A Brief Review on Chemistry of Dibenzosuberenones
Manjunath Moger and Hari N Pati*
Department of Chemistry, Eurofins Advinus Ltd, India
Submission: May 04, 2018; Published: June 05, 2018
*Corresponding author: Hari N Pati, Department of Chemistry, Eurofins Advinus Ltd 21 & 22, Phase II, Peenya Industrial Area, Bangalore-560058, Karnataka, India, Email: harinarayanpati@eurofins.com
How to cite this article: Manjunath M, Hari N P. A Brief Review on Chemistry of Dibenzosuberenones. Organic & Medicinal Chem IJ. 2018; 7(1): 555704. DOI: 10.19080/OMCIJ.2018.07.555704
Abstract
Dibenzosuberenones are tricyclic fused rings and its derivatives are well known anti-depressants. This review describes the synthesis of dibenzosuberenone analogs as well as their biological properties. Some of the well-known anti-depressant drugs derived from dibenzosuberenone and application of these molecules in light emitting diodes and paint industry are described in the review. Attempts were made to summarize the recent developments in suberenone chemistry, its pharmaceutical and other industrial applications.
Keywords: Dibenzosuberenone; Anti-depressants; Anti-microbial; Multidrug modulators
Introduction
Suberane is an acronym for cycloheptane ring and thus, the derived name dibenzosuberane refers to a compound with fused tricyclic framework incorporating a cycloheptane ring and two phenyl rings. Dibenzosuberane [1], dibenzosuberone [2] and Dibenzosuberenone [3], the three key compounds in the series, hereinafter referred to as DBS, are distinguished by their structural differences at positions 1, 4 and 5 of the cycloheptyl ring. To the best of our knowledge, there are no reports on natural occurrence of compounds 1-3 (Figures 1-3).
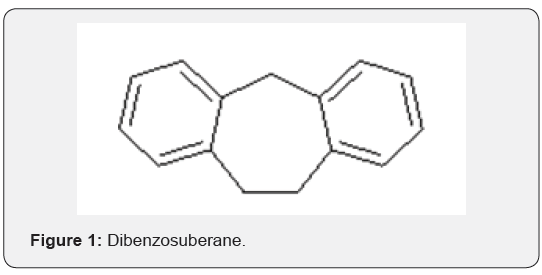
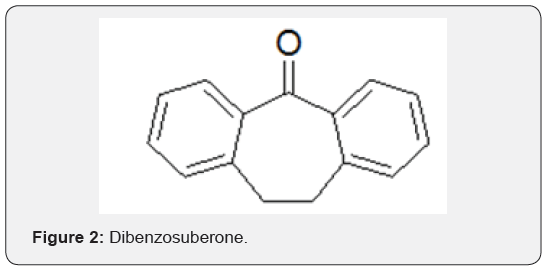
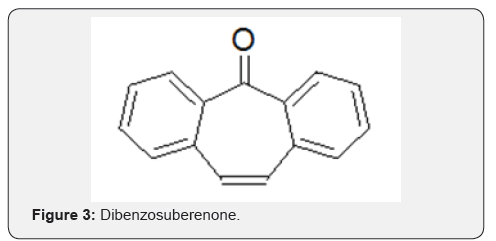
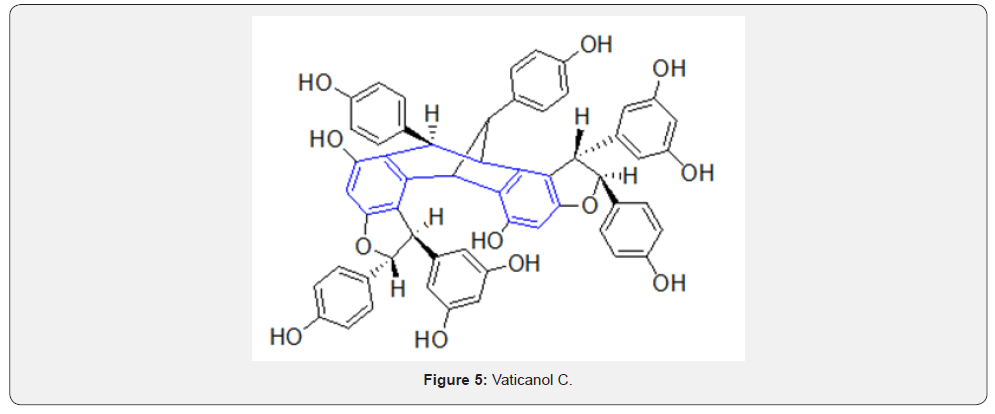
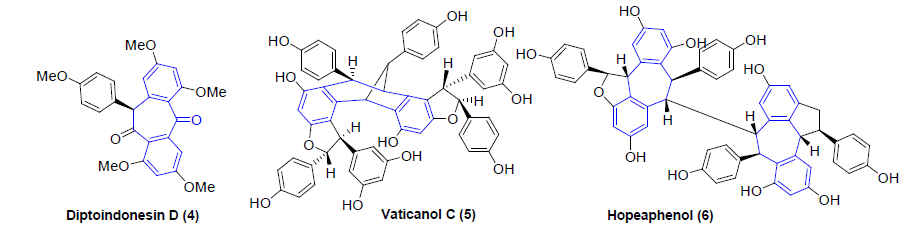
However, there are natural products with the dibenzocycloheptyl ring system as part of their structures, albeit only a few. For example, reserveratrol-based natural products Diptoindonesin D [4], Vaticanol C [5], Hopeaphenol [6] are isolated from Japanese knotweed and tree bark Shorea gibbosa [1] (Figures 4-6).


Preparation of DBS analogues initiated in 1950s, based on synthetic interest [2,3] , and the same in the present could widely be seen in literature as synthetic intermediates. The use of these intermediates drastically increased with the advent of invention of the DBS analogues as psychotropic by Davis and co-workers [4-9]. A class named as tricyclic antidepressants (TCAs) gained utmost popularity. For example, drugs of this class such as amitriptyline [7], [5,10-16] imipramine [8] [17] and noxiptiline [9] [18,19] continue as first line agents to treat depressive disorders. Likewise, several DBS based analogues have been synthesized and tested for wide array of applications [20-25] and the moiety continues to be of interest to synthetic and medicinal chemists.

DBS analogues were previously prepared starting from simple or substituted compounds 1-3, which, in turn, were accessed through multi-step synthetic routes. But currently, compounds 1-3 and a few related analogues are widely available from various commercial sources making the analogue synthesis of DBS more accessible easily. Preparation of the analogues based on DBS skeleton was approached by chemists through different modes of which functionalization of the atoms 1, 4 and 5 of the sevenmembered ring is the key mode which led to majority compounds. In other words, most of the DBS analogues were prepared by modifications of either “double bond” or carbonyl group present on the central “seven-membered ring” or both. Though most of these synthetic details appeared in respective research articles, the comprehensive information dealt with chemistry of DBS is not found in literature. Hence, the current review aims at covering the synthetic efforts of chemists involved in preparation of the DBSbased analogues.
Synthesis
Multi-Step Syntheses of DBS-Based Compounds
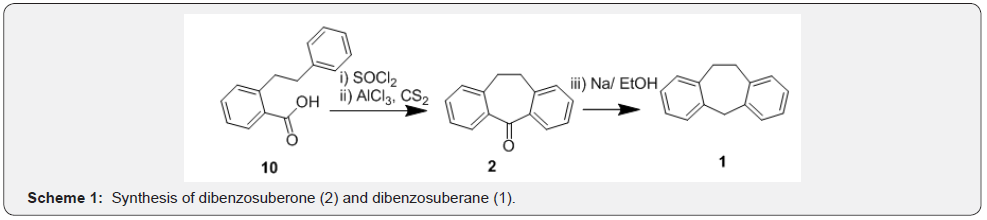
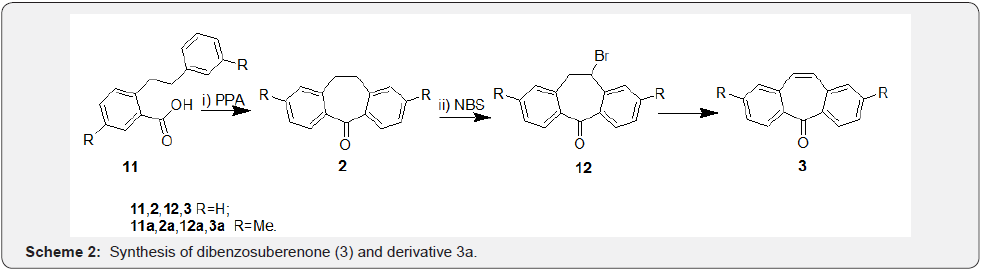
Synthesis of dibenzosuberone (2) was reported first by Triebs and co-workers [26] and later by Cope and co-workers [27]. In their independent syntheses (Scheme 1), 2-phenethyl-benzoyl chloride generated from the corresponding acid 10 was cyclized under Friedal-Craft’s acylation conditions to afford dibenzosuberone (2). Further, authors have achieved deoxygenation of compound 2 in presence of Na/EtOH to afford dibenzosuberane (1). Based on this methodology, several other syntheses appeared and important are the ones dealt with synthesis of dibenzosuberenones. One such strategy was adapted by Glover and co-workers [28] starting from 2-(2-phenylethyl) benzoic acid [11] for synthesis of dibenzosuberenone 3 in two steps. Compound 11 was cyclized to DBS 2 in presence of PPA in the first step which was taken through steps of bromination followed by debromination to afford dibenzosuberenone (Scheme 2). Based on similar strategy, Wild et al. [29] reported synthesis of dimethyl dibenzosuberenone 3a starting from compound 11a. While compound 3 was used as an intermediate in preparation of a stable hydroperoxide intermediate by Stephen and co-workers, [28] compound 3a was used in synthesis of several potential antiviral agents by Wild and co-workers [29].
Rupard et al. [30] has reported another route to access the substituted DBS (Scheme 3). These compounds were prepared as analogues of Clozapine to probe their antipsychotic activities. Synthesis initiated with protection of acid 13 followed by Wittigtype condensation to afford intermediate 14, which upon catalytic hydrogenation followed by ester hydrolysis steps afforded compound 15. Finally, target compound 16 was prepared by PPAmediated cyclization of intermediate 15

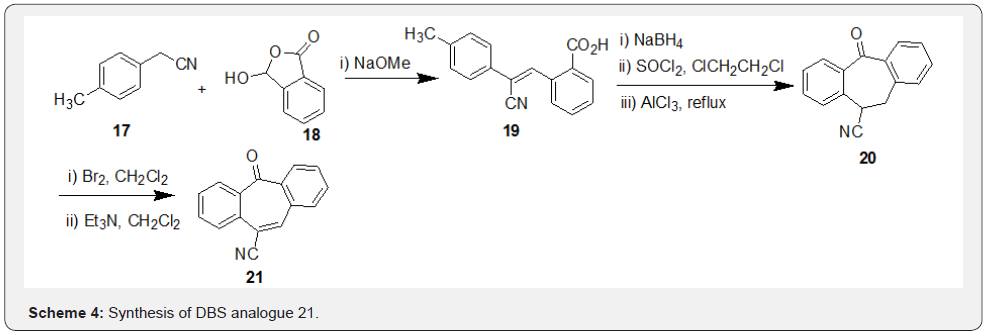
In another work directed towards synthesis of novel angiotensin II receptor antagonists, Kiyama et al. [31] reported a different synthetic route to access the substituted DBS compound 21 as depicted in the Scheme 4. Intermediate 19, prepared by condensation of compounds 17 and 18 in presence of NaOMe, and was subjected to the sequence of steps such as reduction, acid chloride generation followed by cyclization under Friedal-Crafts conditions afforded the intermediate 20. Finally, bromination of 20 followed by dehydro-debromination resulted in synthesis of compound 21.
Kindon et al. [32] achieved synthesis of disubstituted DBS derivative 24, which were prepared as P2-purinoreceptor 7-transmembrane (TM) G-protein coupled receptor antagonists. The synthesis was initiated by preparation of Wittig salt of compound 22 and its further reaction with 3-bromobenzaldehyde to afford a crucial intermediate 23. Finally, sequence of steps including ester hydrolysis, catalytic hydrogenation followed by cyclization mediated under Stille coupling conditions resulted in the synthesis of disubstituted DBS analogue 24 (Scheme 5).
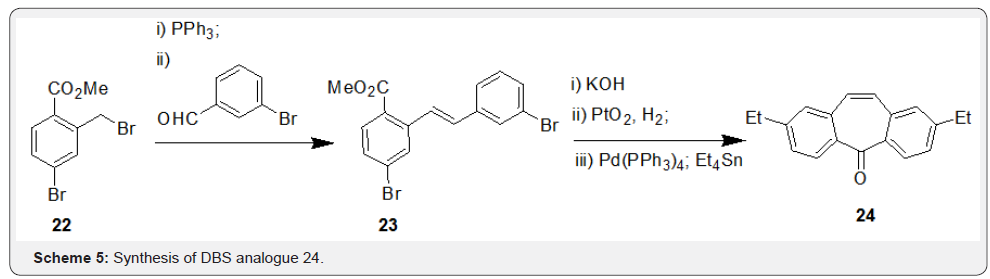
In a recent report, Solveigh et al. [33] has published a synthesis of disubstituted DBS analogue 27 by a different strategy. The synthesis involved a key Wittig coupling of compound 25 and 3-chlorobenzyl chloride to yield 26, which underwent sequence of steps including catalytic hydrogenation followed by cyclization under Friedal-Crafts acylation conditions resulted in the disubstituted DBS analogue 27 (Scheme 6).
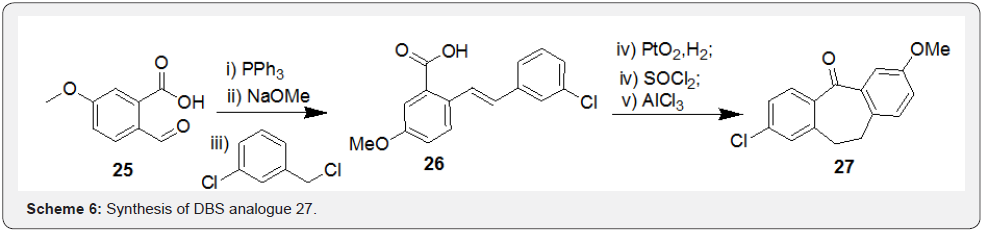
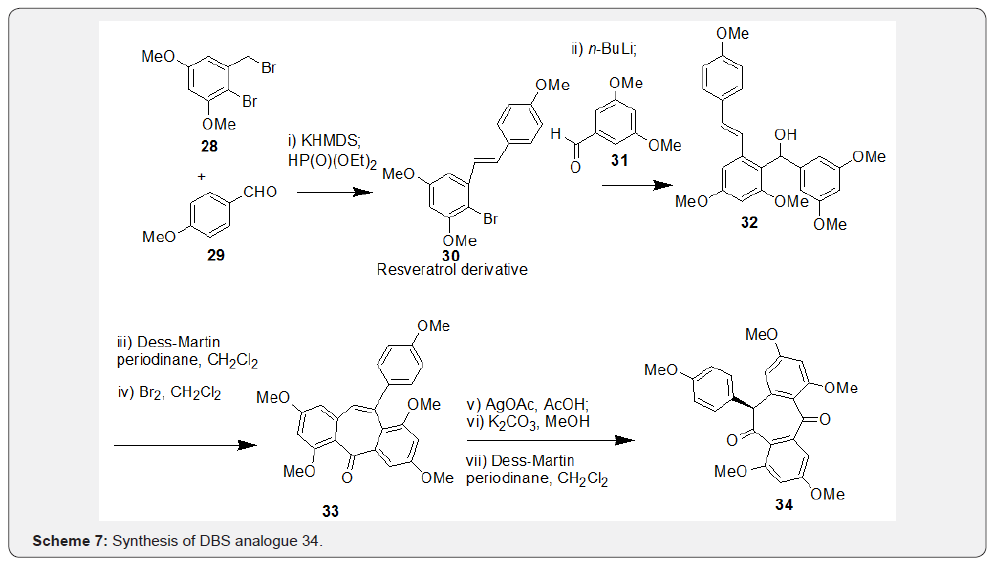
Snyder et al. [34] has reported an article on total syntheses of resveratrol-based natural products wherein, they attempted synthesis of natural product bearing 7-membered carbocycle, i.e. compound 4, remained as a key attraction. Key step of this synthesis includes Horner-Wadsworth-Emmons olefination reaction using compounds 28 and 29 to afford the resveratrol derivative 30. Further, halogen-lithium exchange in compound 30 followed by addition to aldehyde 31 resulted in compound 32. Finally, oxidation of compound 32 followed by treatment with Br2 resulted in assembly of 7-membered ring in compound 33 via a bromonium-induced cascade sequence followed by acid induced phenonium shift to afford non-natural product 34 in multiple steps (Scheme 7).
Reactions of Dibenzosuberenone
By and large, the structural modifications of the DBS performed limit to the chemistry on suberyl ring. In particular, DBS has two important sites for functionalization, by virtue of its structure, namely, the “double bond” and the “carbonyl group”. In the following section, preparation of various analogues of DBS synthesized by modification of the parent skeleton based on reactions of the carbonyl group and the “double bond” and both (miscellaneous reactions) will be addressed.
Functionalization of Carbonyl Group
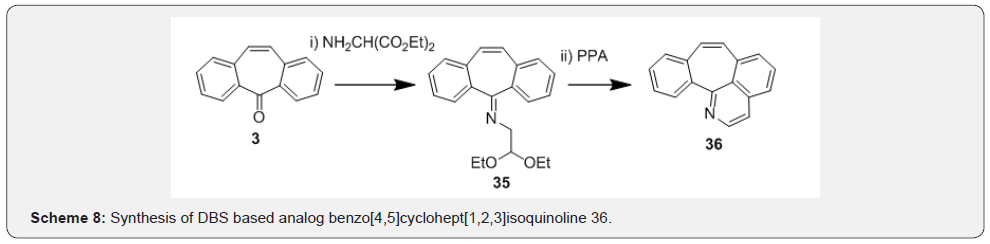
George and co-workers [35] have synthesized new series of benzo[4,5]cyclohept[1,2,3-ij]isoquinolines by condensation reaction of compound 3 with aminoacetaldehyde diethylacetal followed by treatment of the corresponding imines 35 with PPA to afford the tetracyclic compound 36 (Scheme 8).
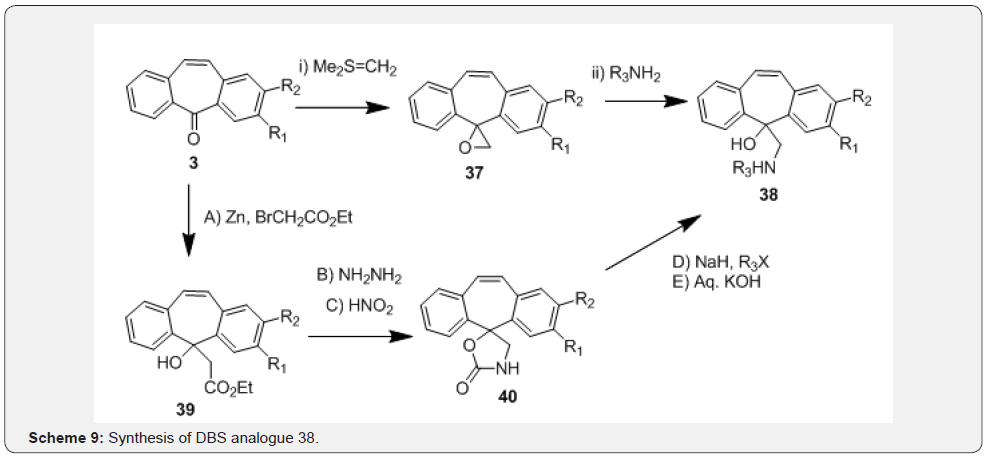
In another work, Coppola and co-workers [36] reported transformations in Dibenzo[a,d]cycloheptene series through two routes. Authors designed these compounds by integrating norepinephrine and dibenzosuberene skeletons resulting in series of 5-aminomethyl-5-hydroxy-5H-dibenzo[a,d]cycloheptene derivatives (Scheme 9). In the first route, compound 3 was converted to an epoxide intermediate 37 in the presence of dimethylsulfonium methylide (Corey’s reagent) followed by ring opening of epoxide with various amines resulting in the desired analogs of compound 38. Alternatively, compound 38 were prepared from series of steps starting from Reformatsky reaction of compound 3 with ethyl bromoacetate to affording initially ethyl ester 39. Treatment of compound 39 followed by treatment of the hydrazide with nitrous acid resulted in spirooxazolidone derivative 40. Finally, N-alkylation followed by alkali hydrolysis of compound 40 resulted in required aminoalcohols of general structure 38 (Scheme 9). Brenner et al. [37] reported a synthesis of N-alkylidenearenesulfenamides 42 using DBS 3 in their investigations on tertiary carbinamines, which are useful precursor to several imino-bridged heterocycles.
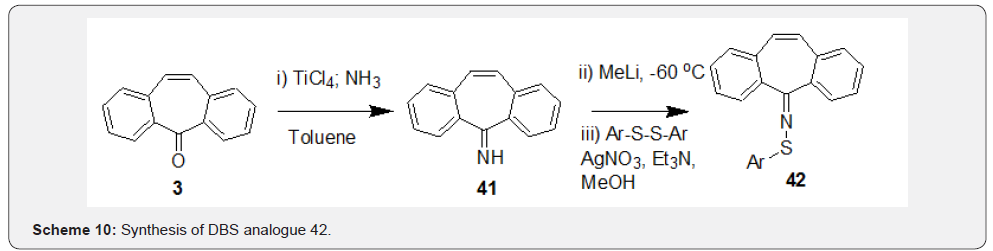
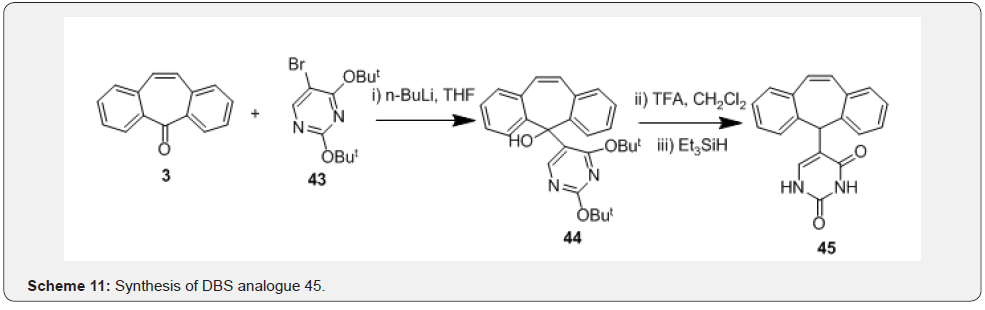
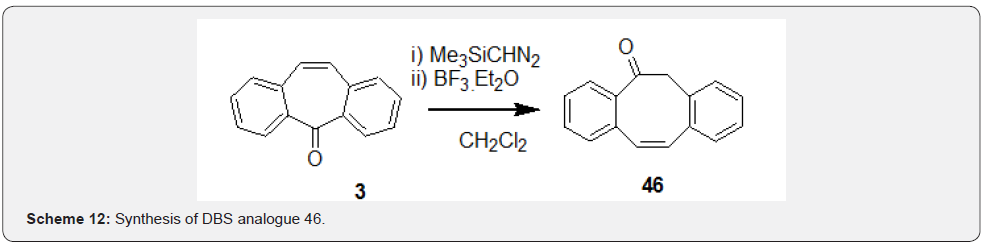
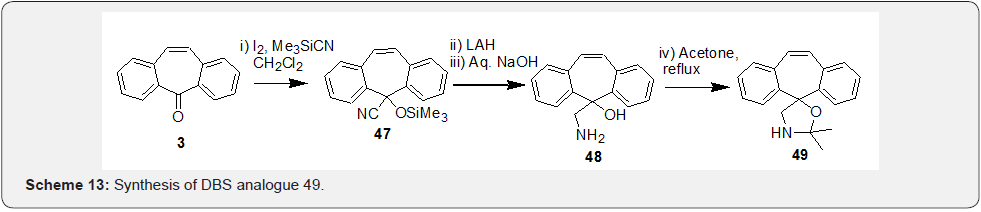
First, a stable ketimine 41 was prepared by condensation reaction of DBS 3 with NH3 in presence of TiCl4. Further, the anion of compound 41 generated in presence of MeLi was treated with corresponding aryldisulfides in presence of Et3N to afford compounds 42 (Scheme 10). Kindon et al. [38] performed modification of carbonyl group of DBS compound 3 as part of their work related to synthesis of 2-oxo-4-thioxopyrimidin-1-ylmethyl heterocyclylcarboxylates as P2-purinoceptor 7-transmembrane G-protein coupled receptor antagonists. Synthesis was initiated by addition of the lithiated compound 43 with DBS 3 to prepare intermediate 44, which underwent t-butoxide deprotection followed by tautomerization to afford compound 45 (Scheme 11). Chaffins and coworkers [39] reported synthesis of dibenzocycloocta-4a,6a-diene-5,11-diyne (46) by treating compound 3 with trimethylsilyl diazomethane in presence of BF3. Et2O to afford compound 46 as a result of ring expansion (Scheme 12). Michellys and coworkers [40] reported synthesis of spirocontaining dibenzocycloheptene-oxazole (49). Synthesis was initiated by treating DBS 3 with I2, followed by TMSCN to prepare intermediate 47, which was subjected to LAH reduction followed by silyl ether deprotection to give compound 48.
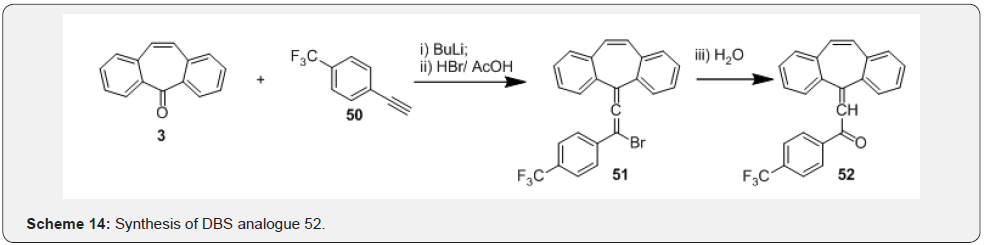
Finally, refluxing compound 48 in acetone afforded the required dibenzocycloheptene-oxazole compound 49 (Scheme 13). These compounds were tested for activities related to modulation of steroid hormone nuclear receptors, and their preparation, pharmaceutical compositions and use. In another work Emilie et al. [41] have prepared dibenzosuberinyledene allenes and have used these intermediates as precursors of dispirotetracenes, diindenotetracenes and 2-phenyl-11bHdibenz[cd,h]azulene. In this work (Scheme 14), synthesis of the dibenzosuberenylidene allenes initiated from the reaction of DBS 3 and the terminal alkyne 50 which yielded the crucial intermediate 51, which upon reduction followed by dehydration afforded the required dibenzosuberinyledene allene 52. These intermediates were even prepared earlier by Banide and co-workers [42] who were interested to know if the tricyclic dibenzo[a,d]cycloheptenyl ring system would stabilize the cations and in preparation of C2- symmetric bis-(dibenzosuberenylidene)cyclobutanes.
Functionalization of Double Bond
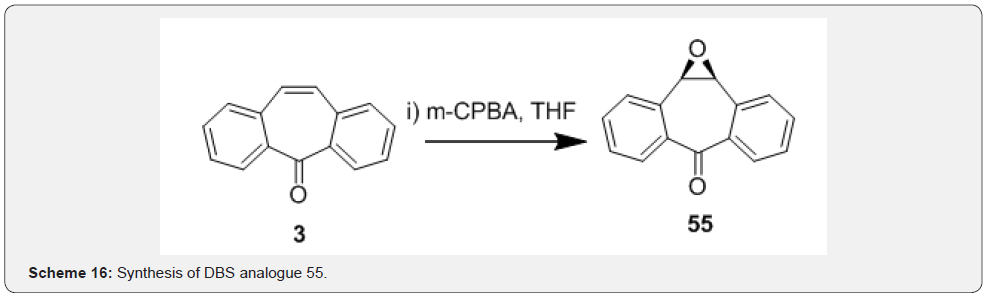
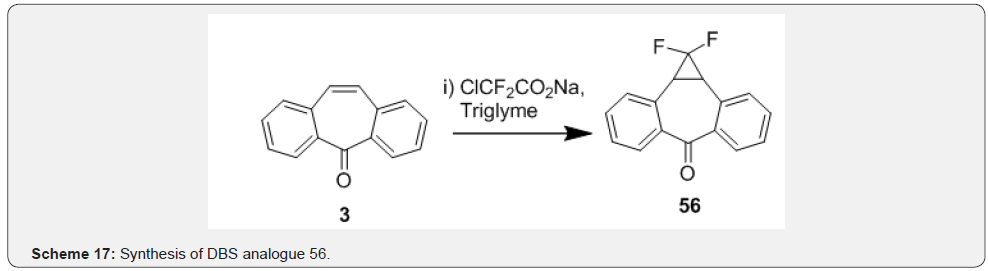
Fantauzzi and coworkers [43] reported the aziridination of dibenzosuberenone 3 with (porphyrin) ruthenium-catalyzed aziridination of olefins using aryl azide 53. Aryl azides have been used as atom-efficient nitrene transfer reagents in the (porphyrin) ruthenium catalyzed amination of olefins. A quantitative yield of 54 in short reaction times was achieved by utilizing aryl azides bearing electron withdrawing groups such as 53 as shown in Scheme 15. Choi and coworkers [44] reported epoxidation of dibenzosuberenone by using metachloroperbenzoic acid and tetrahydrofuran as solvent. The double bond present in the sevenmembered ring of DBS 3 undergoes asymmetric epoxidation to afford product 55 (Scheme 16). Astleford and co-workers [45] treated dibenzosuberenone 3 with sodium chlorodifluoroacetate in triglyme at 180-190°C to obtain 1,1-difluoro-1a,10bdihydrodibenzo[ a,e]cyclopropa[c]cyclohepten-6-one 56. The reaction involved addition of difluorocarbene to olefinic functionality in DBS 3 which resulted in assembly of fused difluorocyclopropyl ring annealated to the DBS skeleton (Scheme 17). Further reactions on compound 56 leading to various novel compounds of biological interest.

Functionalization of Double Bond and Carbonyl Group
In this section reactions starting from dibenzosuberenone involving both functionalities, i.e., “carbonyl group” and “double bond” of the 7-membered ring which led to analogues based on DBS skeleton will be discussed. Majority of the active DBS analogues prepared so far belong to the class of tricyclic antidepressants (TCAs). Dizocilpine (60), a TCA drug developed by Merck, was first prepared by Lamanec and co-workers [46] and later the derivative 61 by Thompson and co-workers [47] using similar strategies. Their syntheses initiated with addition of Grignard reagent to carbonyl group at position ‘5’ leading to tertiary carbinol 57. Further, addition of α-effect nucleophile NH2OH to compound 57 yielded the intermediate 58, which was subjected to trans-annular ring closure in presence of t-BuOK in DMSO to afford compound 59. Finally, hydrogenolysis of hydroxylamine 59 yielded dizocilpine (60) and its derivative 61 (Scheme 18). Recently, Chang et al. [48] followed a different approach for synthesis of dizocilpine (60) which starts with conversion of DBS 3 to aziridine 62 in presence of chloramine-T (Scheme 19). Hydrogenolysis of 62 followed by treatment of the resultant intermediate 63 with MeMgBr afforded compound 64.
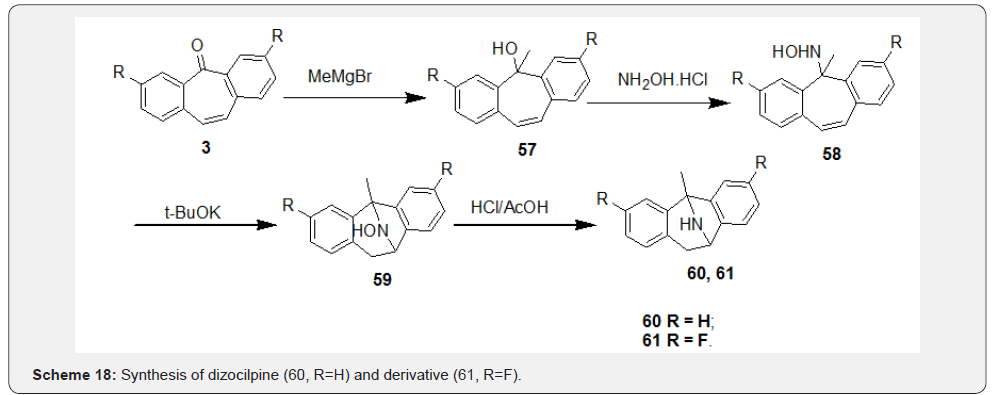
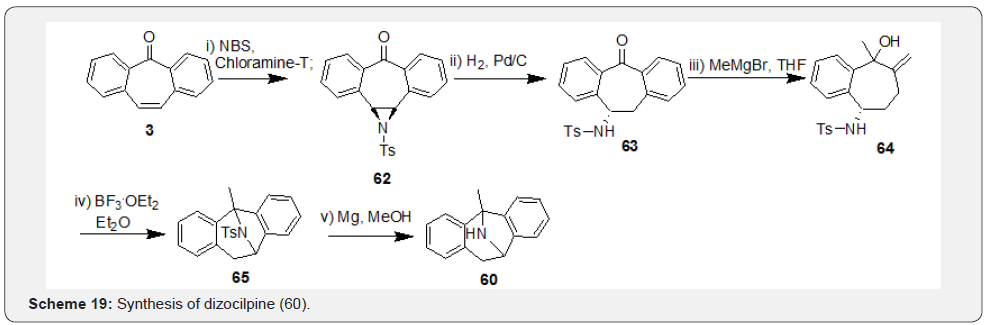
Finally, Lewis acid catalyzed intramolecular ring closure of compound 64 followed by detosylation in compound 65 afforded dizocilpine (60). Pfister et al. [49] synthesized series of methanodibenzosuberylpiperazines as potent multidrug resistance reversal agents based on structure of lead molecule MS-073. Synthesis was initiated with reduction of carbonyl compound 56 to give hydroxyl compound 66 (syn-alcohol), which was subjected to chlorination followed by substitution of ‘Cl’ with formylpiperazine to afford compound 67 (syn/anti mixtures). Finally, base-induced deformylation of 67 followed by treatment with 5-(2,3-epoxypropoxy) quinolone afforded the representative target compound 68 of the series (Scheme 20).

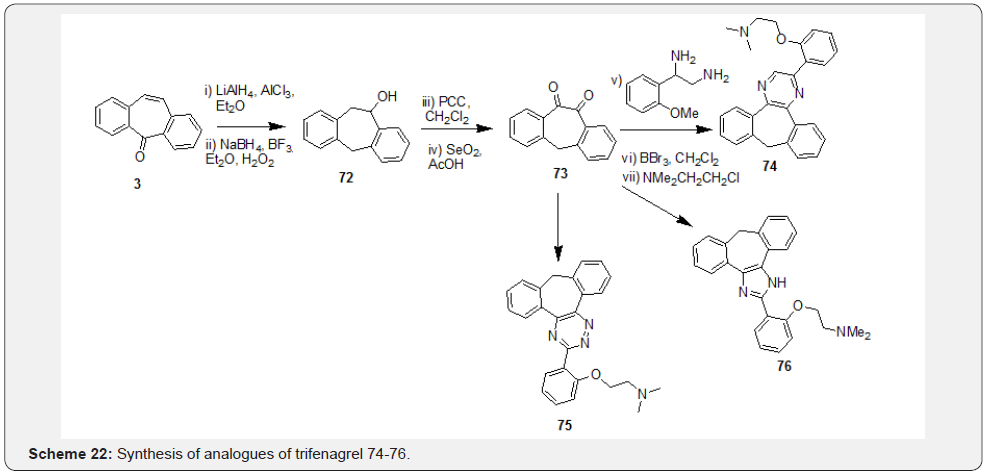
Gonzalez and coworkers [50] reported synthesis of 5-aminocarbonyl-5H-dibenzo[a,d]cyclohepten-5,10-imine starting from DBS 3 through four step process (Scheme 21). Synthesis was initiated with formation of imine of DBS 3 in presence TiCl4 and NH3 gas followed by its treatment with acetone cyanohydrin and catalytic amount NaCN to afford intermediate 69. Treatment of intermediate 69 with Br2 followed by in situ trans-annular ring closure led to bromo derivative 70, which was debrominated in presence of NaCNBH3 to afford product 71. Slusarczyk et al. [51] achieved synthesis of analogues of trifenagrel starting from DBS 3. Their synthesis was initiated with LAH reduction of DBS 3 in presence of AlCl3 (reaction at carbonyl group) followed by hydroboration and oxidation (reaction at double bond) to yield intermediate 72. Further oxidation of secondary alcohol 72 followed by SeO2 mediated oxidation resulted in crucial diketone intermediate 73.

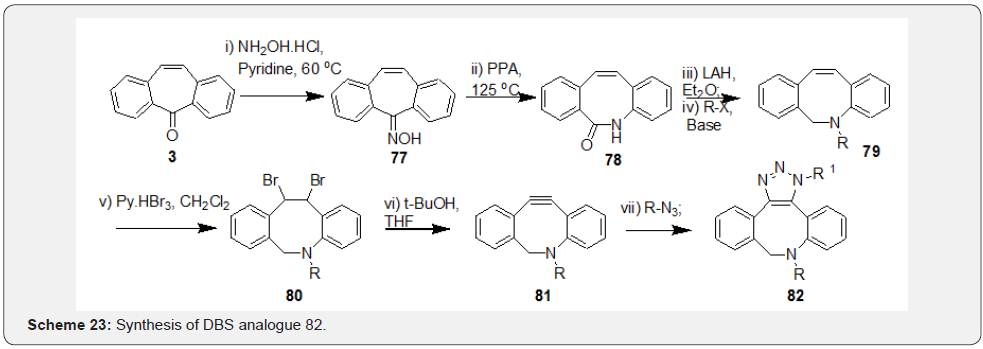
Further, synthesis of the compounds 74-76 was achieved based on the cyclocondensation reaction of a tricyclic 1,2-diketone 73 with an appropriate diamine, hydrazide or an aldehyde to form a pyrazine 74, a 1,2,4-triazine 75 or an imidazole 76, respectively (Scheme 22). Vladimir et al. [52] explored ways of conducting metal-free click chemistry mediated from use of internal alkyne 81 and azide to form triazole 82 in efforts directed at surface immobilization by functionalization of various surfaces. Synthesis of the internal alkyne 81 was initiated from preparation of oxime 77 starting from DBS 3 followed by Beckmann rearrangement of 77 to form the lactam 78. Reduction followed by derivatization of the amine as per the requirement yielded intermediate 79. Dibromination followed by base treatment of compound 80 with t-BuOK resulted in the synthesis of crucial alkyne intermediate 81. Authors have further introduced a biotinylated triazole tag by treating appropriate azide and the cyclooctyne intermediate through a copper-free click chemistry to yield derivatives of general structure 82 (Scheme 23).
Importance of DBS Analogues
Content of the sections 2-3 gives us a glimpse on scope and applications of the tricyclic framework of the 5H-dibenzo [a,d] cycloheptene system in synthesis of novel derivatives. Additionally, the DBS skeleton is known to undergo photochemical reactions and chemists have exploited photochemical properties of the DBS analogues 1-3 for preparing novel complex molecules of synthetic interest [53-55]. Besides, several researchers explored DBS skeleton for preparation of novel analogues that were tested for varied biological activities e.g., Nicholas and co-workers [38] for purinoceptor 7-transmembrane G-protein coupled receptor antagonist activity, Ulens et al. [56] for acetylcholine binding protein and α7 nicotinic receptor ligand binding affinities, Mychajlyszyn and co-workers [57] for antihistamine properties, Coghlan and co-workers; Pierre-Yves and co-workers for steroid hormone nuclear receptor modulation, [58,59] William et al. [60] for farnesyl protein transferase inhibition, Arya and co-workers for anti-inflammation, [61] Wayne and co-workers for N-methyl- D-aspartate antagonist activity, [62] Pauling and co-workers for cytokine biosynthesis inhibition, [63] and Philip and co-workers for anti-convulsant activity [64] and so on.
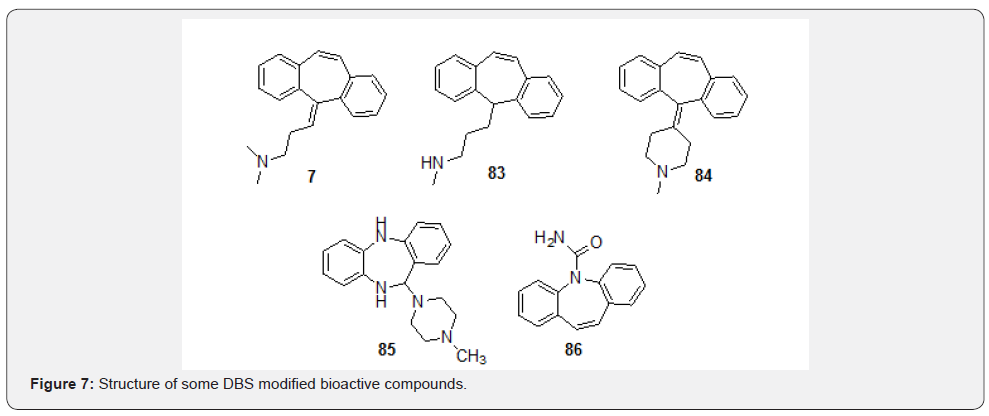
Since the introduction of amitriptyline (7) for the treatment of depression, [65] DBS skeleton has been varied almost infinitely in the search for improved biological activity which led to many second generation potent compounds such as protriptyline (83) and cyproheptadine (84) [66]. Additionally, dibenzosuberenone shares a structural resemblance with a key dibenzazepine intermediate which has been exploited in synthesis of several compounds with anti-depressant properties. Clozapine (85) is a tricyclic dibenzodiazepine derivative which interferes with the binding of dopamine molecules at the D1, D2, D3, D4 and D5 dopamine receptors [67]. Carbamazepine (86) is an anti-epileptic drug used to control grandma and focal seizures besides being a specific analgesic for trigeminal neuralgia (Figure 7) [68].
DBS analogues are known to possess other interesting pharmacological activities. Especially, ability of some DBS analogues to reverse the multi drug resistance which prompted researchers to investigate their application in cancer chemotherapy. In this context, Pfister and coworkers [69] reported LY335979 (68) as a second generation modulator of Pgp which showed significant clinical effectiveness when used in combination with anti-cancer drugs known to be substrates for Pgp. Besides, AR-C118925 (87) is the only current known selective P2Y2 receptor antagonist as a pharmacological reference compound [70,71]. Finally, series of compounds based on DBS skeleton were found to be selective inhibitors of p38 mitogen-activated protein kinase (p38 MAP kinase) with Skepinone-L (88) being known as potent inhibitor of p38 MAP kinase (Figure 8) [72-74].

A series of 10 novel hybrid molecules containing dibenzosuberenone skeleton and substituted oxadiazoles were designed and synthesized by Moger et al. [75] The novel hybrid molecules were screened for their antibacterial and antifungal activities (Figure 9). Some of the molecules exhibited excellent antimicrobial activities.

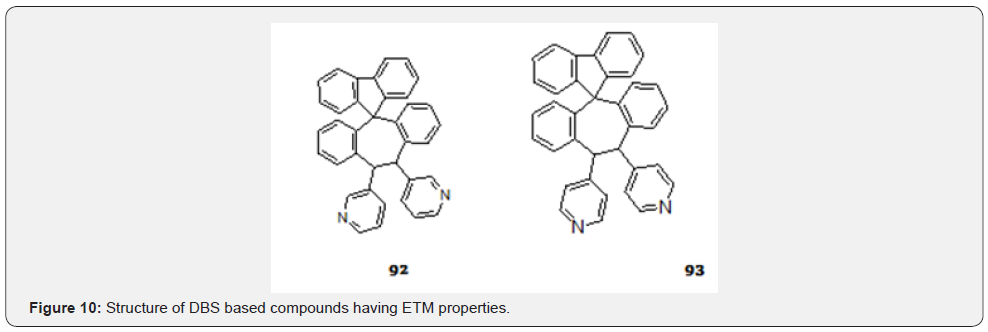
Jeneke et al. [76] reported DBS based compounds (Figure 10) exhibit promising electron transport material (ETM) with excellent exciton as well as hole blocking ability in blue phosphorescent organic light emitting diodes (PhOLED).
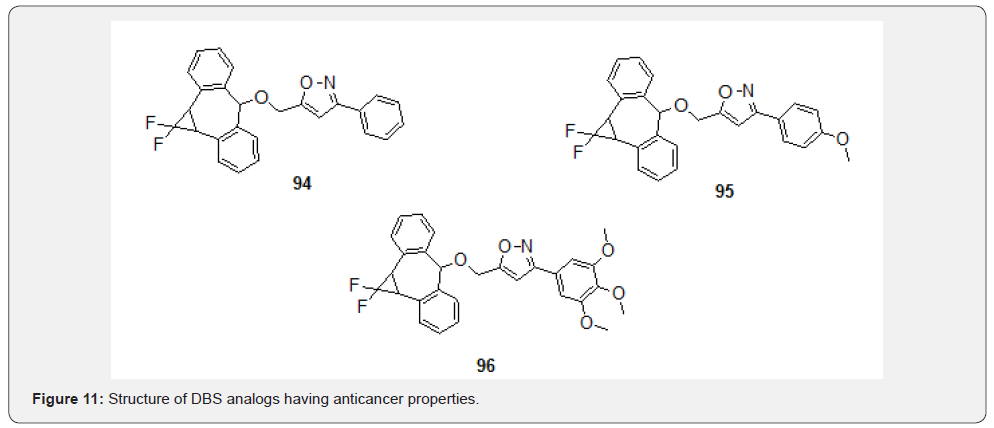
Recently, HN Pati et al. [77] reported synthesis of a series of twelve novel isoxazole analogs containing dibenzosuberane moiety using convergent synthesis approach. The novel DBS analogs were screened for their antiproliferative property against HepG2 and HeLa cell lines. Some of the compounds 94, 95 and 96 were found active against both HepG2 and HeLa cell lines (Figure 11).
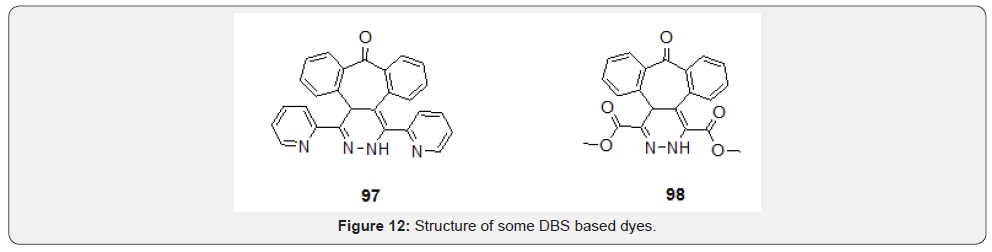
Kocak et al. [78] synthesized dihydropyrazine-appended dibenzosuberenone molecules (97 and 98) using Diels-Alder chemistry (Figure 12). The N-H protons in these structures are fairly acidic and allow fluoride induced deprotonation which leads to a radical color change as well as spontaneous fluorescence quenching. Thus, the novel molecules are found to be potential fluorescent chemosensors which attract special attention in dye chemistry.
Conclusion
This review is an outcome of attempts made towards the collection of recent advances in the synthesis of dibenzosuberenone derivatives. Dibenzosuberenone based tricyclic anti-depressants continue to be the first line agents for depressive disorders. Dibenzosuberenone derivatives are also found effective in cancer treatment and multidrug resistance modulators. Apart from pharmaceutical agents, dibenzosuberenone derivatives attract special attention in dyes and paint industry as well as light emitting diodes. Thus, dibenzosuberenones are the upcoming class of compounds with versatile applications in various industries.
Acknowledgement
Authors are thankful to Eurofins Advinus Ltd. for encouragement to research. Authors are also thankful to Mangalore University for Ph.D. registration of Manjunath Moger.
References
- Scott A, Snyder, Breazzano SP, Lin Y, Zografos AL (2009) Total Synthesis of Diverse Carbogenic Complexity within the Resveratrol Class from a Common Building Block. J Am Chem Soc 131(5): 1753-1765.
- Treibs, Wilhelm, Klinkhammer (1950) Hans J Chem Ber 83: 367.
- Cope AC, Fenton SW (1951) J Am Chem Soc 73: 1668.
- Winthrop SO, Davis MA, Myers GS, Gavin JG, Barber TR (1962) J Org Chem 27: 230.
- Davis MA, Winthrop SO, Stewart J, Sunahara FA, Herr F (1963) NEW PSYCHOTROPIC AGENTS V. DERIVATIVES OF 5-CYANO- AND 5-CARBOXAMIDODIBENZO(A,D)CYCLOHEPTADIENE. J Med Chem 6: 251-255.
- Winthrop SO, Davis MA, Herr F, Stewart J, Gaudry R (1963) NEW PSYCHOTROPIC AGENTS. IV. DERIVATIVES OF DIBENZO(A,D)(1,4) CYCLOOECTADIENE. J Med Chem 6: 130-132
- Davis MA, Sunahara FA, Herr F, Gaudry R (1963) J Med Chem 6: 513.
- Davis MA, Beaulieu G, Watson JR, Charest MP (1966) J Med Chem 9: 860.
- Davis MA, Herr F, Thomas RA, Charest MP (1967) J Med Chem 10: 627.
- McQuay HJ, Moore RA (1997) Br Med J 314: 763.
- Howard M (2005) J Clin Gastroenterol 39: S247.
- Max MB, Culnane M, Schafer SC, Gracely RH, Walter DJ, et al. (1987) R Neurology 37: 589
- Williams JW, Rost K, Dietrich AJ, Ciotti MC, Zyzanski SJ, et al. ( 1999) Arch Fam Med 8: 58.
- Walsh TD (1983) Clin Neuropharmacol. 6, 271.
- Hoffsomer RD, Taub D, Wendler NL (1962) The Homoallylic Rearrangement in the Synthesis of Amitriptyline and Related Systems. J Org Chem 27: 4134-4137.
- Hoffsomer RD, Taub D, Wendler NL (1963) Synthesis of Novel, Potentially Biologically Active Dibenzosuberone Derivatives. J Org Chem 28: 1751.
- Schindler W, Häfliger F (1954) Helv Chim Acta 59: 472.
- Hoffmeister VF, Wutke W, Kroneberg W (1969) Arzneimittel Forsch 19: 846
- Hoffmeister VF (1969) Arzneimittel Forsch 19: 458.
- Thompson JW, Anderson PS, Britcher SF, Lyle TA, Thies JE (1990) J Med Chem 33: 789.
- Ting PC, Lee JF, Solomon DM, Smith SR, Terminelli CA (1995) Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing Agents. Bioorg Med Chem Lett 5: 2749.
- Deblon S, Gruetzmacher H, Maire PF, Schoenberg H (2003) PCT Int Appl 2003048175
- Taljaard B, Taljaard JH, Imrie C, Caira MR (2005) Eur J Org Chem 12: 2607.
- Luo J, Song K, Gu FL, Miao Q (2011) Chem Sci 2: 2029.
- Bayrakceken F, Jomehri BB, Yaman AJ (2001) Ind Chem Soc 78: 234
- Treibs W, Klinkhammer HJ (1950) Chem Ber 83: 367.
- Cope AC, Fenton SW (1951) J Am Chem Soc 73: 1673.
- Glover SA, Goosen A, Mc Cleland CW, Taljaard B, Vogel FRS (1984) Afr J Chem 37: 164.
- Wild H, Hansen J, Lautz J, Paessens A (1993) Eur Pat 589322.
- Rupard JH, Paulis T, Janowsky A, Smith HE (1989) J Med Chem 32: 2261.
- Kiyama R, Honma T, Hayashi K, Ogawa M, Hara M, et al. (1995) T J Med Chem 38: 2728.
- Kindon N, Meghani P, Thom S (1998) PCT Int Appl 9854180.
- Koeberle SC, Fischer S, Schollmeyer D, Schattel V, Grütter C, et al. (2012) Design, Synthesis, and Biological Evaluation of Novel Disubstituted Dibenzosuberones as Highly Potent and Selective Inhibitors of p38 Mitogen Activated Protein Kinase. SA J Med Chem 55: 5868-5877.
- Snyder SA, Breazzano SP, Ross AG, Lin Y, Zografos (2009) Total Synthesis of Diverse Carbogenic Complexity within the Resveratrol Class from a Common Building Block. A L J Am Chem Soc 131: 1753-1765.
- Santroch G, Humber LG (1976) Studies on the benzo[1,2] cyclohepta[3,4,5‐d,e] isoquinoline ring system. J Heterocycl Chem 13: 1049.
- Coppola GM, Hardtmann GE, Koletar G, Kroin S (1981) The chemistry of 3‐azaisotoic anhydrides. Synthesis and reactions of polyaza heterocycles. J Heterocycl Chem 18: 31
- Brenner DG, Cavolowsky KM, Shepard KL (1985) J Heterocycl Chem 22: 805.
- Kindon N, Meghani P, Thom S, PCT (1998) Int Appl 9854180.
- Chaffins S, Brettreich M, Wudl F (2002) Synthesis 9: 1191.
- Michellys PY, Mac CC, Pei W (2010) US7807841 B2.
- Banide EV, O Connor C, Fortune N, Ortin Y, Milosevic S (2010) J Org Biomol Chem 8: 3997.
- Banide EV, Oulie P, McGlinchey M (2009) J Pure Appl Chem 81: 1-17.
- Fantauzzi S, Gallo E, Caselli A, Piangiolino C, Ragaini F (2007) A Mechanistic Investigation of the Ruthenium Porphyrin Catalysed Aziridination of Olefins by Aryl Azides. J of Org Chem 36: 6053.
- Choi MH, Kyung SH (2000) Agric Chem Biotechnol 43: 101.
- Astleford BA, Barnett CJ, Kobierski ME (2003) Wilson TM. US 6570016 B1.
- Lamanec TR, Bender DR, Demarco AM, Karady S, Reamer RA (1988) J Org Chem 53: 1768.
- Thompson WJ, Anderson PS, Britcher SF, Lyle TA, Thies JE (1990) J Med Chem 33: 789.
- Chang MY, Huang YP, Lee TW, Chen YL (2012) Tetrahedron 68: 3283.
- Pfister JR, Makra F, Muehldorf AL, Wu H, Nelson JT (1995) Eigenschaften und Verwendbarkeit des10,11‐Dihydro‐5Hdibenzo[ a,d]cyclohepten‐5‐yl‐Rests(=5‐Dibenzosuberyl‐Rests) alsneueSchutzgruppe für Amine, Aminosäuren, Alkohole, Thiole und Carbonsäuren. Bioorg Med Chem Lett 5: 2473.
- Gonalez J (1998) Carroll FIUS 5831095.
- Slusarczyk M, de Borggraeve WM, Hoornaert G, Robeyns K, van Meervelt L, et al. (2009) Lett Drug Des Disc 6: 478.
- Popik VV, Kuzmin A, Polukhtine A (2012) US20120029186 A1.
- Okamoto M, Fujiwara Y, Oka T, Uemura Y (1988) Chem Pharm Bull 36: 521.
- Fujiwara Y, Sumino M, Nozaki A, Okamoto M (1989) Chem Pharm Bull 37: 1452
- Gad FA, Fahmy AA, El Gazzar (1993) B Egypt J Chem 35: 707.
- Ulens C, Akdemir A, Jongejan A, Elk RV, Bertrand S (2009) J Med Chem 52: 2372
- Mychajlyszyn V, Protiva M Coll Czech (1959) Chem Comm 24: 3955.
- Coghlan MJ, Green JE, Grese TA, Jadhav PK, Matthews DP (2004) PCT Int Appl 2004052847.
- Michellys PY, Mac CC, Pei W (2006) WO2006104594 A2.
- Wndsor WT, Weber PC, Strickland CO, Girijavallabhan VM (2002) PCT Int Appl. 2002080895.
- Arya VP, David J, Fernandes F, Gorhe DS, Grewal RS (1978) J Ind J Chem Sec B 16B: 220.
- Thompson WJ, Anderson PS, Britcher SF, Lyle TA, Thies JE et al. (1990) J Med Chem 33: 789.
- Ting PC, Lee J, Solomon DM, Smith SR, Terminelli CA, et al. (1995) Bioorg Med Chem Lett 5: 2749.
- Magnus PD (1998) PCT Int Appl 9841523.
- Doggrell SA, Vincent LJ (1981) Pharm Pharmacol 33: 720.
- Gordon M (1964) Psychopharmacological Agents, Academic Press, New York, USA, pp. 35-101.
- Kendrick T (1999) Br J Gen Pract 49: 745.
- Goldenberg MM (2010) P&T 35: 392.
- Pfister JR, Makra F, Muehldorf AL, Wu H, Nelson JT, et al. (1995) Bioorg Med Chem Lett 5: 2473.
- Kindon N, Meghani P, Thom S (1998) PCT Int Appl 9854180.
- Brunschweiger A, Müller C (2006) Curr Med Chem 13: 289.
- Koeberle SC, Romir J, Fisher S, Koeberle A, Schattel V, et al. (2012) Nat Chem Bio 8: 141.
- Fischer S, Wentsch HK, Mayer-Wrangowski SC, Zimmermann M, Bauer SM, et al. (2013) J Med Chem 56: 241.
- Storch K, Gehringer M, Baur B, Laufer SA (2014) Med Chem Commun 5: 808.
- Moger M, Satam V, Darshanraj C, Paniraj AS, Gopinath V, et al. (2014) J Braz Chem Soc 25: 104.
- Jenekhe SA, Jeon S, Earmme T (2015) WO 2015/103215.
- Moger M, Pradhan A, Singh A, Darshanraj C, Hindupur RM, et al. (2016) Med Chem Res 25: 449.
- Kocak R, Yildiz D, Bozkaya U, Dastan A, Bozdemir OA (2017) Tet Lett 58: 2981.






























