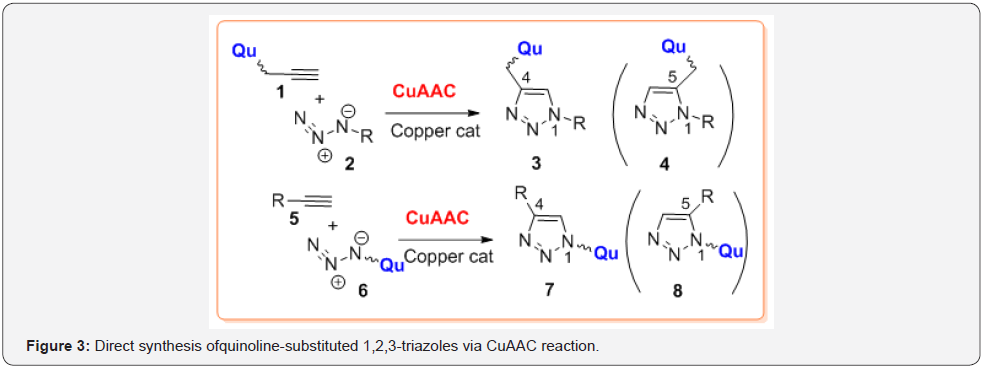
So-called “click” copper-catalyzed azide-alkyne cycloaddition reactions (CuAAC) have become the focus of many chemists and were found wide application in drug synthesis, chemical biology, among others.
Some synthetic aspects of this reaction should be taken into consideration before the preparation of desired quinolinesubstituted 1,2,3-triazole hybrids 3 and 7 [15]:
i. The standard protocol, which consists in using the costefficient salt CuSO4 .5H2O (or Cu(OAc)2 .H2O) and ascorbic acid (sodium ascorbate, NaAsc), is usually realized in a mixture of water and alcohol as solvent (“Sharpless-Fokin conditions”). Water, in combination with other alcohols (MeOH, t-BuOH), THF, DMF, and DMSO, could all be employed as co-solvents for this cycloaddition reaction, enabling a wide range of substrates to be adapted;
ii. Alkynyl quinolines 1, common key intermediates in this transformation, are not available and must be prepared from hydroxy-quinoline derivatives and others;
iii. To improve the utility and manipulability of this process for generation of desired products 3, a microwave-assisted three-component reaction of alkyl halides, sodium azide, and alkynyl quinolines is developed.
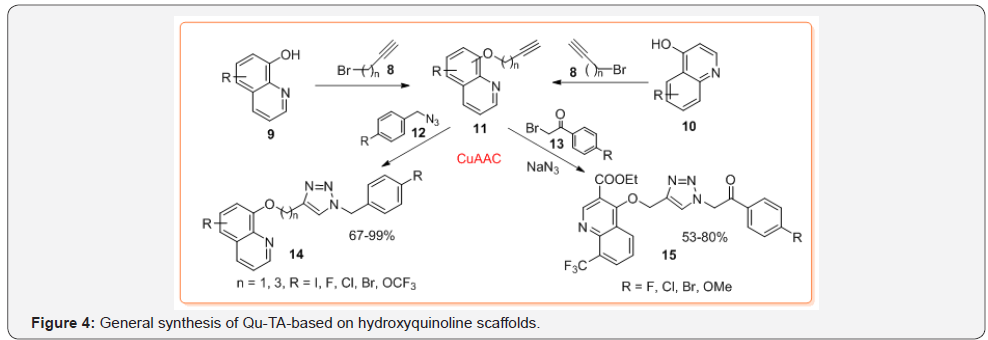
To obtain O-alkynyloxyquinolines 11, respective hydroxyquinolines (mainly, 4(8)-hydroxyquinoline derivatives 9 and 10) are O-alkylated using propargyl bromide 8 or its analogs. Subsequently, solutions of the O-alkynyl- oxyquinolines obtained are usually treated with respective pre-formed azides 12 [20] or in situ forming organoazides from alkyl bromides 13 and NaN3 under reaction condition described above or in an alternative solvents, e.g. PEG-400 [21] that give hybrid products 14,15 in good yields (Figure 4). Noteworthy, that while some 8-hydroxyquinoline derivatives are commercially available, 4-hydroxyquinolines (quinolin-4-ones) must be prepared using common Gould- Jacobs or Conrad-Limpach protocols. In contrast, synthesis of Qu-TA-based on aminoquinolines 20 (mostly, 4-aminoquinoline derivatives) starts with the reaction between 4-chloro (bromo) quinolines 16 and propargyl amine or its derivatives 17 to give N-alkynylaminoquinolines 18. This reaction needs prolonged heating (until 63 h) in a sealed tube at 110 °C. Moreover, applying a CuACC reaction (CuSO4∙5H2O/NaAsc, tert-BuOH-water 1:1, 40 °C) between alkynes 18 and aryl azides 19, final hybrids products 20 are formed in poor to moderate yields [22] (Figure 5).

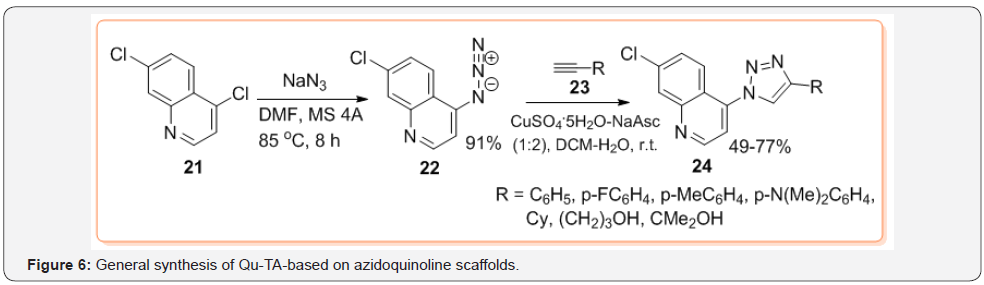
Regarding the synthesis of 1-(quinolinyl)-1,2,3-triazoles 7, among azidoquinolines type 6 (Figure 3), 4-azido-7-chloroquinoline 22, easily obtained from cost-effective and commercially available 2,7-dichloroquinoline 21, is more exploited in synthesis of new Qu-TA hybrid molecules type 24 until now [23] (Figure 6). Although application of CuAAC reaction with non-conventional reaction systems (microwave, ultrasound or flow chemistry, etc.) has given rise to various varieties of triazoles with interesting biological properties, synthesis of conjugated quinoline-1,2,3-triazole hybrids such as 14,15 and 20 is still performed using Sharpless-Fokin catalytic system in solution. Even though some these reactions are efficiently, there is a persistent incentive to find greener alternatives, which would reduce time and energy requirements as well as waste generated by these reactions. Recently, preparation of 4-(quinolinyl)-1,2,3-triazole hybrids 30 via mechanochemical CuAAC reaction of O-alkynyloxyquinolines 28 and aryl azides 29 has been described implementing ball milling technique (Teflon vessel with two stainless-steel milling balls of 7 mm) [24]. The O-alkynyloxyquinolines 28 are prepared in two step common protocol using Conrad-Limpach reaction of anilines 25 with ketoesters 26 to give quinolone derivatives 27, which are treated with propargyl bromide 8 (Figure 7).
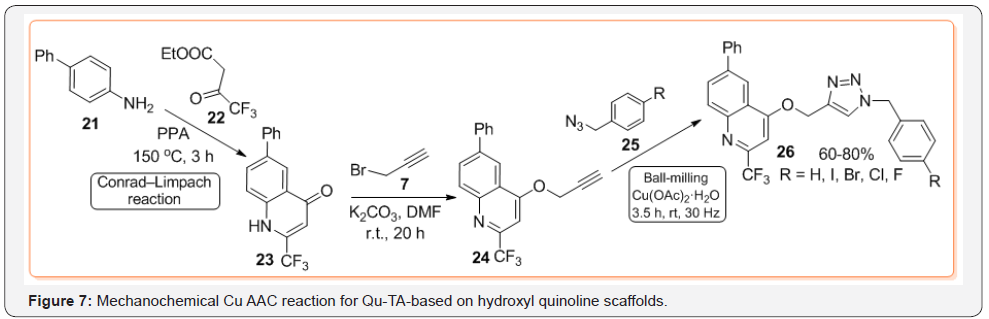
Compared to traditional solvent-based procedures, these mechanochemical reactions proved to be more efficient under solvent-free ball-milling conditions, with ca. 15-fold increase in yields of products. Showing efficacy and environmental importance, ball milling process is becoming clear that this once obscure discipline is converting increasingly mainstream [25].
Another green aspect of CuAAC reaction is the application of glycerol as solvent in the synthesis of 1,2,3-triazoles using a three-component reaction of organic halides, terminal acetylenes, and sodium azide. Like water, glycerol is also naturally available, highly hydrophilic, environmentally friendly, and inexpensive. Thus, a variety of 1,2,3-triazoles were prepared in good to excellent yields (37-99%) with this green solvent. The published procedure involves the use of CuI and diethylamine, which are two easily available reagents as the new catalytic system at room temperature. This simple and low-cost protocol exhibits wide substrate scope and ambient reaction condition and can be easily used for large-scale preparation [26]. Both environmental implementations in this reaction could serve in the synthesis of new Qu-1,2,3-TA hybrids. These multicomponent reactions and/ or click chemistry are important synthetic tools to synthesize such hybrids.
Pharmacological Aspects
Recently obtained 1,2,3-triazole-appended quinolines, including 4-(quinolinyl)-1,2,3-triazole and 1-(quinolinyl)-1,2,3- triazole hybrids as promising lead compounds with large spectra of pharmacological effects, stimulate their further biological investigation. The triazole functionality are usually introduced into privileged N-heterocycles like quinolines because of its favorable properties, including bridge moderate dipole character, hydrogen bonding capability, rigidity and stability under in vivo conditions, which are evidently responsible for their diverse biological activities. Such molecular hybrids could be very suitable in the solution of multiple drugs resistance in malaria, especially cloroquinine (CQ)-resistant P. falciparum which emergence and spread are the major obstacles in the control of this disease.
Malaria remains one of the most serious infectious diseases; it threatens nearly half of the world’s population and led to hundreds of thousands of deaths in 2015, predominantly among children in Africa. Thus, based on general accepted resistance mechanism, which involves two integral membrane proteins, localized in the parasite’s digestive vacuole membrane; - plasmodial P-glycoprotein homolog1 (Pgh1) and CQ resistance transporter (CRT) [10,27], conjugated molecules derived from CQ may be less efficiently extruded by CQ-resistant P. falciparum, having some enzyme effect that prevents them from fitting into the substrate binding site of P. falciparum chloroquine resistance transporter (PfCRT).
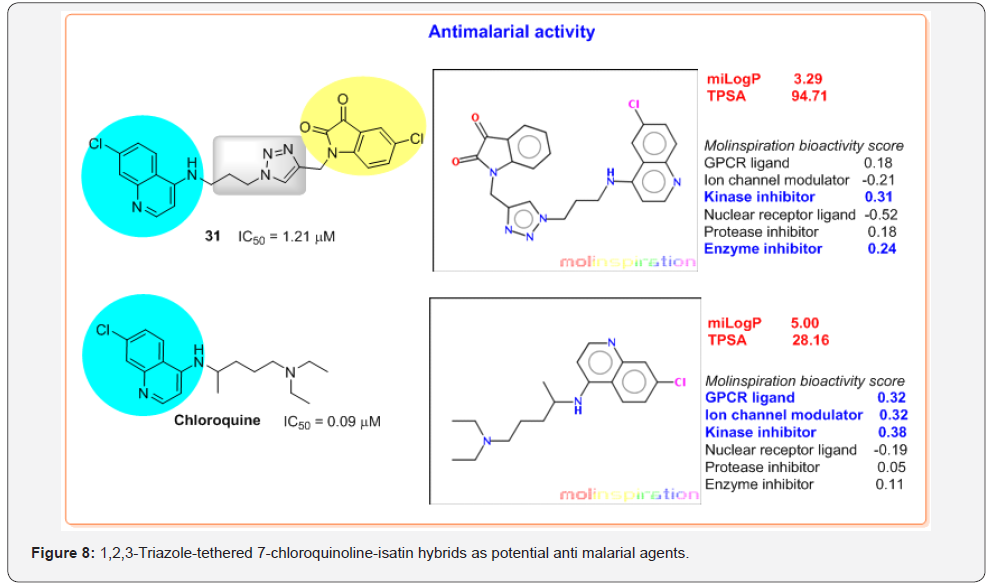
Some of such conjugated molecules are 1H-1,2,3-triazoletethered 7-chloroquinoline-isatin hybrids. The compound 31 with an optimum combination of longer alkyl chain length and chloro substituent at C-5 position of isatin ring displayed the best activity with IC50 value of 1.21 μM against the CQ-resistant P. falciparum W2 strain [28] (Figure 8).
It is well-known that the pharmacological activity of some compounds is strictly related to its hydrophilic/lipophilic nature because to display therapeutic properties they must pass through a series of biological barriers, from the administration site to the site of action.
Therefore, from the physicochemical properties of biologically active compounds, lipophilicity (expressed in LogP, where P is the octanol/water partition coefficient) and molecular polar surface area (TPSA) are major descriptors of great importance and should be determined from the early stages of drug development because they affect basic steps of a drug’s pharmacokinetics and pharmacodynamics, such as absorption, including intestinal absorption, bioavailability, Caco-2 permeability and blood-brain barrier penetration (Lipinski’s Rule of 5 and Veber’s criteria), as well as compound-receptor interactions score for the most important drug targets.
These properties could be easily calculated by diverse software available online. Paying attention to the importance of knowing these physicochemical properties, we used Molinspiration software [29] for the 1,2,3-triazole-tethered 7-chloroquinolineisatin 31 and chloroquine, historical antimalarial drug. Analyzing the results obtained, it can be noted that hybridization of CQ with 5-chloro-isatin linked by 1,2,3-triazole ring produced a drastic change in the calculated lipophilicity, cLogP (miLogP from Molinspiration software) and TPSA parameter (Figure 8).
According to the cLogP values, both quinoline compounds show high bioavailability properties. However, hybrid 31 is a molecule less lipophilic (cLogP = 3.29) than chloroquine (cLogP = 5.00). On the other hand, while TPSA values predict a very good bioavailability for chloroquine (TPSA = 28.16), TPSA parameter of hybrid 31 are higher (TPSA = 94.71) that suggests that this chloroquinoline-isatin hybrid could have some problems of the absorption and barrier penetration. Additionally, according to the predicted bioactivity score, hybridization of CQ with 5-chloroisatin linked by 1,2,3-triazole ring makes to switch biological targets: while chloroquine would affect G protein–coupled receptor (GPCR), ion channels and kinases, hybrid 31 would work as an enzyme and/or kinase inhibitor. These theoretical results evince that Qu-TA hybrid formation is a valuable approach in the development of novel antimalarial (lead) compounds [30,31].
Derivatization of the hydroxyl group from 8-hydroxyquinoline by attaching a 1,2,3-triazole ring presents a good strategy for searching novel molecules against cancer cell lines, because both 8-hydroxyquinoline (or its derivatives) and 1,2,3-triazoles showed interesting cytotoxic properties [32-34]. For example, Clioquinol (5-chloro-7-iodo-8-hydroxyquinoline, CIHQ) and Nitroxoline (8-hydroxy-5-nitroquinoline) have been shown to have anticancer activity both in vitro and in vivo [35,36]. The latter hydroxyquinoline is more potent than the CIHQ suggesting that it may be useful in treating cancer [37]. Moreover, the simple 8-hydroxy-2-quinolinecarbaldehyde showed also potent in vitro cytotoxicity against the human cancer cell lines [38].
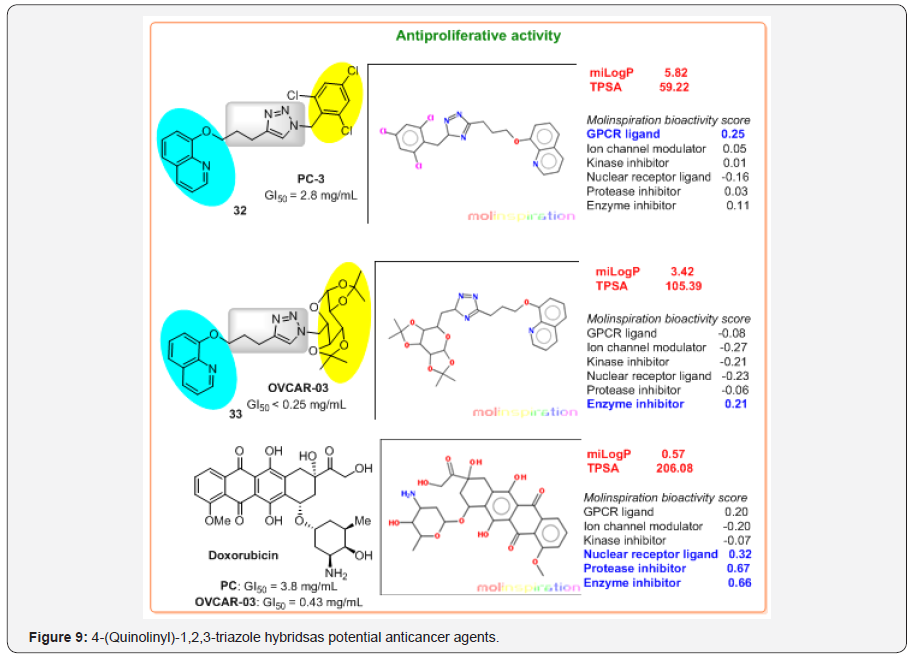
That is why, it is not surprising that new hybrids are available, but it is shocking that so far examples are rare. In 2014, evaluating in vitro for the antiproliferative activity on various cancer cell types: U251 (glioma, CNS), MCF-7 (breast), NCI-ADR/RES (ovarian expressing phenotype multiple drugs resistance), 786-0 (kidney), NCI-H460 (lung, non-small cells), PC-3 (prostate) and OVCAR-03 (ovarian), Alves and co-workers reported at the first time that 4-(quinolinyl)-1,2,3-triazole hybrids based on 8-hydroxyquinoline skeleton and 1,2,3-TA ring containing aromatic or protected sugar moieties should be explored as anticancer agents [39]. Two of these hybrids, trichlorobenzyl-1H-1,2,3-triazol-4-yl-quinoline 32 and α-D-galactopyranosyl-1H-1,2,3-triazol-4-yl-quinoline 33 disclosed potent and selective antiproliferative activity, expressed in 50% cell growth inhibition or a cytostatic effect (GI50) (Figure 9).
The first, “more simple” hybrid 32 showed potent activity against prostate cell lines (PC-3, GI50 = 2.8 mg/mL) compared with activity of Doxorubicin (Adriamycin) (GI50 = 3.8 mg/mL), used in oncologic practice since the late 1960s. However, this hybrid possesses poor selectivity index (SI) value of 1.9 regarding normal cells, human keratinocytes (HaCat). Second hybrid 33 exhibited remarkable GI50 value (GI50 < 0.25 mg/mL) against ovarian cancer (OVCAR-03) cells, indicating that it was even more active than Doxorubicin (GI50 = 0.43 mg/mL). This hybrid presented the high selectivity with SI value of >117.6 for ovarian cancer cell that suggests the potential use of this compound for future in vivo tests. However, it showed weak antiproliferative activity against other cancer cell lines.
The physicochemical parameters of the selected hybrids 32,33, Doxorubicin as reference anticancer drug and probable enzyme activity scores for the most important drug targets obtained through Molinspiration software [29], gave an interesting information that should be commented:
a) Doxorubicin, natural antitumor antibiotic, is a tetracyclic molecule with much less lipophilic property (cLogP = 0.57), e.g. with good H2O solubility, while hybrid compounds 32,33 are more lipophilic molecules (cLogP = 3.42-5.82);
b) Between these hybrid molecules there is only a structural difference (trichlorobenzyl fragment versus protected sugar moiety), which is responsible for their molecular properties;
c) Although both hybrids showed a potent in vitro antiproliferative activity overcoming activity of Doxorubicin, their TPSA values specify some problems of the absorption and barrier penetration;
d) Chemical nature of second pharmacophores in these hybrids makes to change biological targets predicting GPCR ligand capacity for compound 32 and kinase inhibitor property for hybrid 33 indicating that these compounds would possess diverse mode of antitumor action. Interestingly, predicted drug-receptor interactions for Doxorubicin are more pronounced and very different to those of hybrids 32,33 (Figure 9).
e) This simple and brief analysis could help to design better new Qu-1,2,3-TA hybrids with antitumor properties.
The frequency of human fungal infections has increased drastically worldwide, in particular, systemic mycoses that are very difficult to diagnose and treat [40]. Cancer patients with metastasis, AIDS patients infected by HIV and organ transplant patients, all these immunocompromised patients have a high probability of invasive fungal infections, especially opportunistic invasive mycoses like candidiasis [41]. It is well-known that within the limited antifungal drug collection, the azole antifungals are the most frequent class used to treat Candida infections and one of the most commonly prescribed antifungal drugs used for most C. albicans infections is Fluconazole, a small molecule based on two 1H-1,2,4-triazole rings (Figure 10).
As Fluconazole (FLC) is fungistatic rather than fungicidal, the treatment provides the opportunity for acquired resistance to develop in the presence of this antifungal [42]. Therefore, there is a need for developing antifungal agents with novel structure and mode of action. In this context, quinoline-substituted 1,2,3-triazolebased molecules are suitable models. Abid and co-workers at the first time designed and synthesized 8-hydroxyquinolines bearing 1,2,3-triazole ring as potent inhibitors of Candida species [43,44]. Their research showed that two hybrids of this type, 34 and 35 (Figure 10) have potent IC50 values for C. albicans ATCC 90028 (standard) and C. albicans D15.9 (fluconazole resistant) strains and significant inhibitory effect on the growth of FLC-sensitive as well as FLC-resistant C. albicans strains (Table 1). Interestingly, growth curve and time kill curve studies indicated that hybrid 34 has fungicidal nature while compound 35 was fungistatic. Curiously, Fluconazole is fungistatic and may be fungicidal, depending on the concentration. Moreover, both hybrid compounds showed significant inhibition in ergosterol biosynthesis like does Fluconazole.

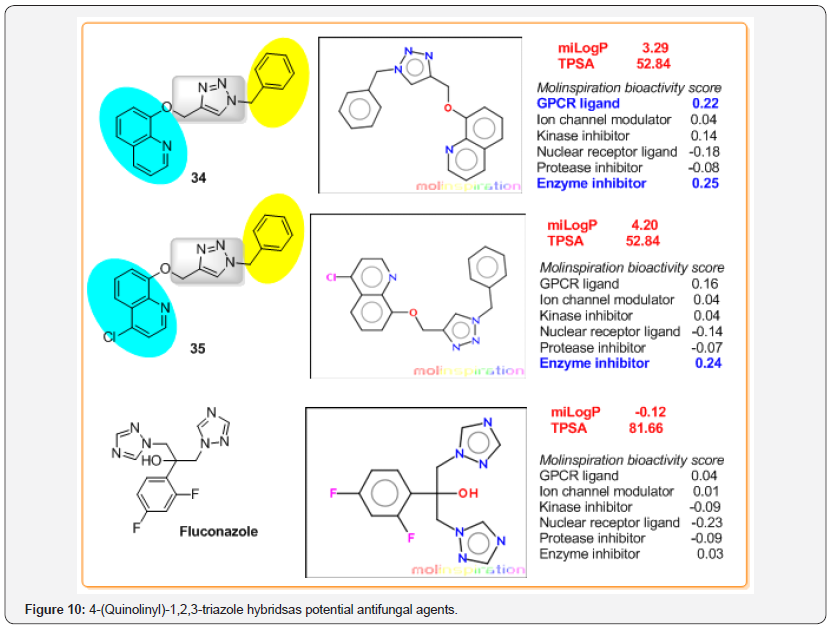
Noteworthy, that both compounds 34 and 35 were significantly less cytotoxic than Fluconazole. Analyzing their physicochemical properties obtained in silico study, we could note that calculations predict negative partition coefficient values (cLogP = -0.12) of Fluconazole indicating that its very high solubility, while both hybrid compounds 34,35 possess appropriate cLogP values (cLogP < 5) according to the Lipinski’s rule. Interestingly, in practice, Fluconazole, white crystalline solid is slightly soluble in water, but has high oral efficacy caused by high solubility in gastric juice [45]. When cLogP and TPSA parameters for these hybrids are compared, the role of chlorine atom, present in quinoline ring (comp. 35), can be seen: the presence of Cl enhances lipophilicity, but does not change polar surface area of the molecule although chlorine atom is highly electronegative atom.
Another interesting detail is that these hybrids would affect various receptor interactions, whereas the predicted FLC-receptor interaction scores are minimal. However, it should be commented that there are plenty of examples available for such prediction violation amongst the existing drugs. Precisely, majority of violations come from antifungals and others like antibiotics, vitamins, and cardiac glycoside [46].
Conclusion
The molecular hybridization strategy offers the possibility of building a single molecular framework containing quinoline and triazoles rings with modified selectivity profile, different and/or dual modes of action and reduced undesired side effects. These molecular hybrids are especially interesting for the development of new prototypes in the treatment of cancer and parasitic diseases including malaria, protozoan and fungal infections. Due to synthetic versatility of catalyzed azide-alkyne cycloaddition reactions, it can be expected that these efficient reactions and rational design based on in silico study could provide diverse novel quinoline-triazole prototypes for innovating drugs in the near future. It is evident that the future is optimistic and that this type of design will lead to important anticancer and antiparasitic drugs with higher activity, better selectivity and lower toxicity than the traditional combination therapies.
References
- Beruvé G (2016) An overview of molecular hybrids in drug discovery. Expert Opin Drug Discov 11 (3): 281-305.
- Viegas-Junior C, Danuello A, da Silva Bolzani V, Barreiro EJ, Fraga CAM (2007) Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem 14(17): 1829-1852.
- Nepali K, Sharma S, Sharma M, Bedi PMS, Dhar KL (2014) Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem 77: 422- 487.
- Xiao ZP, Wang XD, Wang PF, Zhou Y, Zhang JW, et al. (2014) Design, synthesis, and evaluation of novel fluoroquinolone–flavonoid hybrids as potent antibiotics against drug-resistant microorganisms. Eur J Med Chem 80: 92-100.
- Cavalli A, Bolognesi ML (2009) Neglected tropical diseases: multitarget- directed ligands in the search for novel lead Candidates against Trypanosoma and Leishmania. J Med Chem 52(23): 7339-7359.
- Hu YQ, Gao C, Zhang S, Xu L, Xu Z, et al. (2017) Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur J Med Chem 139: 22-47.
- Corson TW, Aberle N, Crews CM (2008) Design and applications of bifunctional small molecules: why two heads are better than one. ACS Chem Biol 3(11): 677-692.
- Saadeh HA, Mubarak MS (2017) Hybrid Drugs as Potential Combatants Against Drug-Resistant Microbes: A Review. Curr Top Med Chem 17(8): 895-906.
- Morphy R, Rankovic Z (2005) Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J Med Chem 48(21): 6523-6543.
- Kouznetsov VV (2013) New Quinoline-based Multiple Ligands in Antimalarial Drug Development. In Banet AC, Brassier PE (Eds.), Antimalarial Drug Research and Development Nova Biomedica, New York, USA, pp. 69-128.
- Chung PY, Bian ZX, Pun HY, Chan D, Chan ASC, et al. (2015) Recent advances in research of natural and synthetic bioactive quinolines. Future Med Chem 7(7): 947-967.
- Faraz KM, Garima V, Wasim A, Akranth M, Mumtaz AM, et al. (2017) Synthetic Trends Followed for the Development of 1,2,3-Triazole Derivatives. Inter J Drug Develop Res 9(2): 22-25.
- Agalave SG, Maujan SR, Pore VS (2011) Click chemistry: 1,2,3-triazoles as pharmacophores. Chem Asian J 6(10): 2696-2718.
- Dhee D, Singh V, Shankar R (2017) Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg Chem 71: 30-54.
- Kouznetsov VV, Vargas Méndez LI, Zubkov F (2017) Recent Advances in the Synthesis of Bioactive Quinoline-Based 1, 2, 3-Triazoles via Cu- Catalyzed Huisgen 1, 3-Dipolar Cycloaddition (“Click Reaction”). Mini Rev Org Chem 13(6): 488-503.
- Huisgen R (1963) 1, 3‐Dipolar cycloadditions. Past and future. Angew Chem Int Ed Engl 2(10): 565-598.
- Tornøe CW, Christensen C, Meldal M (2002) Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J Org Chem 67(9): 3057-3064.
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed 41(14): 2596-2599.
- Rasmussen LK, Boren BC, Fokin VV (2007) Ruthenium-Catalyzed Cycloaddition of Aryl Azides and Alkynes. Org Lett 9(26): 5337-5339.
- Irfan M, Aneja B, Yadava U, Khan SI, Manzoor N, et al. (2015) Synthesis, QSAR and antiCandidal evaluation of 1,2,3-triazoles derived from naturally bioactive scaffolds. Eur J Med Chem 93: 246-254.
- Garudachari B, Isloor AM, Satyanarayana MN, Fun HK, Hegde G (2014) Click chemistry approach: Regioselective one-pot synthesis of some new 8-trifluoromethylquinoline based 1,2,3-triazoles as potent antimicrobial agents. Eur J Med Chem 74: 324-332.
- Fisher GM, Tanpure RP, Douchez A, Andrews KT, Poulsen SA (2014) Synthesis and Evaluation of Antimalarial Properties of Novel 4-Aminoquinoline Hybrid Compounds. Chem Biol Drug Des 84(4): 462-472.
- Pereira GR, Brandão GC, Arantes LM, de Oliveira Jr HA, de Paula RC, et al. (2014) 7-Chloroquinolinotriazoles: Synthesis by the azide-alkyne cycloaddition click chemistry, antimalarial activity, cytotoxicity and SAR studies. Eur J Med Chem73: 295-309.
- Tireli M, Maračić S, Lukin S, Kulcsár MJ, Žilić D, et al. (2017) Solvent-free copper-catalyzed click chemistry for the synthesis of N-heterocyclic hybrids based on quinoline and 1, 2, 3-triazole. Beilstein J Org Chem 13: 2352.
- Andersen J, Mack J (2018) Mechanochemistry and organic synthesis: from mystical to practical. Green Chem 20(7): 1435-1443.
- Guo S, Zhou Y, Dai B, Huo C, Liu C, et al. (2018) CuI/Et2NH-Catalyzed One-Pot Highly Efficient Synthesis of 1, 4-Disubstituted 1, 2, 3-Triazoles in Green Solvent Glycerol. Synthesis 50(11): 2191-2199.
- Petersen I, Eastman R, Lanzer M (2011) Drug‐resistant malaria: Molecular mechanisms and implications for public health. FEBS Lett 585(11): 1551-1562.
- Raj R, Singh P, Singh P, Gut J, Rosenthal PJ, et al. (2013) Azide-alkyne cycloaddition en route to 1H-1, 2, 3-triazole-tethered 7-chloroquinolineisatin chimeras: synthesis and antimalarial evaluation. Eur J Med Chem 62: 590-596.
- http://www.molinspiration.com
- Vandekerckhove S, Dhooghe M (2015) Quinoline-based antimalarial hybrid compounds. Bio org Med Chem 23(16): 5098-5119.
- Hu YQ, Gao C, Zhang S, Xu L, Xu Z, et al. (2017) Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur J Med Chem 139: 22-47.
- Oliveri V, Vecchio G (2016) 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur J Med Chem 120: 252-274.
- Xu H, Chen W, Zhan P, Liu X (2015) 8-Hydroxyquinoline: a privileged structure with a broad-ranging pharmacological potential. Med Chem Comm 6(1): 61-74.
- Prachayasittikul V, Pingaew R, Anuwongcharoen N, Worachartcheewan A, Nantasenamat C, et al. (2015) Discovery of novel 1, 2, 3-triazole derivatives as anticancer agents using QSAR and in silico structural modification. Springer Plus 4(1): 571.
- Oliveri V, Giuffrida ML, Vecchio G, Aiello C, Viale M (2012) Gluconjugates of 8-hydroxyquinolines as potential anti-cancer prodrugs. Dalton Trans 41(15): 4530-4535.
- Cao B, Li J, Zhou X, Juan J, Han K, et al. (2014) Clioquinol induces prodeath autophagy in leukemia and myeloma cells by disrupting the mTOR signaling pathway. Sci Rep 4: 5749.
- Jiang H, Taggart JE, Zhang X, Benbrook DM, Lind SE, et al. (2011) Nitroxoline (8-hydroxy-5-nitroquinoline) is more a potent anti-cancer agent than clioquinol (5-chloro-7-iodo-8-quinoline). Cancer Lett 312(1): 11-17.
- Chan SH, Chui CH, Chan SW, Kok SH, Chan D, et al. (2013) Synthesis of 8-hydroxyquinoline derivatives as novel antitumor agents. ACS Med Chem Lett 4(2):170-174.
- De O Freitas LB, Borgati TF, de Freitas, RP, Ruiz AL, Marchetti GM, et al. (2014) Synthesis and antiproliferative activity of 8-hydroxyquinoline derivatives containing a 1,2,3-triazole moiety. Eur J Med Chem 84: 595- 604.
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. (2012) Hidden killers: human fungal infections. Sci Transl Med 4(165): 165rv13.
- Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, et al. (2017) Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol 7: 2173.
- Berkow EL, Lockhart SR (2017) Fluconazole resistance in Candida species: a current perspective. Infect Drug Resis 10: 237-245.
- Irfan M, Aneja B, Yadava U, Khan SI, Manzoor N, et al. (2015) Synthesis, QSAR and anti-Candida evaluation of 1,2,3-triazoles derived from naturally bioactive scaffolds. Eur J Med Chem 93: 246-254.
- Irfan M, Alam S, Manzoor N, Abid M (2017) Effect of quinoline based 1,2,3-triazole and its structural analogues on growth and virulence attributes of Candida albicans. PloS One 12(4): e0175710.
- Park HJ, Kim MS, Lee S, Kim JS, Woo JS, et al. (2007) Recrystallization of fluconazole using the supercritical antisolvent (SAS) process. Inter Journal Pharm 328(2): 152-160.
- Kadam RU, Roy N (2007) Recent trends in drug-likeness prediction: A comprehensive review of in silico methods. Indian J Pharm Sci 69(5): 609-615.






























