Effects of Levetiracetam on Childhood-onset Refractory Epilepsy Classified by Level of Intellectual Disability
Tomohiro Nakayama1-3*, Saki Saeki1, Naoko Nakayama3, Haruka Ooguro1, Junko Nakayama1, Hiroshi Maruyama3 and Nobuaki Iwasaki1,2
11Department of Pediatrics, Ibaraki Prefectural University of Health Sciences Hospital, 4733 Ami, Ami-cho, Inashiki-gun, Ibaraki, Japan 300-0331
2Center of Medical science, Ibaraki Prefectural University of Health Sciences, 4669-2 Ami, Ami-cho, Inashiki-gun, Ibaraki, Japan 300-0394
33Department of Pediatrics, Matsudo clinic, 1-3, Nakai-cho, Matsudo, Chiba 270-2242
Submission: August 10, 2023; Published: August 23, 2023
*Corresponding author: Tomohiro Nakayama, Center of Medical science, Ibaraki Prefectural University of Health Sciences, 4669-2 Ami, Ami-cho, Inashiki-gun, Ibaraki, Japan 300-0394
How to cite this article: Tomohiro Nakayama*, Saki Saeki, Naoko Nakayama, Haruka Ooguro, Junko Nakayama, Hiroshi Maruyama and Nobuaki Iwasaki. Effects of Levetiracetam on Childhood-onset Refractory Epilepsy Classified by Level of Intellectual Disability. Open Access J Neurol Neurosurg 2023; 18(3): 555988. DOI: 10.19080/OAJNN.2023.18.555988.
Abstract
The efficacy of levetiracetam (LEV) for childhood-onset refractory epilepsy was retrospectively evaluated from medical records, based on the level of intellectual disability. The study sample included 84 patients (68 adults, 16 children) with childhood- onset refractory epilepsy who had been treated with LEV (dose: adult, 1000 mg/day; children, 30 mg/kg/day) for approximately 1.7 years. The responder rates (≥ 50% and ≥ 75% reduction in seizure frequency from baseline) were 85.7% and 42.9% for focal seizures in adults with normal intelligence; the responder rates for focal seizures in adults with intellectual disabilities were average 69.0% and 6.9%, respectively. Serious adverse events were not observed. A high level (≧75%) of reduction can be expected for focal seizures in adults with normal intelligence but cannot be expected in adults with intellectual disabilities. Further studies are required to investigate these.
Keywords: Childhood-onset; Refractory epilepsy; Levetiracetam; Intellectual disability
Abbreviations: LEV: Levetiracetam; LTG: Lamotrigine; TPM: Topiramate; GMFCS: Gross Motor Function Classification System
Introduction
Refractory epilepsy is a condition in which seizures do not stop despite treatment with two or three more appropriate antiepileptic drugs for at least two years, with the patient’s daily life interrupted, accounting for 20-30% of all patients with epilepsy [1]. Recently, new antiepileptic drugs, such as gabapentin (July 2006), topiramate (TPM) (August 2007), lamotrigine (LTG) (December 2008), and levetiracetam (LEV) (September 2010) have been approved in Japan. These drugs are expected to be superior to existing drugs in terms of ease of use due to fewer drug interactions [2] and are increasingly used as additional treatments for refractory epilepsy.
LEV has been reported to be highly effective in the treatment of refractory epilepsy, with seizure reduction rates of 50% or greater ranging between 25-74% [3-7] and complete seizure suppression rates of 6–38% [3-5,8]. In our previous study of 162 patients with childhood-onset refractory epilepsy at our institution, the rates of seizure reduction of ≥ 50% were 51.1% in adults and 44.0% in children, and the rate of complete seizure suppression was 0.0% in both groups. Although the maintenance dose may not be sufficient, the seizure reduction rate of 50% or more was as high as previously reported; however, the complete seizure control rate was lower than that reported in previous studies.
Many patients with intellectual and physical disabilities attend our institution. On this premise, we hypothesized that the severity of intellectual and physical impairment contributes to the lower efficacy of LEV. Therefore, we planned to study the efficacy of LEV in the treatment of childhood-onset refractory epilepsy by first selecting a group of patients who received a relatively high dose of LEV and secondarily based on the level of intellectual and physical impairment.
Subjects and Methods
From September 2010 to August 2013, 187 patients (100 males and 87 females) were treated with LEV at the Department of Pediatrics, Ibaraki Prefectural University of Health Sciences Hospital, and Matsudo Clinic. The subjects were patients with childhood-onset refractory epilepsy who were eligible for followup.
Before its approval for use in children in Japan, we explained to the patients and their parents that LEV had already been approved for use in children overseas who have partial seizures or generalized seizures. LEV was used in children in Japan after gaining approval in May 2013. Among these patients, those who were 16 years of age or older at the start of LEV prescription were classified as adults and those younger than 16 years of age were classified as children.
Sixty-eight adults (34 males and 34 females) and 16 children (8 males and 8 females) were selected from the adult and pediatric groups, respectively, who received LEV of 1,000 mg/ day or more in the adult group and 30 mg/kg/day or more per body weight in the pediatric group. The patient background included the number of antiepileptic drugs, LEV initial dose, LEV maintenance dose, and LEV maintenance dose per body weight. The percentage of seizure frequency reduction (≥50% and ≥75%) from baseline was retrospectively classified into focal and generalized seizures based on the 2010 proposed revision of the International Classification of Epileptic Seizure Types. Before introducing LEV, baseline seizure frequency was calculated from the 6 month average seizure frequency. Next, we examined the rate of seizure frequency reduction using intelligence quotient classification (based on the Tanaka-Binet test), gross motor function classification system (GMFCS) level, and motor paralysis site. The changes in seizure frequency were evaluated at the following five levels: 1. 100% seizure frequency reduction; 2.75- 99% seizure frequency reduction; 3. 50-74% seizure frequency reduction; 4. 25-49% seizure frequency reduction; and 5.0-24% seizure frequency reduction.
This study was approved by the Ethics Committee of Ibaraki Prefectural University of Health Sciences. The cases at the Matsudo Clinic were reviewed by the university on behalf of the authors. Approval for off-label use was obtained from the Ethics Committee of the Ibaraki Prefectural University of Health Sciences.
Results
Patient background
The starting age of LEV prescription was 33.9±10.3 years old in adults and 9.3±3.8 years old in children. The number of antiepileptic drugs used was 4.6±1.2 in adults and 4.3±0.8 in children. The mean duration of treatment was 20.5±9.1 months in adults and 20.3±13.0 months in children. The initial dose of LEV was 430± 220 mg/day in adults and 270± 170 mg/day, 11.0± 6.9 mg/kg/day in children, and the maintenance doses were 1500± 570 mg/day in adults and 1250± 530 mg/day in children. The maintenance dose per body weight was 27±13 mg/kg/day in adults and 51±14 mg/kg/day in children.
Efficacy of LEV
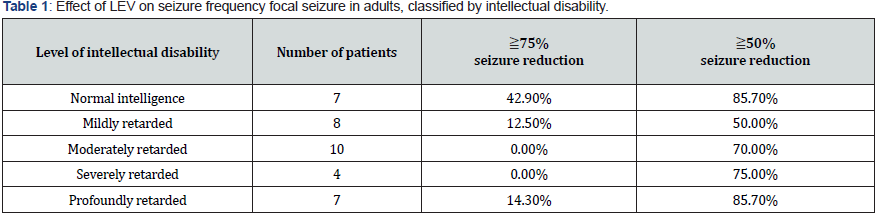
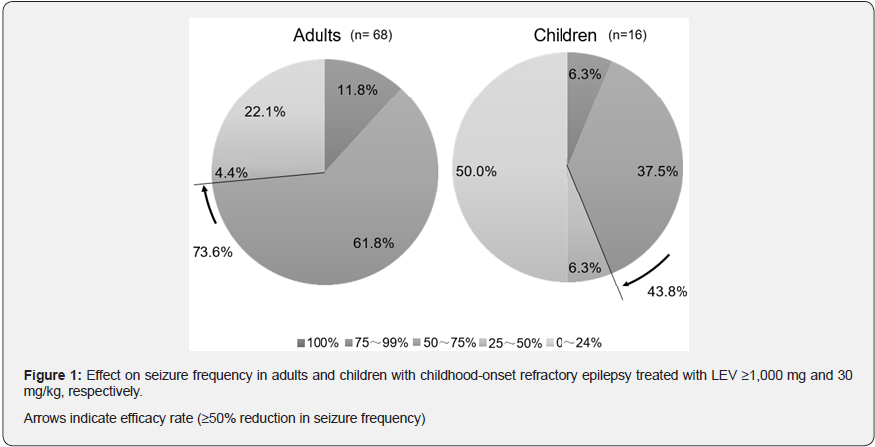
The 50% or greater seizure frequency reduction rates were 73.5% and 43.8% in adults and children, respectively, and the 75% or greater reduction rates were 11.8% and 6.3% in adults and children, respectively (Figure 1). The seizure frequency reduction rates classified according to intellectual disability in adults are shown in Table 1. In adults with normal intelligence, the rates of ≥50% and ≥75% reduction in focal seizures were 85.7% and 42.9%, respectively. The rate of ≥75% seizure reduction was higher than that in the other groups. In adults with intellectual disabilities, ≥50% and ≥75% seizure reduction in focal seizures were average 69.0%, ranging from 50.0% to 85.7%, and average 6.9%, ranging from 0% to 14.3%, respectively. Statistical analysis was not performed due to the insufficient number of the patients. The rates of ≥50% and ≥75% seizure reduction for generalized seizures in adults and for both focal and generalized seizures in children varied, and there was no clear trend in the rates in each group (data not shown). The seizure frequency reduction rates classified by the GMFCS level and motor paralysis (no paralysis, hemiplegia, paraplegia, and tetraplegia) were not significantly different between the groups (data not shown).
Safety
Adverse effects included somnolence, drowsiness, irritability, headache, insomnia, decreased vitality, itchy skin, and wandering in adults, and increased arousal and somnolence in children. No significant or new drug reactions were observed.
Discussion
LEV is assumed to exert anticonvulsant effects by acting on synaptic vesicle protein 2A (SV2A) in neurons. It is classified as a second-generation antiepileptic drug and its mechanism of action is completely different from that of conventional antiepileptic drugs. It does not mediate P450 hepatic cytochrome metabolism and does not interact with other drugs [9]. It is expected to be effective for myoclonus epilepsy because its structure is similar to that of piracetam, a drug used to treat myoclonus [10]; however, it was found to be effective as a treatment for partial seizures in children and adults worldwide. In Japan, the drug was approved for the treatment of partial seizures in children in May 2013, and monotherapy became available in February 2015. It is now widely used clinically. The seizure reduction rate of 50% or more for refractory epilepsy is 25-74% [4- 7], and the complete seizure suppression rate is as high as 6-38% [4-8]. The efficacy of LEV for myoclonic seizures in Lennox-Gastaut syndrome [11], Dravet syndrome [12], and epileptic encephalopathy with continuous spike-and-wave during sleep wave [13,14] has been demonstrated.
On the other hand, a multicenter network study of antiepileptic medicine reported that LEV is the most commonly used antiepileptic drug but is also the most frequently discontinued drug [15], suggesting that there may be many cases in which the efficacy is not as good as expected.
In this study, we investigated the efficacy of LEV on seizure frequency in a relatively high-dose group. Although the rate of seizure frequency reduction of 50% or more was as high as that previously reported, the rate of complete seizure suppression was 0%, and the rate of seizure frequency reduction of 75% or more was relatively low, ranging from 6.3 to 11.8%. In addition, when the results were re-evaluated by intellectual disability, the rate of 50% or more seizure reduction rate in adults with focal seizures in both the normal and retarded intelligence groups was as high as previously reported, but the rate of 75% or more seizure reduction rate was high only in the normal intelligence group. Opp et al. [6] reported that intellectual disability was associated with limited efficacy and discontinuation of LEV, which is consistent with the results of the present study.
In the present study, we also followed the patients for a long period, averaging 1.7 years (20 months). Although the initial effect was significant in the early stage, the seizure suppression rate decreased from 50% to 19% after 1 year [16], and the initial effect may have diminished.
Conclusion
In conclusion, LEV can be expected to be effective for the treatment of focal refractory childhood-onset epilepsy with 75% or greater seizure reduction in the mentally normal adult group. However, maintaining 75% or greater seizure control for a long time in many patients with intellectual disabilities is difficult, even if LEV is initially effective. Further investigation is needed to determine the efficacy of LEV classified by level of intellectual disabilities.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None of the authors has any conflict of interest to disclose.
Acknowledgments
We would like to thank the patients and their parents. We would like to thank Editage (www.editage.com) for English language editing.
References
- Kwan P, SC Schachter, MJ Brodie (2011) Drug-resistant epilepsy. N Engl J Med 365(10): 919-926.
- Kanner AM, Ashman E, Gloss D, Harden C, Bourgeois B, et al. (2018) Practice guideline update summary: Efficacy and tolerability of the new antiepileptic drugs II: Treatment-resistant epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 91(2): 82-90.
- Shorvon SD, Löwenthal A, Janz D, Bielen E, Loiseau P (2000) Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia 41(9): 1179-1186.
- Peake D, Mordekar S, Gosalakkal J, Mukhtyar B, Buch S, et al. (2007) Retention rate of levetiracetam in children with intractable epilepsy at 1 year. Seizure 16(2): 185-189.
- Giroux PC, Salas-Prato M, Theoret Y, Carmant L (2009) Levetiracetam in children with refractory epilepsy: lack of correlation between plasma concentration and efficacy. Seizure 18(8): 559-563.
- Opp J, Tuxhorn I, May T, Kluger G, Wiemer-Kruel A, et al. (2005) Levetiracetam in children with refractory epilepsy: a multicenter open label study in Germany. Seizure 14(7): 476-484.
- von Stuelpnagel C, Holthausen H, Kluger G (2007) Long-term use of Levetiracetam in patients with severe childhood-onset epilepsy. Eur J Paediatr Neurol 11(6): 341-345.
- Kelly K, Stephen LJ, Brodie MJ (2004) Levetiracetam for people with mental retardation and refractory epilepsy. Epilepsy Behav 5(6): 878-883.
- Carreno M (2007) Levetiracetam. Drugs Today (Barc) 43(11): 769-794.
- Genton P, Van Vleymen B (2000) Piracetam and levetiracetam: close structural similarities but different pharmacological and clinical profiles. Epileptic Disord 2(2): 99-105.
- De Los Reyes EC, Sharp GB, Williams JP, Hale SE (2004) Levetiracetam in the treatment of Lennox-Gastaut syndrome. Pediatr Neurol 30(4): 254-256.
- Chiron C, Dulac O (2011) The pharmacologic treatment of Dravet syndrome. Epilepsia 52 Suppl 2: 72-75.
- Capovilla G, Beccaria F, Cagdas S, Montagnini A, Segala R, et al. (2004) Efficacy of levetiracetam in pharmacoresistant continuous spikes and waves during slow sleep. Acta Neurol Scand 110(3): 144-147.
- Aeby A, Poznanski N, Verheulpen D, Wetzburger C, Van Bogaert P (2005) Levetiracetam efficacy in epileptic syndromes with continuous spikes and waves during slow sleep: experience in 12 cases. Epilepsia 46(12): 1937-1942.
- Ibrahim GM, Rutka JT, Snead 3rd OC (2013) Network analysis reveals patterns of antiepileptic drug use in children with medically intractable epilepsy. Epilepsy Behav 28(1): 22-25.
- Grosso S, Cordelli DM, Franzoni E, Coppola G, Capovilla G, et al. (2007) Efficacy and safety of levetiracetam in infants and young children with refractory epilepsy. Seizure 16(4): 345-350.






























