Synthesis of Styryl Derivatives of Pyrimidines and of Pyrido[1, 2-A] Pyrimidine
A Harutyunyan*1,2, G Ghukasyan2, A Grigoryan1 and G Danagulyan1,2
1Institute of Fine Organic Chemistry, Armenia, Yerevan
2Russian-Armenian University, Armenia, Yerevan
Submission: July 10, 2017; Published: July 14, 2017
*Corresponding author: Arthur Harutyunyan, Doctor of Science in Chemistry and Bio-organic Chemistry, Institute of Fine Organic Chemistry, Russia, Tel: 00374 93 785590; Email: harutyunyan.arthur@yahoo.com
How to cite this article: A Harutyunyan, G Ghukasyan, A Grigoryan and G Danagulyan. Synthesis of Styryl Derivatives of Pyrimidines and of Pyrido [1, 2-A] Pyrimidine. Organic & Medicinal Chem IJ. 2017; 3(1): 555602. DOI: 10.19080/OMCIJ.2017.03.555602.
Short Communication
The styryl derivatives of pyrimidines and pyrido [1,2] pyrimidine are heterocyclic isosters of stilbenes, possesses different types of biological activity [1-3], and are also in the focus of research on obtaining new materials with optical- absorption, emission, luminescent and other properties [4]. Two series of heterocyclicstyrile derivatives were synthesized by us: 2-(2-phenyl-1-ethenyl-, 4-phenyl-1,3-butadienyl)-5-substituted- 4-methyl-1,6-dihydro-6-pyrimidinones, in which the conjugated double bond system included both phenyl rings (compounds 1) and the heterocyclic isatin nucleus (compounds 2) and new 4-oxo-2-[(E)-2-aryl-1-ethenylpyrido[1,2-a]-pyrimidines 3 (Figure 1).
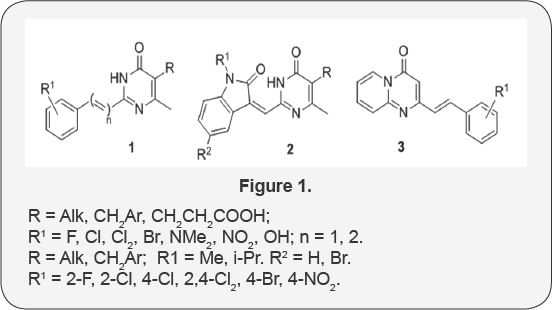
Synthesis of compounds 1, 2 was carried out by the interaction of 5-substituted-2,4-dimethyl-1,6-dihydro-6-pyrimidinones with aromatic aldehydes and substituted isatin under co-heating conditions in the presence of ZnCl2. It was shown that the reaction proceeded regioselectively with the involvement of the methyl group at position 2 of the pyrimidine ring, and the formation of derivatives with unsaturated side chains with (E) -configuration in the case of ethylene bonds, (1E, 3E) -configuration in the case of denies and with (Z) configuration of the 1-methylisatin derivatives. 2-[(E)-2-Arylvinylpyrido [1,2-a]pyrimidines3 were synthesized by interaction of 2-methyl-4H-pyrido[1,2-a] pyrimidin-4-one with aromatic aldehydes in methanol in the presence of sodium methoxide. The structure of the synthesized compounds was proved by 1H, 13C NMR spectroscopy and NOESY. Antibacterial and anti mono aminoxidase properties of synthesized compounds have been studied.
References
- VM Sviripa, W Zhang, AG Balia, OV Tsodikov, JR Nickell, et al. (2014) 2',6'-Dihalostyrylanilines, pyridines, and pyrimidines for the inhibition of the catalytic subunit of methionine S-adenosyltransferase-2. J Med Chem 57(14): 6083-6091.
- S Nagarajan, P Shanmugavelan, M Sathishkumar, R Selvi, A Ponnuswamy (2014) Synthesis, Crystal Structure and DFT Studies of 4-(1-Benzyl-5- methyl-1H-1,2,3-triazol-4-yl)-6-(o-tolyl) pyrimidin-2-amine. J Med Chem 57(14): p 6083.
- Katritzky AR, (1995) Advances in Heterocyclic Chemistry. In: (Eds.) elsevier,USA, (63): p 106.
- S Achelle, F Robin-le Guen (2013) Tetrahedron Lett 54 (33): p. 4491.






























