Assessing Behavioral and Psychological Symptoms of Dementia in a 3xTg-AD Mouse Model of Alzheimer’s Disease
Subash Bhandari1,2, Hongyuan Xu1, Belinda Chen1, Stefan Basilio1, Parsa Sartipi1, Hailee Sontag1, Wen Shen1,2, Rui Tao1,2, Howard Prentice1,2,3* and Jang-Yen Wu1,2,3*
1Department of Biomedical Sciences, Charles E. Schmidt College of medicine, Florida Atlantic University, Boca Raton, FL 33431, USA
2Program in Integrative Biology, Florida Atlantic University, Boca Raton, FL, 33431, USA
3Center of Complex systems and Brain Sciences, Florida Atlantic University, Boca Raton, FL, 33431, USA
Submission: December 21, 2023; Published: January 04, 2024
*Corresponding author: Jang-Yen Wu, Department of Biomedical Sciences, Charles E. Schmidt College of medicine, Florida Atlantic University, Boca Raton, FL 33431, USA Howard Prentice, Center of Complex systems and Brain Sciences, Florida Atlantic University, Boca Raton, FL, 33431, USA
How to cite this article: Subash B,, Hongyuan X, Belinda C, Stefan B, Parsa S, et al. Assessing Behavioral and Psychological Symptoms of Dementia in a 3xTg-AD Mouse Model of Alzheimer’s Disease. Open Access J Neurol Neurosurg 2024; 18(4): 555996. DOI: 10.19080/OAJNN.2024.18.555996.
Abstract
Alzheimer’ disease (AD) is characterized by progressive deposition of amyloid plaques and neurofibrillary tangles along with corresponding severity of neuronal loss, cognitive decline and neuropsychiatric changes. The non-motor neuropsychiatric changes, that are commonly called behavioral and psychological symptoms of dementia (BPSD), are key contributors to enhanced caregiver distress as well as the institutionalization of dementia patients. There is a scarcity of animal models that have been adequately characterized in terms of neuropsychiatric disorders to enable assessment of drug efficacy for treating these changes. The present study characterizes the behavioral and psychological symptoms of dementia using Force plate actometer assay in the 3xTg-AD mice, an animal model that exhibits comprehensive progressive pathology that is similar to AD patients. One-year old 3xTg-AD mice exhibited elevated levels of anxiety like behaviors and stereotypies along with reduced levels of power in the frequency range associated with neuropsychiatric abnormalities. Combinations of force/time analysis, power spectra, tremor index, number of low mobility bouts, and stereotypies scores were analyzed and these measurements demonstrated that 3xTg-AD mice represent an excellent animal model for drug feasibility studies addressing BPSD.
Keywords: Behavioral and psychological symptoms of dementia; BPSD; 3xTg-AD; Alzheimer’s disease; Behavior; Low mobility bouts; Tremor; Stereotypy; Force plate actometer; Open field test
Abbreviations: AD: Alzheimer’s disease; BPSD: Behavioral and Psychological Symptoms of Dementia; COF: Center of Force; COP: Center of Pressure; ET: Essential Tremor; FPA: Force Plate Actometer; LMB: Low Mobility Bouts; NFT: Neurofibrillary Tangles; WT: Wild-Type
Introduction
Alzheimer’s disease (AD), a chronic neurodegenerative disease of the CNS system that affects more than 55 million people worldwide, is expected to reach 115 million people by 2050 in the absence of disease-modifying therapies [1,2]. Alzheimer’s disease and its associated dementia are not only emotional and physical burdens to patients, their families, and caregivers but also major financial costs to communities worldwide. Insoluble amyloid senile plaques and neurofibrillary tangles (NFTs) are constant neuropathological features of both early-onset and late-onset AD. In the early stage of disease progression, AD patients show accumulation of abnormal amyloid plaques and progressively express hyperphosphorylated tau and subsequent inflammation and neuronal death leading to progressive cognitive decline. However, the exact mechanism of this progression is the subject of active investigations. Multiple hypothesis of disease progression includes amyloid cascade hypothesis, tau hypothesis, cholinergic hypothesis and cerebral vascular dysfunction/hypoxia. Cerebral blood flow measurements determined by arterial spin-labelling (ASL) quantification in mild cognitive impairment patients were found to lie between levels found in control subjects and AD patients [3]. Furthermore, in an investigation focusing on vascular factors predisposing to cerebrovascular disease, silent cerebral infarctions were found to contribute to the severity of cognitive decline in AD [4]. Hypoxia increases beta amyloid aggregation in addition to tau pathology and hypoxia can induce pathological calcium -homeostasis dysfunction in neurons though L-type calcium channel activation that is dependent on the presence of beta-amyloid [5].
Cognitive decline is central to AD pathology but importantly AD patients also exhibit, to varying degrees, behavioral and psychological symptoms of dementia (BPSD), such as depression, anxiety, agitation, and apathy. Multiple studies have reported that many AD patients exhibit at least one symptom of BPSD, and these symptoms start to develop early during disease progression in some cases [6,7,8]. Caregiver distress and institutionalization in AD patients are more closely related to BPSD than to cognitive impairment [9]. While different animal models have been used to elucidate the pathology, progression and cognitive decline in Alzheimer’s disease, there is a scarcity of information concerning the use of these animal models to study symptoms of BPSD. Similarly, very few drugs are currently available for clinical use that specifically target the alleviation of BPSD in AD and related dementia. Hence, reliable and simple animal models for studying BPSD symptoms to assess the efficacy of potential drugs for treating BPSD in patients with AD are highly desirable.
BPSD are characterized as noncognitive symptoms associated with AD patients. In a cross-sectional, observational study conducted on AD patients in Spain, patients with higher BPSD had significantly greater incidences of delusions, hallucinations, delirium, and tremors [10]. Another study reported that tremors are prevalent in the majority of AD patients (56%) [11]. A 3xTg-AD mouse model has been demonstrated by multiple studies to exhibit BPSD, including neophobia [12,13,14], in the corner test; anxiety-like behavior, such as increased freezing in the open field test [13,14] and T-maze [14]; and freezing and stereotyped rearing in the open field test [15]. It has also been recently reported that the Circling index (bizarre behavior) and flotation index during Morris water maze are considered symptoms of BPSD, and a study [16] showed that 3xTg-AD mice exhibit these symptoms. Another study reported that 3xTg-AD mice exhibit increased freezing and unusual shaking/tremoring behavior [17], a symptom of BPSD. Here, we present our investigation of BPSD symptoms in aged 3xTg-AD mice and assess the feasibility of using 3xTg-AD mice for therapeutic intervention in AD using Force plate actometer test (FPA).
Materials and Methods
Animals
A triple-transgenic mouse model of Alzheimer’s disease (B6;129-Tg (APPSwe, tauP301L) 1Lfa Psen1tm1Mpm mice – 3xTg-AD and their corresponding wild-type (WT) controls (C57BL/6 × 129/Sv, F2 generation, B6129SF2/J)- WT were purchased from Jackson Laboratory and housed under standard laboratory conditions (temperature 23 ± 1 °C, a 12-hour day-night cycle, unrestricted access to food and water). Mice were approximately 12 months old at the time of the behavioral test. All procedures were conducted according to the guidelines outlined in the institutional Animal Care and Use Committee and approved by the university for the use and care of animals at Florida Atlantic University.
Behavioral Testing apparatus: Force Plate Actometer (FPA)
Behavioral tests were conducted using a Force Plate Actometer (FPA) (BASi, West Lafayette, IN, USA). The FPA is an open field chamber invented by late Dr. Stephen C. Fowler and his colleague Troy Zaccone at the University of Kansas in the early 2000s. The actometry and data processing methods used were described in the original 2001 paper published in the Journal of Neuroscience Methods [18,19]. In brief, FPA is a computer-based device with great temporal (0.01 s resolution), force resolution (0.33 g resolution) and spatial resolution (1 mm resolution) consisting of a carbon fiber/Nomex composite material, measuring 24 × 24 cm surrounded by a clear polycarbonate cage (15 cm high) with a polycarbonate top perforated with ventilation holes and housed in a sound-attenuating cabinet [20]. As rodents move in this open field chamber, FPA records precise information about the location of the center of force (COF), the force exerted, the force and mass variations, and the direction and proximity of the animal from a reference point. These collected measurements are used to detect rhythmicities of variations displayed during movements that are quantified for behavioral analysis associated with power spectra, force variations as a function of frequency, whole-body tremor and tremor indices and stereotypies.
To acclimate the mice to the FPA chamber, each mouse was introduced to the chamber for 30 minutes for 2 consecutive days. On the third day, each mouse was reintroduced for 30 minutes, and the data were collected only on the third day. We used wild-type (n=11) and 3xTg-AD (n=9) mice for these behavioral tests. Raw data for the whole duration of the experiment, as well as parameter-defined data and low mobility bouts, (LMB; defined as the period of time during which mice did not travel outside the 15 mm radius for 10.24 seconds), were analyzed to determine differences in 3xTg-AD mice with respect to their background control regarding tremor, stereotypies, power, spatial confinement statistics score as well as frequency and duration of LMBs.
Power spectra
As mice move through the force plate, FPA collects data on the forces exerted in three dimensions, namely, the X-axis, Y-axis, and center of pressure (COP). These data were recorded through the four sensitive force transducers found underneath the force plate at each corner of the chamber. FPA then uses the fast Fourier transform to convert these force data into a frequency domain representation generating power spectra [18,21]. The generated power spectra illustrate the distribution of power across different frequencies, with higher values indicating greater activity at those frequencies. Power spectra can provide valuable information on movement patterns within and between different experimental groups. Low-frequency power spectra (0-4 Hz) are associated with slow, low-amplitude movement behaviors such as head bobbing and postural adjustments, and spectra at frequencies between 4 and 12 Hz are associated with low-speed gaits such as walking, lateral walking, stop and go gait, hopping, crawling, sniffing and grooming. Higher-frequency power spectra (12-25 Hz) are associated with running or other high-speed movements, such as jumping, grooming or involuntary movements. Studies have used power spectra data from FPA to infer subtle changes associated with neurological disorders such as Parkinson’s disease and Huntington’s disease and to illustrate the effect of drugs between groups.
Tremor
Tremor is a characteristic hallmark of neurological disorders such as Parkinson’s disease and Huntington’s disease and implies CNS dysfunction. Although action tremors are more common in diseases such as Parkinson’s disease, essential tremor (ET), especially during immobility movements and posture, are more common adult movement disorders [22] and are reported to be more closely associated with dementia patients [23,24] and cognitive impairment-associated conditions [25,26]. Studies have suggested a possible link between AD and ET [27,28,29], including a study in which action (nonrest) tremors were significantly worse in AD patients than in controls [27]. Moreover, cognitive dysfunction is becoming more well recognized as a characteristic of ET, as these cognitive abnormalities are especially common in the domains of executive function and memory. Mice are reported to exhibit essential tremors, especially at rest, within the same range as humans (4-12 Hz); however, these tremors could depend upon the underlying causes. For instance, in a mouse model of Parkinson’s disease, harmaline-induced tremors occur in the 10-16 Hz range.
FPA analysis defines tremor as a period of no locomotion following freezing in which the animal displays a full body of sudden jerky movement lasting up to 10 seconds. The force generated by mouse movement was recorded by four force transducers on each corner of the FPA. These force signals are filtered to isolate frequency components associated with tremors, followed by analysis to extract information such as peak amplitude, frequency, and duration of tremor bursts. FPA then combines this information into a single score called the tremor index. A higher tremor index indicates greater intensity and frequency of tremor. This score can be a valuable tool for assessing behavioral and physiological symptoms in animal models of neurological disorders.
Stereotypies
The highly repetitive, rhythmic behaviors of rodents without locomotion, such as nose poking, sniffing, walking, lateral walking, stop-and-go gait, hopping, crawling, sniffing, head bobbing, and licking, are collectively called stereotypies. FPA analysis defines stereotypy as the intensity of behaviors occurring in one place. FPA can machine score these behaviors to determine the effect of drugs or disorders in rodents. FPA uses and extracts different parameters, such as the center of force (COF) trajectory, force movement and variation, and time budget, which is defined as the percentage of time spent in stereotypical behavior as a function of total exploration time. For stereotypical analysis, the FPA measurement defines a threshold movement amplitude of the COF and any movement exceeding that threshold as a part of stereotypy. Also included in the analysis for determining the stereotypy score are features such as the distance traveled during stereotypy, the movement area and the frequency enclosed by the COF trajectory and the mean velocity during the COF trajectory. FPA collectively uses these data to calculate the comprehensive focus stereotypy score [21,30,20]. A higher focused stereotypical score is associated with more intense and frequent stereotypy.
Spatial confinement statistics score
The spatial confinement statistics score is used in rodent behavioral tests to analyze behaviors such as movement patterns, the center of activity, and spatial memory. The force plate actometer calculates spatial confinement statistics scores by comparing the movement variations of a mouse to those of a theoretical rodent that explores every sector of the actometer force plate at a given time. For example, a score of one hundred would mean that a mouse did not move beyond a single sector in each period (10.24 s). Mice with a higher spatial confinement score are considered to have a behavioral deficit.
Low mobility Bouts (LMB)
A low mobility band (LMB) is defined as the period in the FPA chamber where the COF of a mouse does not travel outside of a 15 mm radius for at least 10.24 seconds (one frame). LMB data were extracted from the raw experimental data recorded by the FPA and analyzed for tremor, stereotypical score, number and frequency of bouts, amount of time spent engaging in LMB behaviors, and spatial confinement statistics. LMB data can provide valuable insights into mice’s level of activity and distress and can be used to characterize behaviors due to neurological conditions, the effects of drugs and so forth. LMB data from FPA are more selective and sensitive for differentiating subtle differences between genetic conditions and disease stages.
Statistical Analysis
Once the data were primarily collected, analyzed, and extracted from FPA software (version 10.1.1), differences among groups were evaluated using two-tailed t tests. All the data are presented as the mean ± SEM. We used an alpha value of 0.05 or less to indicate statistical significance. **** p≤0.0001, *** p≤0.001, ** p≤0.01, * p≤0.05, ns p≥0.05.
Results
3xTg-AD mice exhibit altered force time series and power
Rodent behaviors such as grooming, scratching, tremors, and flanking often involve rhythmic force time signals, and these signals are collected by force sensors in the FPA and are displayed as force time series, as illustrated in Figure 1. Figure 1 shows a representative force‒time graph of one WT mouse and one 3xTg-AD mouse throughout the entire experiment (30-minute test). Analysis of the force–time series between groups revealed greater baseline force and force variation in 3xTg-AD mice (average baseline force of 56.4 g) than in wild-type mice (average baseline force of 43.2 g) (Figure 1). The actometer converts these force time series of each mouse to power spectra over the 0 to 25 frequency domain using fast Fourier transformation as illustrated in Figure 2A. Both 3xTg-AD and WT mice manifested a peak frequency in the 5-7 Hz range, the range associated with increased activity and WT mice exhibited greater peaks and hence greater activity than 3xTg-AD mice [31]. We employed a parametric two-tailed paired t test to determine whether there was a difference in power between groups in each frame (10.24 s). The test showed that 3xTg-AD mice had a significantly decreased level of power (50% reduction) across the entire frequency spectrum (****, p < 0.0001; two-tailed, unpaired t test) (Figure 2A) and that was also evident when measuring total power (**, p =0.0097; two-tailed, unpaired t test) as shown in Figure 2B. We then analyzed differences in the power and tremor indices associated with stereotypes as well as different types of tremors including essential tremor. An unpaired two-tailed t test indicated a significantly elevated tremor index in 3xTg-AD mice compared to WT mice at 0 to 4 Hz (**, p < 0.0046; two-tailed, unpaired t test); 4 to 12 Hz (**, p < 0.0072; two-tailed, unpaired t test); and 2 to 25 Hz (**, p < 0.0040; two-tailed, unpaired test) frequency range (Figure 2C-E).
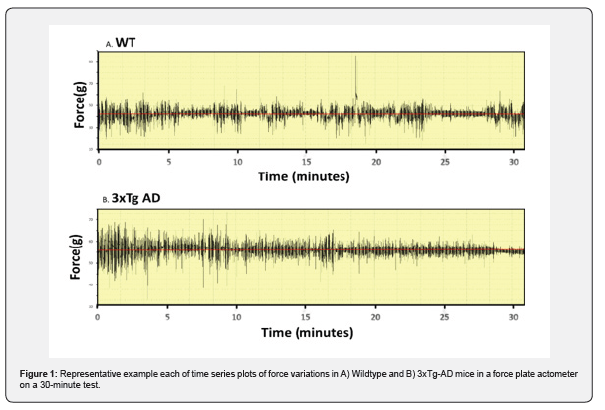
3xTg-AD mice exhibited elevated tremor at one year
The differences in tremor between wild-type and transgenic mice were subsequently examined. Our analysis of tremors in the 4-12 Hz range, which corresponds to the frequency range in AD patients with essential tremor [32,33] (Figure 2G), revealed significantly elevated tremor indices (**, p < 0.0019; two-tailed, unpaired t test). The tremor index difference was even more pronounced in the 2-25 Hz frequency range (Figure 2H) (***, p < 0.0002; two-tailed, unpaired t test), implying that 3xTg-AD mice may exhibit different types of tremors, potentially combinations of resting, essential tremors.
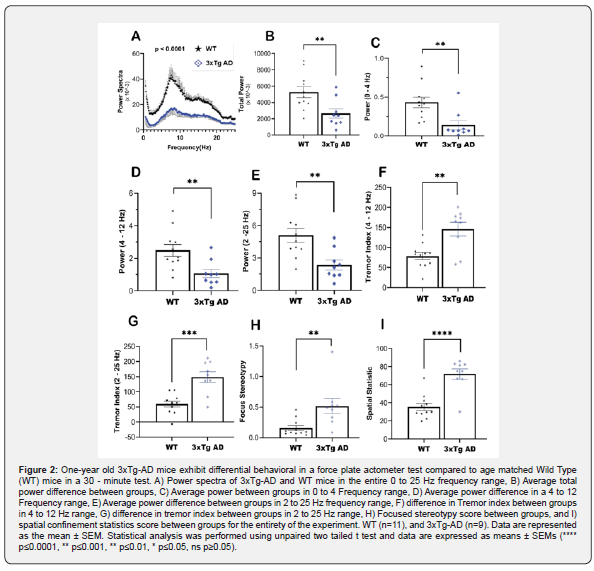
3xTg-AD mice exhibit elevated focused stereotypy and spatial confinement scores
Stressful situations are known to induce stereotypic behavior in species. Stereotypies are regarded as BPSD [34,15], and it has been reported that 3xTg-AD mice exhibit symptoms of BPSD by six months. We used FPA to generate an automated focused stereotypy and spatial statistical score to compare the baseline level of stereotypies in 1-year-old wild-type and transgenic 3xTg-AD mice. The results (Figure 2F) showed that, compared with wild-type mice, transgenic mice had significantly elevated levels of stereotypies (**, p = 0.070; two-tailed; unpaired t test) and spatial confinement statistics score (****, p<0.0001; two-tailed; unpaired t test).
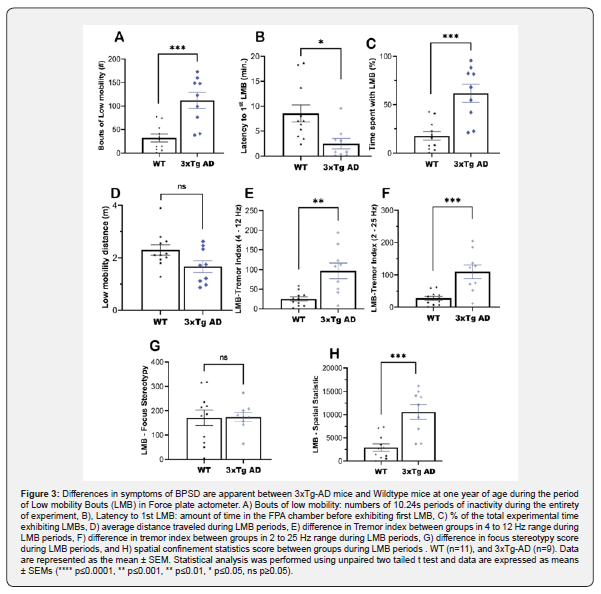
3xTg-AD mice exhibit differential stereotypical behaviors during periods of LMBs
Low-mobility bouts provide valuable information about the temporal components of inactivity. To assess these behaviors in 3xTg-AD mice, entire experimental timeline raw data from FPA were filtered using the following criteria: the COFs of mice not traveling outside of a 15-millimeters radius for at-least 10.24s. These criteria were used to extract information such as the tremor index, stereotypy, spatial confinement statistics score and frequency of bouts (Figure 3). These statistics were assessed to determine the behavioral deficiencies exhibited by 3xTg-AD mice compared to WT mice. In average, one-year-old 3xTg-AD mice were exhibited 112 incidences of LBMs compared to 32 for WT mice during the experimental period (30-minute test) (***, p = 0.0003; two-tailed, unpaired t test) (Figure 3A). The latency to first LMB was 2.52 minutes for 3xTg-AD mice compared to 8.53 minutes for WT (*, p = 0.011; two-tailed; unpaired t test), and these mice spent a significantly greater portion of their experimental time exhibiting LMB (62%) than did WT (18%) mice (*, p = 0.0003; two-tailed; unpaired t test) (Figure 3B-C). During LMB, 3xTg-AD mice were less inclined to locomote than were WT mice, but the difference was not statistically significant (ns, p = 0.0547; two-tailed, unpaired t test (Figure 3D). We then analyzed tremor indices and found similar trends in tremors at both 4-12 Hz and 2-25 Hz. 3xTg mice exhibited a statistically significant increase in tremor severity at 4-12 Hz (**, p = 0.0014; two-tailed; unpaired t - test) and 2-25 Hz (***, p = 0.0007; two-tailed; unpaired t test) (Figure 3E-F). However, the elevation in stereotypy that were apparent during the whole experimental timeline (Figure 2F) were not observed during LMBs (ns, p = 0.9297; two-tailed, unpaired t test), even though the spatial confinement statistics score retained significance (***, p = 0.0002; two-tailed, unpaired t test) (Figure 3 G-H).
Discussion
While the majority of research on Alzheimer’s disease has focused on cognitive deficits in mouse models, very few mouse models have been used to assess neuropsychological disorders associated with dementia/Alzheimer’s disease patients. In amyloid- and tau-related AD mouse models that have been studied for BPSD-like behaviors, there is no clear mechanism-based evidence to support the use of these models for therapeutic interventions to treat symptoms of BPSD. Like in human AD patients, multiple different AD mouse models are reported to exhibit variety of classes of heterogeneous behaviors, suggesting a mechanism-based origin of different BPSD-associated disorders. It is our belief that extensively studying BPSD-like behaviors in existing AD mouse models could lead us to identify the neuronal basis of BPSD-like behaviors that can be utilized for therapeutic interventions. 3xTg-AD mice harbor the PS1M146V, APPSWE, and tauP301L transgenes and exhibit plaque and tangle pathology as well as cognitive decline with age [35,36,37]. Synaptic dysfunctions, including long-term potentiation, precede plaque and tangle formation [38,39,36]. These progressive pathologies closely resemble the pathologies exhibited by AD patients. Hence, 3xTg-ADhas become one of the most important mouse models for elucidating the mechanism of cognitive impairment and AD progression. 3xTg-AD mice have also been reported to exhibit progressive neuropsychiatric dysfunctions, as observed in AD patients. 3xTg-AD mice exhibit progressive behavioral deficits that correspond to cellular and intracellular brain abnormalities. Within 6 months, they exhibit reduced exploratory activity, and these abnormalities persist and deteriorate with age [40]. The same study demonstrated that these mice also had persistent impairment in novel object recognition starting at 4 months and that these changes were directly correlated with age-dependent elevation of amyloid monomers in the hippocampus as well as a reduction in hippocampal brain volume. Other studies have confirmed these findings and furthermore have reported additional abnormalities by 6 months, including retention deficits and long-term memory impairments [41,36], impairment in long-term potentiation and basal synaptic transmission disruption [36], signs of neophobia and anxiety-like behavior, and impairment in long-term spatial memory [42,13].
Between 6 and 12 months of age, these mice exhibited elevated episodes of slow-wave sleep [43], spatial learning and memory impairment in the Barnes Maze test [44], elevated levels of anxiety, decreased levels of exploratory behavior or neophobia and locomotion, and increased freezing behavior [45,37]. These behavioral aberrations are correlated with extensive tau pathology in different brain regions, including the hippocampus and cortex [35]. Between 12 and 18 months of age, amyloid plaque pathology can be found in almost all brain regions, including the extracellular posterior cortex [35], and mice at this stage exhibit severe neuropsychological symptoms, such as depressive behavior in forced swim and tail suspension tests [46] and signs of social disinhibition and withdrawal potentially associated with synaptic alteration [47]; these behaviors seem to be associated with progressive AD-related pathologies. Therefore, we hypothesized that 3xTg-AD mice would be a suitable animal model for treating BPSD-associated AD disorders.
The current study identified 3xTg–AD mice as a suitable animal model for studying the behavioral and psychological symptoms of dementia. Our study demonstrated and confirmed some of the previous findings that 3xTgAD mice at 12 months of age exhibit various neuropsychiatric and nonmotor deficits, such as anxiety, depression, social isolation, and social withdrawal. The force plate actometer test seems to be sensitive enough to successfully capture these neuropsychiatric changes in this model. The data acquisition was completely automated, and hence, human error and bias were removed from the data recording and analysis. 3xTg-AD mice exhibited differential power spectra in the 0–25 Hz frequency range compared to those of WT mice; moreover, 3xTg-AD mice exhibited significantly reduced power across the entire frequency range as well as within the specific frequencies of 0 to 4 Hz, 4–12 Hz, and 2–25 Hz. 3xTg-AD mice showed at least a 50% reduction in power in each of the frequency ranges, signifying the severity of neurological disorders and a reduction in mobility and overall activity, implying progressive functional decline, as has also been extensively reported [47,48,49,36,46]. AD patients and mice generally exhibit essential tremors, especially at low mobility, in the 4-12 Hz frequency range [23,32]. A decrease in power in those frequency ranges in 3xTg-AD mice directly corresponded with an increase in tremor in the 4-12 Hz range as well as an increase in stereotypy and spatial confinement. Studies that have used force plate actometers for behavioral tests have consistently shown that harmaline induces tremor in a mouse model of Parkinson disease at a frequency of 10-15 Hz with peak power in the 10-12 Hz range [50,51,52]. We observed that both our 3xTg-AD mice and WT mice exhibited peak power and peak tremor indices in the 6-8 Hz range, consistent with essential tremors. The focus stereotypy score can quantify patterns of spatial, temporal, and topographic domains of behavior [53]. The elevated Focus stereotypy and spatial confinement of 3xTg-AD mice imply that these mice are engaging in repetitive, purposeless behaviors such as head bobbing, licking, and grooming at an elevated rate with heightened freezing, implying anxiety-like behaviors, as reported elsewhere [45,37].
These mice also exhibited frequent occurrences of low mobility bouts, which are common in rodents with neuropsychiatric disorders. These bouts occurred more frequently in 3xTg-AD mice than in control mice starting at very early timepoints (within first 3 minutes compare to 9 minutes for WT). These kinds of frozen movements and their frequency could characterize motor disability but can also signify complex behavioral deficits such as anxiety, stress, or neurological disorders. Therefore, we further characterized these bouts to determine whether they were simply age-related motor issues or underlying causes of BPSD observed in AD patients. Further analysis demonstrated that there was no significant difference in movement ability (measured by locomotor distance traveled) during low-mobility bouts compared to that of WT individuals, suggesting an association between bout frequency and nonmotor behavioral disorders. Furthermore, during these bouts, we observed an elevated index of tremor that was apparent in the 0-25 Hz frequency range, suggesting the association of the frequency and duration of these bouts with freezing, anxiety, and possibly apathy. Interestingly, there was no difference in the stereotypy score between the WT and 3xTg-AD mice during these low mobility moments even though spatial confinement was significantly elevated. Further study is warranted to understand whether this particular difference can be attributed to frequency variations in LMB moments or whether the significant difference observed in focused stereotypy in the raw score was a result of the frequency of stereotypy and not the intensity of stereotypical behavior in each frame (10.24 s).
Conclusion
Taken together, the reductions in power, as a measure of general activity and speed of that activity, in 3xTg-AD mice with corresponding increases in frequency and duration of inactivity (low mobility bouts), combined with increased incidence of stereotypy, spatial confinement, and index of tremor, suggest that 3xTgAD mice aged 12 months exhibit neuropsychiatric symptoms similar to those observed in patients with AD dementia. These data support the use of 3xTg-AD as a model for investigating therapeutic interventions for BPSD associated with Alzheimer’s disease.
Conflict of Interest
The authors declare no competing interest.
Funding
This research was funded in parts by grants from Florida Department of Health, James and Esther King Biomedical Research Program of Florida (#6JK-08).
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- (2022) Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 18(4): 700-789.
- Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, et al. (2022) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7(2): e105-e125.
- Binnewijzend MAA, Benedictus MR, Kuijer JPA, Van Der Flier WM, Teunissen CE (2016) Cerebral perfusion in the predementia stages of Alzheimer’s disease. Eur Radiol 26(2): 506-514.
- Song IU, Kim JS, Kim YI, Eah KY, Lee KS (2007) Clinical significance of silent cerebral infarctions in patients with Alzheimer disease. Cogn Behav Neurol 20(2): 93-98.
- Lall R, Mohammed R, Ojha U (2019) What are the links between hypoxia and Alzheimer’s disease? Neuropsychiatric Disease and Treatment 15: 1343-1354.
- Cerejeira J, Lagarto L, Mukaetova-Ladinska EB (2012) Behavioral and Psychological Symptoms of Dementia. Front Neurol 3: 73.
- Kim B, Noh GO, Kim K (2021) Behavioural and psychological symptoms of dementia in patients with Alzheimer’s disease and family caregiver burden: A path analysis. BMC Geriatrics 21(1): 160.
- Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE (2013) The Association of Neuropsychiatric Symptoms in MCI with Incident Dementia and Alzheimer Disease. Am J Geriatr Psychiatry 21(7): 685-695.
- Kosel F, Pelley JMS, Franklin TB (2020) Behavioural and psychological symptoms of dementia in mouse models of Alzheimer’s disease-related pathology. Neurosci Biobehav Rev 112: 634-647.
- Fernández M, Gobartt AL, Balañá M (2010) Behavioural symptoms in patients with Alzheimer’s disease and their association with cognitive impairment. BMC Neurol 10(1): 87.
- Marin DB, Green CR, Schmeidler J, Harvey PD, Lawlor BA, et al. (1997) Noncognitive Disturbances in Alzheimer’s Disease: Frequency, Longitudinal Course, and Relationship to Cognitive Symptoms. J Am Geriatr Soc 45(11): 1331-1338.
- Giménez-Llort L, Rivera-Hernández G, Marin-Argany M, Sánchez-Quesada JL, Villegas S (2013) Early intervention in the 3xTg-AD mice with an amyloid β-antibody fragment ameliorates first hallmarks of Alzheimer disease. Mabs 5(5): 665-677.
- Roda AR, Esquerda-Canals G, Martí-Clúa J, Villegas S (2020) Cognitive Impairment in the 3xTg-AD Mouse Model of Alzheimer’s Disease is Affected by Aβ-ImmunoTherapy and Cognitive Stimulation. Pharmaceutics 12(10): 944.
- Torres-Lista V, López-Pousa S, Giménez-Llort L (2019) Impact of Chronic Risperidone Use on Behavior and Survival of 3xTg-AD Mice Model of Alzheimer’s Disease and Mice with Normal Aging. Front Pharmacol 10: 1061.
- Baeta-Corral R, Giménez-Llort L (2014) Bizarre behaviors and risk assessment in 3xTg-AD mice at early stages of the disease. Behav Brain Res 258: 97-105.
- Castillo-Mariqueo L, Giménez-Llort L (2020) Indexes for flotation and circling, two non-search behaviors in the water maze, sensitive to d-galactose–induced accelerated aging and Alzheimer’s disease. Behav Brain Res 377: 112229.
- Bareiss SK, Johnston T, Lu Q, Tran TD (2022) The effect of exercise on early sensorimotor performance alterations in the 3xTg-AD model of Alzheimer’s disease. Neurosci Res 178: 60-68.
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E (2001) A force-plate actometer for quantitating rodent behaviors: Illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods 107(1): 107-124.
- Zarcone TJ (2022) Neuroscience and Actometry: An example of the benefits of the precise measurement of behavior. Brain Res Bullet 185: 86-90.
- Pes R, Godar SC, Fox AT, Burgeno LM, Strathman HJ, et al. (2017) Pramipexole enhances disadvantageous decision-making: Lack of relation to changes in phasic dopamine release. Neuropharmacol 114: 77-87.
- Fowler SC, Miller BR, Gaither TW, Johnson MA, Rebec GV (2009) Force-plate quantification of progressive behavioral deficits in the R6/2 mouse model of Huntington’s disease. Behav Brain Res 202(1): 130-137.
- Louis ED, Ottman R, Hauser WA (1998) How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 13(1): 5-10.
- Benito-León J, Louis ED, Bermejo-Pareja F (2006) Elderly-onset essential tremor is associated with dementia. Neurology 66(10): 1500-1505.
- Thawani SP, Schupf N, Louis ED (2009) Essential tremor is associated with dementia. Neurology 73(8): 621-625.
- Duane DD, Vermilion KJ (2002) Cognitive deficits in patients with essential tremor. Neurology 58(11): 1706.
- Prasad S, Shah A, Bhalsing KS, Kumar KJ, Saini J, et al. (2019) Abnormal hippocampal subfields are associated with cognitive impairment in Essential Tremor. J Neural Transm 126(5): 597-606.
- Gnanalingham KK, Byrne EJ, Thornton A, Sambrook MA, Bannister P (1997) Motor and cognitive function in Lewy body dementia: Comparison with Alzheimer’s and Parkinson’s diseases. J Neurol Neurosurgery Psychiatry 62(3): 243-252.
- LaRoia H, Louis ED (2011) Association between Essential Tremor and Other Neurodegenerative Diseases: What Is the Epidemiological Evidence? Neuroepidemiol 37(1): 1-10.
- Tsolaki M, Kokarida K, Iakovidou V, Stilopoulos E, Meimaris J, et al. (2001) Extrapyramidal symptoms and signs in Alzheimer’s disease: Prevalence and correlation with the first symptom. Am J Alzheimer’s Dis Other Demen 16(5): 268-278.
- Godar SC, Mosher LJ, Strathman HJ, Gochi AM, Jones CM, et al. (2016) The D1CT‐7 mouse model of Tourette syndrome displays sensorimotor gating deficits in response to spatial confinement. Br J Pharmacol 173(13): 2111-2121.
- Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC (2003) Aged Fischer 344 rats exhibit altered orolingual motor function: Relationships with nigrostriatal neurochemical measures. Neurobiol Aging 24(2): 259-266.
- Pan MK, Ni CL, Wu YC, Li YS, Kuo SH (2018) Animal Models of Tremor: Relevance to Human Tremor Disorders. Tremor Other Hyperkinet Mov 8: 587.
- Sharma S, Pandey S (2016) Approach to a tremor patient. Ann Indian Acad Neurol 19(4): 433-443.
- Ambrée O, Touma C, Görtz N, Keyvani K, Paulus W, et al. (2006) Activity changes and marked stereotypic behavior precede Abeta pathology in TgCRND8 Alzheimer mice. Neurobiol Aging 27(7): 955-964.
- Oddo S (2003) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24(8): 1063-1070.
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, et al. (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 39(3): 409-421.
- Sterniczuk R, Antle MC, LaFerla FM, Dyck RH (2010) Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: Part 2. Behavioral and cognitive changes. Brain Res 1348: 149-155.
- Belfiore R, Rodin A, Ferreira E, Velazquez R, Branca C, et al. (2019) Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 18(1): e12873.
- Clemons GA, Silva AC, Acosta CH, Udo MSB, Tesic V, et al. (2022) Protein arginine methyltransferase 4 modulates nitric oxide synthase uncoupling and cerebral blood flow in Alzheimer’s disease. J Cell Physiol 10.1002.
- Chiquita S, Ribeiro M, Castelhano J, Oliveira F, Sereno J, et al. (2019) A longitudinal multimodal in vivo molecular imaging study of the 3xTg-AD mouse model shows progressive early hippocampal and taurine loss. Hum Mol Genet 28(13): 2174-2188.
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM (2005) Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 45(5): 675-688.
- Pietropaolo S, Feldon J, Yee BK (2008) Age-dependent phenotypic characteristics of a triple transgenic mouse model of Alzheimer disease. Behav Neurosci 122(4): 733-747.
- Benthem SD, Skelin I, Moseley SC, Stimmell AC, Dixon JR, et al. (2020) Impaired Hippocampal-cortical interactions during sleep in a mouse model of Alzheimer’s disease. Curr Biol 30(13): 2588-2601.
- Stover KR, Campbell MA, Van Winssen CM, Brown RE (2015) Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav Brain Res 289: 29-38.
- Giménez-Llort L, Blázquez G, Cañete T, Johansson B, Oddo S, et al. (2007) Modeling behavioral and neuronal symptoms of Alzheimer’s disease in mice: A role for intraneuronal amyloid. Neurosci Biobehav Rev 31(1): 125-147.
- Romano A, Pace L, Tempesta B, Lavecchia AM, Macheda T, et al. (2015) Depressive-Like Behavior Is Paired to Monoaminergic Alteration in a Murine Model of Alzheimer’s Disease. Int J Neuropsychopharmacol 18(4): pyu020.
- Bories C, Guitton MJ, Julien C, Tremblay C, Vandal M, et al. (2012) Sex-Dependent Alterations in Social Behaviour and Cortical Synaptic Activity Coincide at Different Ages in a Model of Alzheimer’s Disease. Plos One 7(9): e46111.
- Castillo-Mariqueo L, Giménez-Llort L (2022) Impact of Behavioral Assessment and Re-Test as Functional Trainings That Modify Survival, Anxiety and Functional Profile (Physical Endurance and Motor Learning) of Old Male and Female 3xTg-AD Mice and NTg Mice with Normal Aging. Biomedicines 10(5): 973.
- Filali M, Lalonde R, Theriault, P, Julien C, Calon F, et al. (2012) Cognitive and non-cognitive behaviors in the triple transgenic mouse model of Alzheimer’s disease expressing mutated APP, PS1, and Mapt (3xTg-AD). Behav Brain Res 234(2): 334-342.
- Iseri PK, Karson A, Gullu KM, Akman O, Kokturk S, et al. (2011) The effect of memantine in harmaline-induced tremor and neurodegeneration. Neuropharmacology 61(4): 715-723.
- Kosmowska B, Wardas J, Głowacka U, Ananthan S, Ossowska K (2015) Pramipexole at a Low Dose Induces Beneficial Effect in the Harmaline‐induced Model of Essential Tremor in Rats. CNS Neurosci Ther 22(1): 53-62.
- Ossowska K, Głowacka U, Kosmowska B, Wardas J (2015) Apomorphine enhances harmaline-induced tremor in rats. Pharmacol Repor 67(3): 435-441.
- Fowler SC, Birkestrand B, Chen R, Vorontsova E, Zarcone T (2003) Behavioral sensitization to amphetamine in rats: Changes in the rhythm of head movements during focused stereotypies. Psychopharmacology 170(2): 167-177.






























