Abstract
Survival in ALS is highly variable, ranging from a few months to many years. Population-based prospective registries showed that up to a third of the patients die within one year from diagnosis [1-3]. In this short communication we present our long-term observations (~15 years) in terms of severe adverse events and survival rates, in patients treated with autologous mesenchymal stem cells, who participated in 3 previous open-label studies, in our center at Hadassah Medical Organization in Jerusalem, Israel.
Keywords: Cell transplantations; Population; Mortality
Manuscript
Survival in ALS is highly variable, ranging from a few months to many years. Population-based prospective registries showed that up to a third of the patients die within one year from diagnosis [1-3]. Multivariable analysis revealed that age over 75 years, interval between symptom onset and diagnosis below 7 months and Functional Rating Score below 31 points were independent factors predicting early death [4]. The cumulative probability of overall survival was reported to be 78% at 12 months, 56% at 24 months, and 32% at 48 months, with a median survival time from onset to death, 39.2 months [2]. However, 20% of ALS patients may remain alive for more than 5 years and up to 10% for >10 years, from diagnosis. Older age and bulbar onset are consistently reported to be associated with a worse outcome [5]. The rates of decline in ALS Functional Rating Scale-Revised score (ALS-FRS-R), grip strength and FVC, were also shown as significant risk factors for mortality [6]. In a large meta-analysis, high serum levels of neurofilaments light chains (NFL), the presence of dementia (of frontotemporal-FTD type), the rate of change in ALSFRS, a bulbar-respiratory subtype (<85% in forced vital capacity-FVC), were the strongest predictors of poor prognosis [7]. In the largest observational multi-center study [8], the combination of the above parameters was shown to predict the non-tracheostomized survival time, which was considered as very long when exceeded 8 years. On the other hand, younger age, limb (and not bulbar) onset and longer diagnostic delay were associated with prolonged survival [9].
In this short communication we present our long-term observations (~15 years) in terms of severe adverse events and survival rates, in patients treated with autologous mesenchymal stem cells, who participated in 3 previous open-label studies, in our center at Hadassah Medical Organization in Jerusalem, Israel. The two first trials included a total of 26 ALS patients who were treated with a single dose of autologous MSC-NTF (“NurOwn” cells, Brainstorm R) (NCT01051882 and NCT01777646, 6 received intramuscular-IM treatments; 20 received treatments intrathecally-IT, either IT or IT+ IM) [10]. In a third trial [11,12], 20 patients were treated in an open study (NCT04821479), with repeated IT administrations of autologous MSC-NG01 (Neurogenesis R). Safety and survival data from all patients who received IT treatments in these 3 trials, are summarized in this paper. Patients from the first two (Brainstorm R) trials [10], who were treated with a single IT- or IT+IM-injection of MSC-NTF (NurOwn), were followed for up to 15 years from diagnosis. In total there were 20 patients in this cohort, 6 from the MSC-NTF-001-IL trial (who received NurOwn IT) and 14 from the MSC-NTF-002-IL study (who were treated with both IT and IM injections). 9 out of these 20 IT-treated patients remain alive today (up to 18 years post diagnosis) (last follow up: Nov 2023). The median survival for all patients was 7.88 years. The average ALSFRS-R score of the long-survivors (i.e., patients still alive), at study inclusion was 31, with a median of 33, indicating a better effect on the subpopulation with higher ALSFRS scores. No unexpected adverse events were observed in these participants, providing evidence of the long-term safety of IT administered MSC (Table 1A and Figure 1A).
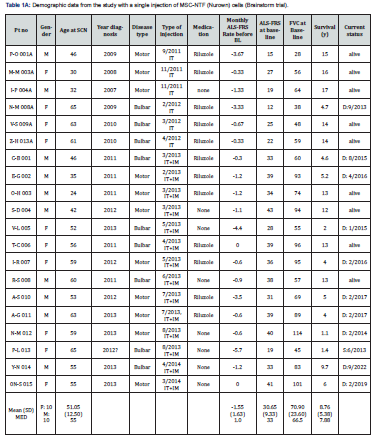
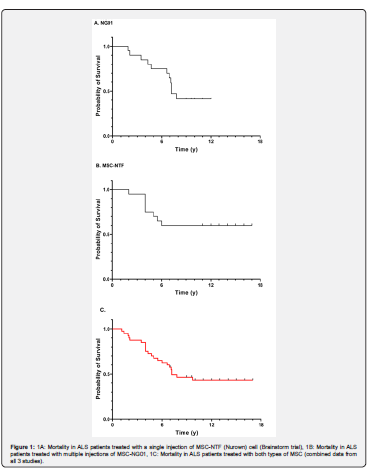
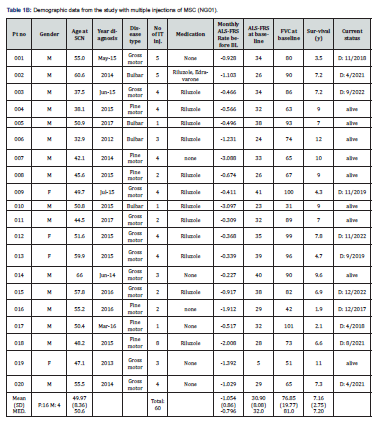
From the second trial, 20 patients (16 males, 4 females) with a mean age of 50.4 years and a mean ALSFRS-R score of 31.6 and of FVC 77.7, were followed for 12 years after disease onset (Table 1B). The patients were treated with a median of 3.5 (range:1-8) intrathecal injections of MSC-NG01 at Hadassah Center. Nine out of these 20 patients (45%) are still alive after >10 years (last follow up: Nov 2023). Two of them have undergone gastrostomy and mechanical ventilation and 2 more, continue to be gastrostomized and intubated as they were at the beginning of the trial. Two of the patients committed assisted suicide. The median survival period for all patients in this study, was 7.2 years (Table 1B and Figure 1B). Interestingly, 11 out of 20 patients in the Nurown studies and 9 of the 20 in the NG01-trial, had an FVC score at inclusion, of less than 75% which was shown as a bad predictor for ALS progression and death [6,7]. Nine patients in the Nurown trials and 4 in the NG01 study, had a bulbar onset (7/13 still, alive) and 8/20 a low ALSFRS-R score (of <30) inclusion, which also represent bad prognostic factors for long-term survival in ALS [6,7]. The mean monthly progression rate in ALSFRS before inclusion to the studies, was: -1.56±1.63 in the MSC-NTF Nurown trials and -1.05±0.86 in the MSC-NG01 (Neurogenesis R) trial; such rates of progression are considered high and were reported to have a negative impact on survival [8].
Combining the data of the 40 patients treated intrathecally with MSC from all three trials, we found that ~45% of the patients remain alive for up to 18 years (Figure 1C). It is important to emphasize, that despite the similarities in the treatment-procedure (intrathecal injection of MSC-based cell therapy) and the observed effects on survival, there were certain differences between the above studies, in terms of the type of cells used (MSC-NTF vs MSC-NG01), the number of injections (one in the Nurown studies and >1 in the NG01 trial) and also in the length of follow up which was longer in the Nurown-trials (up to 18 years, vs up to 12 years). In conclusion, our data represents the first long-term observations of ALS patients treated with autologous mesenchymal stem cells intrathecally. The finding of survival exceeding 12-18 years in ~45% of our treated patients, even in those with bad prognostic indicators (such as a low ALSFRS score and FVC function and bulbar onset of ALS), seem to deviate from the known epidemiological studies in ALS, but there is no sound way to directly compare data from different cohorts of patients. These long-term data, along with the lack of significant adverse events, may provide an important insight for the future of stem cell therapies in ALS and the proper selection of the best candidates for such treatment protocols. Larger real-world studies are needed to confirm these observations.
References
- Logroscino G, Traynor BJ, Hardiman O, Chio A, Couratier P, et al. (2008) Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry 79(1): 6-11.
- Millul A, Beghi E, Logroscino G, Micheli A, Vitelli E, et al. (2005) Survival of patients with amyotrophic lateral sclerosis in a population-based registry. Neuroepidemiology 25(3): 114-119.
- Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, et al. (2009) Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler 10(5-6): 310-323.
- Wolf J, Safer A, Wöhrle JC, Palm F, Nix WA, Maschke M, et al. (2014) Factors predicting one-year mortality in amyotrophic lateral sclerosis patients - data from a population-based registry. BMC Neurology 14(1): 197.
- Chiò A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. (2009) Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler 10(5-6): 310-323.
- Armon C, Moses D (1998) Linear estimates of disease progression as predictors of survival in patients with ALS entering clinical trials. J Neurol Sci 160 Suppl 1: S37-S41.
- Su WM, Cheng YF, Jiang Z, Duan QQ, Yang TM, et al. (2021) Predictors of survival in patients with amyotrophic lateral sclerosis: A large meta-analysis. Ebio Medicine 74: 103732.
- Westeneng HJ, Debray TPA, Visser AE, van Eijk RPA, Rooney JPK, et al. (2018) Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol 17(5): 423-433.
- Czaplinski A, Yen AA, Appel SH (2006) Amyotrophic lateral sclerosis: early predictors of prolonged survival. J Neurol 253(11): 1428-1436.
- Petrou P, Gothelf Y, Argov Z, Gotkine M, Levy YS, et al. (2016) Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients with Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol 73(3): 337-344.
- Petrou P, Kassis I, Yaghmour NE, Ginzberg A, Karussis D (2021) A phase II clinical trial with repeated intrathecal injections of autologous mesenchymal stem cells in patients with amyotrophic lateral sclerosis. Front Biosci (Landmark Ed) 26(10): 693-706.
- Georgoulopoulou E, Fini N, Vinceti M, Monelli M, Vacondio P, et al. (2013) The impact of clinical factors, riluzole and therapeutic interventions on ALS survival: a population-based study in Modena, Italy. Amyotroph Lateral Scler Frontotemporal Degener 14(5-6): 338-345.






























