Tenecteplase Compared to Alteplase in a Larger Wake-up Stroke Cohort
Dr. Hassan Khan Ahmed MD1,2*
1Neuroscience Research Group, Stavanger University Hospital, Stavanger, Norway
2University of Bergen, Bergen, Norway
Submission: November 25, 2024; Published: November 29, 2024
*Corresponding author: Hassan Khan Ahmed Neuroscience Research group, Stavanger University Hospital, 4068 Stavanger, Norway, Email: hka786@yahoo.com
How to cite this article: Dr. Hassan Khan Ahmed MD. Tenecteplase Compared to Alteplase in a Larger Wake-up Stroke Cohort. Open Access J Neurol Neurosurg 2024; 19(3): 556011. DOI: 10.19080/OAJNN.2024.19.556011.
Abstract
Tenecteplase is a newer thrombolytic agent with possible clinical and pharmacological advantages compared to alteplase. In NOR-TEST, a randomized-controlled study, both thrombolytics had a similar safety profile as well in Wake-up stroke (WUPS) patients. In order, to assess both thrombolytics in a bigger WUPS cohort, we merged WUPS patients treated in NOR-TEST with WUPS patients treated after the trial and reanalyzed the patient outcome.
. Methods: WUPS patients were included in Nor-Test based on DWI/FLAIR mismatch and randomly assigned (1:1) to receive intravenous tenecteplase 0·4 mg/kg or alteplase 0·9 mg/kg. The clinical outcome was measured by the National Institutes of Health Stroke Scale (NIHSS), functional outcome by the modified Rankin Scale (mRS). After the termination of the trial one center continued treating the patients with tenecteplase while another center switched back to alteplase. Patients with a pre-stroke mRS>1 were excluded from the analysis.
Results: Total 114 patients were included in this study. Clinical outcome was measured in 97 patients with prestroke mRS≤1. Of them, 38 (39%) were treated with tenecteplase. There was neither a difference in neurological improvement 24h after admission, (alteplase: 41 (69.5%), tenecteplase: 21 (55.3%); (p=0.154) nor any difference in mRS at day 90; (alteplase: 39 (66.1%), tenecteplase: 22 (57.9%); p=0.414). Symptomatic intracranial hemorrhage was detected on control MRI/CT in one tenecteplase patient (n=1; 2.6%, p=0.392).
Conclusion: Compared to alteplase, tenecteplase appears to have similar efficacy and safety profile in WUPS patients selected by DWI-FLAIR mismatch at admission MRI, also in a bigger WUPS patient cohort.
Keywords: Acute ischemic stroke, Wake-up Stroke, Magnetic Resonance Imaging (MRI), DWI-FLAIR-mismatch
Introduction
Intravenous thrombolysis (IVT) is an effective, established treatment in acute ischaemic stroke if administered within 4.5h of symptom onset, but the IVT effect decreases with time [1]. Today alteplase, which is a genetically modified product of recombinant tissue plasminogen activator with superior pharmacodynamic and pharmacokinetic properties, is the established and approved thrombolytic agent used in acute stroke patients [2,3]. Nevertheless, alteplase has its limitations such as short half-life, low recanalisation rate and risk of intracranial hemorrhage [4,5].
Up to 25 % stroke patients wake up with neurological deficit also called wake-up stroke (WUPS) [6]. To select stroke patients eligible for IVT treatment MRI imaging is used, guided by mismatch between weighted imaging (DWI) and Fluid Attenuated Inversion Recovery (FLAIR) on MRI (MRI mismatch) [6].
In NOR-TEST, comparing 0.4 mg/kg tenecteplase with 0.9 mg/kg alteplase, tenecteplase showed similar efficacy without increasing the rate of intracerebral bleedings [7]. In our previous study, a small substudy of NOR-TEST trial, where total 40 WUPS patients were treated with either alteplase (24 patients) or tenecteplase (16 patients) with an overall low stroke severity measured in NIHSS, respectively NIHSS 4.5 vs 6, showed no difference in clinical outcome (mRS 0-1) at day 90, and no ICH or deaths were detected in either alteplase or tenecteplase group [8]. Due to a small patient data, it was concluded that more investigation is required [8]. Still limited data is available about the efficiency and safety profile in WUPS patients selected to IVT by MRI mismatch. In order, to assess alteplase and tenecteplase in a bigger WUPS cohort, we merged WUPS patients treated in NOR-TEST with WUPS patients treated after the trial and reanalyzed the patient outcome.
Methods
Study design and patients
In this study both WUPS patient treated in NOR-TEST and after NOR-TEST were included. NOR-TEST was a multicenter, randomized, open-label, blinded endpoint, phase III trial, designed to investigate safety and efficacy of tenecteplase 0.4 mg/kg versus alteplase 0.9 mg/kg in patients with acute ischemic stroke. Inclusion of NOR-TEST patients occurred between Sept 1, 2012 and Sep 30, 2016 and from Oct 1, 2016 to Oct 31, 2019 after the NOR-TEST trial.
Inclusion criteria: patients had to be >18 years, living independently pre-stroke and had to be admitted within 4.5 hours of stroke onset with a measurable neurological deficit on the National Institutes of Health Stroke Scale (NIHSS).
Along with stroke patients with Known Symptom Onset (KOS), also patients with symptoms on awakening or unknown onset were included in NOR-TEST. They underwent randomization based on FLAIR-DWI mismatch and the treatment of thrombolysis were randomly assigned (1:1) with either tenecteplase (0.4 mg/kg to a maximum of 40 mg as a single bolus intravenously) or alteplase (0.9 mg/kg to a maximum of 90 mg, 10% as an initial bolus, followed by 90% as 1h intravenous infusion). Detailed inclusion and exclusion criteria are published elsewhere [7].
In order, to gain a larger WUPS corhort, WUPS patients treated with thrombolysis after the termination of NOR-TEST trial were also included in the current study. One stroke center, Stavanger University Hospital, switched back to alteplase treatment (0.9 mg/kg to a maximum of 90 mg), whereas another stroke center, Haukeland University Hospital, continued treating acute stroke patients with tenecteplase (0.4 mg/kg to a maximum of 40 mg). 24-48 hours after the treatment a control CT or MRI was performed. The radiological assessment was done by the local neuroradiologists. Intracranial hemorrhage and symptomatic intracerebral hemorrhage were defined in according to respectively ECASS I and ECASS III criteria [2,9].
Functional outcomes were measured with modified Rankin Scale at day 7 or at discharge if discharged earlier and finally after 3 months by telephone consultation or as an outpatient consultation performed by qualified stroke staff. Improvement of neurologic deficits were defined as an improvement of NIHSS 4 points within 24 hours as compared to admission NIHSS or NIHSS 0 at 24 hours; mRS of 0 or 1 at 90 days was defined as a favorable functional outcome.
Statistics: SPSS Statistics version 24 was used to complete all statistical analysis. Mann-Whitney test, Chi square test, and Fisher’s exact test were used to examine both baseline variables and variable changes.
Results
In NOR-TEST 1107 patients were signed up and of these 1100 patients were included after 7 patients were excluded either because informed consent was withdrawn or reconsideration of thrombolysis eligibility. Of these, 45 patients were included as WUPS. Five patients did not present an ischemic lesion on DWI, but still treated with IVT on clinical suspicion of stroke. These patients were subsequently classified as stroke mimics and excluded from further safety and efficacy analysis [8].
All 40 WUPS patients (24 treated with alteplase and 16 with Tenecteplase) from NOR-TEST were included in this study. In order to achieve a larger WUPS cohort in this current study, further 74 WUPS patients were included after the completion of the NOR-TEST trial. Of these, 44 patients were treated with alteplase 0.9 mg/kg in Stavanger University Hospital while 30 patients were treated with tenecteplase 0.4 mg/kg in Haukeland University Hospital.
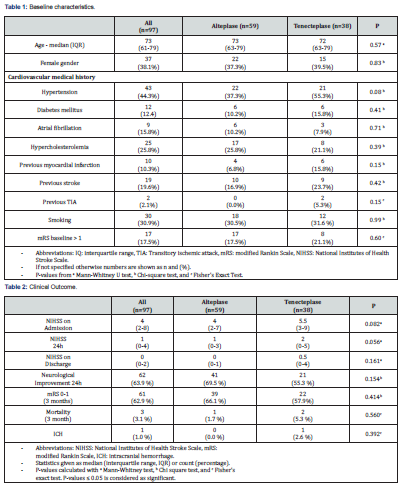
By this, the total WUPS cohort reached 114 WUPS patients. Clinical outcome was measured in 97 WUPS patients with pre-stroke mRS≤1 while 17 patients presented a pre-stroke > 1 and excluded from the clinical analysis. A median age of 73 (61-79) was seen in the total WUPS cohort and no significant difference was seen in age in the treatment groups; (p=0.57). Female patients were represented with (n=37) (38.1%). No significant difference was seen in the other baseline characteristics in any of the treatment groups (Table 1).
The median NIHSS of the entire cohort at admission was 4 (IQR 2-8). No significant difference was seen between alteplase and tenecteplase in NIHSS at admission: (4 (2-7), 5.5 (3-9); p=0.082), in NIHSS at 24h: (1 (0-3), 2 (0-5); p=0.056) or NIHSS at discharge: (0 (0-1), 0.5 (0-4); p=0.161).
Significant difference was not seen in neurological improvement at 24h after admission, (alteplase: 41 (69.5 %), tenecteplase: 21 (55.3 %); p=0.154) nor any difference in mRS at 3 months; (alteplase: 39 (66.1 %), tenecteplase: 22 (57.9 %); p=0.414). Intracranial hemorrhage was detected on control MRI/CT in one tenecteplase patient; (p=0.392) and one patient died in the alteplase group while 2 patients died in the tenecteplase group, respectively within the first 3 months; (p=0.560) without exhibiting any significant difference (Table 2).
Discussion
Patients in this WUPS cohort were included in the NOR-TEST and after the termination of the NOR-TEST trial. We did not find any significant differences in neurological improvement or favorable clinical outcome at three months between patients treated with either alteplase or tenecteplase. Neither any significant differences in hemorrhagic complications or deaths in any of the treatment groups. In this WUPS cohort patients were selected to IVT treatment based on MRI DWI-FLAIR imaging [10].
Current WUPS cohort is relatively small in size with an overall low stroke severity in NIHSS (Altp: 4, Tnc: 5.5) making it difficult to elucidate clinical effects, as patients with small infarction on admission tend to get a good functional outcome independent of whether or not they are treated with reperfusion therapy [11].
Five WUPS patients were initially enrolled in NOR-TEST and treated with IVT on clinical suspicion without any lesion on DWI, but later excluded from the study as stroke mimics and not included in further statistical analysis. None of these patients experienced an ICH [8].
Our study has some shortcomings. Basically, a relatively low number of WUPS patients included and treated with either alteplase or Tenecteplase. Additionally presenting a low NIHSS score, respectively 4 and 5.5 which tend to improve clinically regardless of IVT treatment [11].
The first 40 patients in this study were included in NOR-TEST which was a randomized study, whereas the last 74 patients were included after the NOR-TEST trial was terminated and was not part of a randomized control trial which might weaken the study protocol. Still the follow up CT/MRI, measurement of clinical outcomes with NIHSS and scoring the functional outcome with mRS at 3 months was performed in the same way as in the NOR-TEST trial [7,8]. This study shows that the treatment of tenecteplase compared with alteplase has similar efficacy and safety profile, and being comparable with our previous study [8].
Selection of WUPS patients with a traditional MRI DWI/FLAIR, might exclude patients who would otherwise benefit from IVT treatment encouraging a modified MRI mismatch concept [12-14]. CT selection of WUPS patients might have similar safety and efficacy compared to MRI selection [6,12,15]. However, the role of CT in WUPS patients has been investigated in the TWIST trial by selecting WUPS patients with non-contrast CT [16]. No increased ICH complications were seen, but the trial failed to document better functional outcome in the treatment group, encouraging studies of other imaging modalities.
CT perfusion criteria has been used to select also WUPS patients [17]. However, the concept has low sensitivity in posterior strokes and possibly excluded lacunar stroke syndromes [18]. In the extend-IA TNK-2 study, tenecteplase dosages before EVT, concluded that patients with large vessel occlusion ischemic treated with 0.40 mg/kg did not significantly improve cerebral reperfusion prior to EVT compared to patients treated with 0.25 mg/kg [19]. The NOR-TEST trial had showed in minor strokes, similar clinical effect and safety profile of tenecteplase 0.4 mg/kg compared to alteplase 0.9 mg/kg [7]. The NOR-TEST II trial aimed to test non-inferiority of tenecteplase 0.4 mg/kg versus alteplase 0.9 mg/kg in moderate and severe strokes. NOR- TEST II was terminated prior to completion due to a significant larger number of Symptomatic Intracranial Hemorrhages (sICH) and mortality rate [20].
In conclusion, this current extended WUPS cohort treated with either tenecteplase or alteplase exhibited equivalent neurological improvement and favorable clinical outcome at 3 months in the treatment groups. One ICH was observed in the tenecteplase group and none in the alteplase group, exhibiting no significant difference. Further one death was observed in the alteplase group and two deaths in the tenecteplase group within the first 3 months after IVT treatment, also without showing significant difference. Our study supports selection of WUPS patients to thrombolytic treatment with either alteplase or tenecteplase in acute ischemic stroke. Our study further encourages ongoing trials testing different dosages of tenecteplase and different imaging modalities in acute ischemic stroke including in known onset stroke and wake-up stroke.
Acknowledgements
NOR-TEST is funded by the Research Council of Norway as part of the project number 229006 “The Norwegian Stroke Project: Expanding therapeutic options in acute cerebral ischaemia and haemorrhage”.
Ethical approval
This study was approved by the Regional Committee for Medical and Health Research Ethics, the Norwegian Centre for Research Data and the local hospital authorities. WUPS patients treated with IVT in NOR-TEST and consecutively thereafter were included. The need for informed consent was waived by the Regional Committee for Medical and Health Research Ethics, the Norwegian Centre for Research Data and the local hospital authorities.
Data Availability
All data generated or analyzed in this study can be accessed on request.
Sources and funding
Research Council of Norway.
Disclosures
Nothing to disclose.
Declaration of Conflicting Interest
The Author declare that there is no conflict of interest.
References
- Meretoja A, Keshtkaran M, Saver JL, Tatlisumak T, Parsons MW, et al. (2014) Stroke thrombolysis: save a minute, save a day. Stroke 45(4): 1053-1058.
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, et al. (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359(13): 1317-1329.
- National Institute of Neurological D, Stroke rt PASSG (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24): 1581-1587.
- Bhatia R, Hill MD, Shobha N, Menon B, Bal S, et al. (2010) Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 41(10): 2254-2258.
- Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, et al. (2016) Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol 15(9): 925-933.
- Kurz MW, Advani R, Behzadi GN, Eldoen G, Farbu E, et al. (2017) Wake-up stroke- Amendable for thrombolysis-like stroke with known onset time? Acta Neurol Scand 136(1): 4-10.
- Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, et al. (2017) Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol 16(10): 781-788.
- Ahmed HK, Logallo N, Thomassen L, Novotny V, Mathisen SM, et al. (2020) Clinical outcomes and safety profile of Tenecteplase in wake-up stroke. Acta Neurol Scand 142(5): 475-479.
- Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, et al. (1995) Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 274(13): 1017-1025.
- Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, et al. (2011) DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol 10(11): 978-986.
- Ferrari J, Reynolds A, Knoflach M, Sykora M (2021) Acute Ischemic Stroke With Mild Symptoms-To Thrombolyse or Not to Thrombolyse? Front Neurol 12: 760813.
- Odland A, Saervoll P, Advani R, Kurz MW, Kurz KD (2015) Are the current MRI criteria using the DWI-FLAIR mismatch concept for selection of patients with wake-up stroke to thrombolysis excluding too many patients? Scand J Trauma Resusc Emerg Med 23: 22.
- Jakubicek S, Krebs S, Posekany A, Ferrari J, Szabo J, et al. (2019) Modified DWI- FLAIR mismatch guided thrombolysis in unknown onset stroke. J Thromb Thrombolysis 47(2): 167-173.
- Ahmed HK, Mathisen SM, Kurz K, Dalen I, Logallo N, et al. (2024) Thrombolysis in wake-up stroke based on MRI mismatch. J Neurol Sci 466: 123265.
- Barreto AD, Fanale CV, Alexandrov AV, Gaffney KC, Vahidy FS, et al. (2016) Prospective, open-label safety study of intravenous recombinant tissue plasminogen activator in wake-up stroke. Ann Neurol 80(2): 211-218.
- Eltoft A, Wilsgaard T, Roaldsen MB, Soyland MH, Lundstrom E, et al. (2022) Statistical analysis plan for the randomized controlled trial Tenecteplase in Wake-up Ischaemic Stroke Trial (TWIST). Trials 23(1): 421.
- Campbell BCV, Ma H, Ringleb PA, Parsons MW, Churilov L, et al. (2019) Extending thrombolysis to 4.5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet 394(10193): 139-147.
- Garcia-Esperon C, Visser M, Churilov L, Miteff F, Bivard A, et al. (2021) Role of Computed Tomography Perfusion in Identification of Acute Lacunar Stroke Syndromes. Stroke 52(1): 339-343.
- Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, et al. (2020) Effect of Intravenous Tenecteplase Dose on Cerebral Reperfusion Before Thrombectomy in Patients With Large Vessel Occlusion Ischemic Stroke: The EXTEND-IA TNK Part 2 Randomized Clinical Trial. JAMA 323(13): 1257-1265.
- Kvistad CE, Naess H, Helleberg BH, Idicula T, Hagberg G, et al. (2022) Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol 21(6): 511-519.






























