Unlocking the Clinical Manifestations and Classifications of Locked-In Syndrome: A Comprehensive Review
Maria Alejandra Nieto-Salazar1, Sharima del Milagro Kanahan Osman2, Hritvik Jain3, Ronald Mauricio Blanco Montecino4, Jhon Navarro Gonzalez5, María Isabel Murillo Pineda6, Felix Ricardo Bonilla Bonilla4, Walter Manuel Robles Negrillo7, Peggie Crisalida Mendoza Robles8, Rossy I Valecillos Paez9, Kevin Josue Acevedo Gomez4, Mayra Rebeca Dominguez de Ramirez4 and Alejandra María Aleman6
1Juan N. Corpas University, Colombia
2Universidad de Carabobo, Venezuela
3All India Institute of Medical Sciences (AIIMS), India
4Universidad de El Salvador, El Salvador
5Universidad del Zulia, Venezuela
6Universidad Católica de Honduras, Honduras
7Universidad San Juan Bautista, Perú
8Universidad San Martin de Porres, Perú
9Universidad Central de Venezuela, Venezuela
Submission: July 26, 2023; Published: August 04, 2023
*Corresponding author: Maria Alejandra Nieto-Salazar, Larkin Community Hospital, 100 Parrott Drive, Shelton, CT 06484, USA
How to cite this article: Maria Alejandra N-S, Sharima d M K O, Hritvik J, Ronald M B M, Jhon Navarro G, et al. Unlocking the Clinical Manifestations and Classifications of Locked-In Syndrome: A Comprehensive Review. Open Access J Neurol Neurosurg 2023; 18(3): 555987. DOI: 10.19080/OAJNN.2023.18.555987.
Abstract
Locked-in syndrome is a rare neurological condition characterized by complete paralysis of all voluntary muscles except for the muscles controlling eye movement. It is caused by damage to the ventral part of the brainstem, often resulting from vascular events. The syndrome is devastating, as patients are fully conscious but unable to communicate verbally or move any body part except for vertical eye movements and blinking. LIS can be classified into subtypes based on the extent of motor involvement, including classic, incomplete, total, and total with anarthria. Diagnosis is challenging but relies on clinical presentation and neuroimaging studies. Differential diagnosis is essential to distinguish LIS from similar conditions with altered consciousness. Prognosis varies depending on the underlying cause, with younger age associated with better outcomes at the onset. Rehabilitation can improve motor function and communication capabilities, leading to a higher quality of life for LIS patients. Early recognition and accurate diagnosis are crucial for implementing appropriate management strategies and improving patient outcomes. Further research is needed to explore therapeutic interventions for enhancing recovery and functional independence in LIS patients.
Keywords: Locked-in syndrome; Neurological condition; Complete paralysis; Vascular events; Stroke
Abbreviations: LIS: Locked-in Syndrome; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; FMRI: Functional Magnetic Resonance Imaging; PET: Positron Emission Tomography; EEG: Electroencephalogram; EMG: Electromyography; CSF: Cerebrospinal Fluid; MS: Multiple Sclerosis
Introduction
Gliomas compose the most common group of intrinsic tumours of the central nervous system (CNS) and can be divided into diffuse gliomas and “non-diffuse” gliomas, which demonstrate a more circumscribed pattern of growth. In 2016 published revised fourth edition of the WHO Classification of CNS tumours, several new pathohistological entities have been introduced in the group of “non-diffuse” gliomas and neuronal-glial tumours, such as anaplastic pleomorphic xanthoastrocytoma, RELA fusion-positive ependymoma and diffuse leptomeningeal glioneuronal tumour (DLGT) [1], which represents a rare type of tumour with sparse cases and an ambiguous prognosis [2,3]. DLGT has previously been diagnosed as disseminated oligodendroglial-like leptomeningeal tumour, dysembryoplastic neuroepithelial tumour-like neoplasm, meningeal gliomatosis, diffuse leptomeningeal neurocytoma, diffuse leptomeningeal gangliocytoma, and diffuse leptomeningeal oligodendrogliomatosis [4] and is now assigned to the category of neuronal and mixed neuronal-glial tumours [5]. DLGT is often diagnosed in children, more often boys, and has an accelerating course with a few cases occurring in adulthood [4,6]. Due to its infrequency and minor studies, different views of clinical, pathohistological and neuroradiological characteristics persist; DLGT has not yet been assigned a WHO grade [3,6]. DLGT can mimic a chronic infection, such as meningitis [7], or leptomeningeal carcinomatosis, which complicates the diagnosis [4,8]. The clinical presentation of DLGT depends on the area involved and it can manifest as paraesthesia, seizures or symptoms of hydrocephalus, such as headache and vomiting [8]. A combination of the fusion of KIAA1549 and the serine/threonine protein kinase B-raf (BRAF) and deletion of the short arm of chromosome 1 (1p) or/and the long arm of chromosome 19 (19q) is present in molecular genetic studies [6]. The most often discovered imaging features are diffuse abnormal nodular leptomeningeal growth with no evidence of a primary intraparenchymal focus, which can be seen in the basal cisterns, posterior fossa, Sylvian fissures, brainstem and the spinal cord, often associated with cystic T2 hyperintense lesions on MRI [4,8].
Pathohistological features of DLGT show a low mitotic index with a low to moderate density and cellular features that are similar to those of oligodendrogliomas [5,8], however, multiple patients often have a non-diagnostic initial biopsy due to limited tumour material [8]. Opposite to oligodendrogliomas, DLGT is negative for the presence of IDH 1 and 2 mutations [5]. DLGT demonstrates histological and immunohistochemical features of glial and neuronal differentiation with an expression of synaptophysin, OLIG2 and S-100 [9]. In the majority of cases, cytology of the cerebrospinal fluid (CSF) is negative for tumour cells, despite the extensive leptomeningeal involvement [8]; it involves mild hyperproteinaemia and lymphocyte pleocytosis [10]. Patients with a poor prognosis are often older at the time of diagnosis and have multiple site lesions and hydrocephalus, whereas those with a stable disease have a higher rate of 1p/19q codeletion [5]. Currently, no therapeutic guidelines exist, patients are treated by use of various chemotherapy regimens with or without radiation therapy and biological agents, namely carboplatin, bevacizumab, and temozolomide [9,11]. Craniospinal radiation has been proven to improve clinical outcomes and slow disease progression [11].
Introduction
Locked-in syndrome is a rare and devastating neurological condition characterized by complete paralysis of all voluntary muscles except for the muscles controlling eye movement. It occurs due to damage to the ventral part of the pons, usually caused by vascular events such as stroke, brainstem hemorrhage, or brainstem tumors. This leads to the disruption of descending motor tracts, resulting in the inability to move any part of the body except for vertical eye movements and, in some cases, blinking [1]. The physiopathology of locked-in syndrome involves lesions in the ventral part of the pons, explicitly affecting the corticospinal tracts and corticobulbar tracts. These lesions lead to a disconnection between the brainstem and the spinal cord, resulting in the loss of voluntary motor control. However, the cranial nerves responsible for eye movement, including the oculomotor, trochlear, and abducens nerves, remain intact, allowing communication through vertical eye movements [1,2].
The epidemiology of this syndrome is challenging to determine accurately due to its rarity. The prevalence and incidence are low, and its occurrence varies across different populations [3]. Risk factors for developing locked-in syndrome are primarily related to the underlying causes, such as vascular events (e.g., ischemic stroke, brainstem hemorrhage) or brainstem tumors. The clinical presentation is characterized by complete paralysis of voluntary muscles, sparing only vertical eye movements and blinking. Patients are conscious and aware of their surroundings but cannot communicate verbally or move any body part. Diagnosis is primarily based on clinical presentation and neuroimaging studies, which reveal brainstem lesions in the ventral pons [1,4]. Locked-in syndrome can be classified into different categories based on the extent of motor involvement. Complete locked-in syndrome refers to patients with no residual voluntary motor function. In contrast, incomplete locked-in syndrome refers to cases with some preserved voluntary motor function, such as limited facial movements or control over vertical eye movements [2,3,5].
Finally, treatment primarily focuses on supportive care and communication strategies, such as using eye-tracking devices or specialized computer interfaces to enable communication. Physical and occupational therapy may also be provided to maintain muscle tone and prevent complications of immobility [1,5]. The prognosis for locked-in syndrome varies depending on the underlying cause and extent of motor involvement, but in many cases, the condition remains stable or slowly progresses over time [2]. This narrative review aims to provide an overview of locked-in syndrome, focusing on its clinical presentation and classification. By understanding this syndrome's unique features and subtypes, clinicians can improve early identification and implement appropriate management strategies to enhance the quality of life for affected individuals.
Epidemiology & Physiopathology
Since locked-in syndrome (LIS) is an uncommon disorder, there is no exact estimation of its incidence or prevalence [6]. Some literature has documented that LIS syndrome has an average onset age that generally ranges from 30 to 50 years. The condition is slightly predominant in males [7]. Many patients with vascular etiology LIS had comorbid conditions such as hypertension, major artery atherosclerosis, and diabetes [8]. In classical LIS, it has as characteristic the loss of movement in the four extremities and inertia. In partial LIS, the patient still has some motor function. The complete LIS is the one that has the most severe manifestations because, in addition to the quadriplegia, they cannot blink or have a vertical look; this prevents communication [9].
The pathophysiology of locked-in syndrome is explained by damage to the corticosteroid and corticobulbar tracts, which run parallel to each other through the cerebral peduncles of the midbrain and are located in the ventral part of the protuberance and the spinal bulb [6]. The lattice activation system, located dorsally, is responsible for wakefulness and is not damaged by such ventral lesions that cause clogging syndrome [6,10]. The corticosteroid tract is crucial for voluntary movements of the body and limbs. At the same time, cortical-ulnar fibers are responsible for voluntary non-oculomotor movements of muscles innervated by the caudal cranial nerves [6].
Patients have tetraplegia due to damage to the corticosteroid tracts. The vertical look is not affected (classic and partial) due to the nucleus's location in the mesencephalon's rostral portion [11]. Medial and lateral gaze paralysis are common. They may also have diplopia and blurred vision. Patients with LIS appear to be unaware of their environment. However, functional MRI tends to show regular activity. Anartria is caused by paralysis of the facial-glossium-pharyngeal-laryngeal muscles and damage to corticobulbar fibers [12]. Lastly, breathing is often affected in these patients when the lateral segment is involved. The sensory system is preserved chiefly due to the lateral and posterior location of the sensory pathways [13].
Clinical Presentation
Locked-in syndrome is a devastating condition with a unique and distinct clinical presentation. Patients are fully conscious and aware of their surroundings. However, they experience profound paralysis of all voluntary muscles except for the muscles controlling vertical eye movements and blinking. The syndrome is often caused by damage to the ventral part of the pons, disrupting the descending motor tracts that carry signals from the brain to the spinal cord and peripheral muscles [14]. This disruption results in the complete loss of voluntary motor control throughout the body, rendering the individual unable to move any body part, including limbs and facial muscles. Despite the severe paralysis, patients retain full cognitive function and intact cranial nerve function responsible for eye movements and blinking. This allows them to establish communication through vertical eye movements and, in some cases, blinking in response to specific questions or commands. For instance, patients can answer "yes" or "no" questions by looking up or down, respectively, or blinking in response to simple commands [10,14].
Locked-in patients typically present with quadriplegia, paralysis affecting all four limbs, and complete facial immobility, often referred to as "facial immobility with preserved vertical eye movements." Preserving vertical eye movements and blinking becomes a crucial channel for communication with the outside world. Patients can use eye movements to direct their gaze toward objects or individuals and may employ blinking patterns to convey simple messages [15]. Given their immobility and the inability to speak or produce sounds, patients with locked-in syndrome may experience immense frustration, depression, and anxiety due to communication challenges and the reliance on others for primary care needs. Family members and caregivers play a crucial role in interpreting the patient's eye movements and understanding their needs and preferences [16,17].
The clinical presentation of locked-in syndrome is strikingly different from conditions that cause similar degrees of paralysis, such as a coma or vegetative state, where patients lack consciousness and responsiveness to external stimuli. In contrast, locked-in patients remain fully conscious. They can engage in cognitive activities, feel pain, and emotionally respond to their environment, but they cannot express themselves verbally or physically [18].
It is crucial to be vigilant in identifying locked-in syndrome and recognizing the preserved cognitive function, as this may prevent misdiagnosis or misunderstanding of the patient's condition. Early and accurate diagnosis can lead to appropriate communication strategies, supportive care, and rehabilitation interventions that improve the quality of life for patients with locked-in syndrome and facilitate their interactions with the world [14,18].
Subtypes & Classification
Locked-in syndrome can be classified into subtypes based on the extent of preserved motor function and the level of paralysis experienced by the individual. The subtypes of locked-in syndrome include classic, incomplete, total, and total with anarthria. Differentiating between the subtypes of locked-in syndrome is essential for providing appropriate care and communication strategies [19].
Classic Locked-in Syndrome: in this subtype, the patient experiences near-complete paralysis of all voluntary muscles except for vertical eye movements and blinking. They are fully conscious and aware of their surroundings but cannot move any body part or speak. Communication is primarily achieved through vertical eye movements or blinking in response to questions or commands. Classic locked-in syndrome is typically caused by damage to the ventral part of the pons, affecting the descending motor tracts responsible for voluntary movements [19,20].
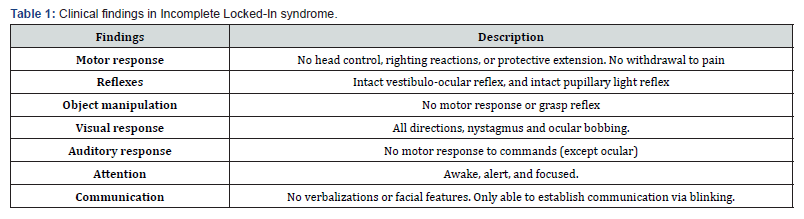
Incomplete Locked-in Syndrome: in this subtype, there is some limited motor function beyond eye movements and blinking. Patients may have partial control over specific muscles, such as the head or facial muscles, and vertical eye movements. This subtype can result from less severe brain stem damage or partial sparing of the motor tracts. Communication capabilities may vary depending on the degree of preserved motor function [21]. The most common examination findings for this LIS subtype are summarized in Table 1.
Total Locked-in Syndrome: this is the most severe form of locked-in syndrome, where the individual experiences complete paralysis of all voluntary muscles, including the eye muscles. In this state, patients cannot move their eyes vertically or horizontally, and they cannot blink. Despite the profound paralysis, these patients maintain full consciousness and cognitive function, making the diagnosis challenging without advanced communication technology [21-23].
Total Locked-in Syndrome with Anarthria: it is a subtype where the individual can move their eyes vertically but cannot speak due to the loss of voluntary control over the vocal muscles. These patients can use vertical eye movements and assistive communication devices to convert eye movements into speech [20,23].
Assessment & Diagnosis
Diagnosing locked-in syndrome can often be difficult since the condition shares many features with other mute conditions. Medical states such as coma, persistent vegetative state, akinetic mutism, and catatonic states are the main confounding conditions [24]. Recent literature supports a need for standardized diagnostic procedures to confirm Locked-in syndrome (LIS). Unfortunately, this often relies on imaging which may not show pathological findings even if other LIS symptoms are present. So, a diagnosis of LIS is not usually made until approximately two months after onset. Clinical observation, CT of the brain, MRI, fMRI, PET, electroencephalogram (EEG), electromyography (EMG), and angiography are obligatory tests for accurate diagnosis [25]. These tests can also rule out damage elsewhere in the brain [26]. The diagnosis can be missed if unresponsive patients' voluntary vertical eye movement is not assessed [27].
The physical and neurologic examination of cranial nerves can show impairment of horizontal eye movement and sparing of vertical eye movements. There is also the involvement of cranial nerves 5, 7, 9, 10, 11, and 12, which presents as a bilateral sensory deficit to the face, bilateral peripheral facial palsy, absent gag reflex, weak tongue movement, and neck weakness. Hearing is often preserved in LIS; however, patients often do not have the means to communicate due to paralysis. In this case, hearing and the integrity of the cranial nerve 8 may be tested by a patient's response to verbal communication with vertical eye motions in the form of "yes and no" responses. Both the pupillary reflex and the vestibulocochlear reflex may be preserved in LIS. Testing of motor strength, sensory deficits, and reflexes should all be done to localize the lesion's site and monitor for progress or improvement. The early recognition of the symptoms of LIS may mitigate the lag between injury onset and the time of formal diagnosis. Serial monitoring of patients should be standard, assessment of alertness by eye movement, spontaneous or voluntary limb movements, withdrawal to noxious stimuli, and reflexes. Examining the reflexes can point to an upper motor neuron involvement, like in hyperreflexia, or lower motor neuron involvement in hyporeflexia or areflexia. A positive Babinski reflex indicates corticospinal damage, usually located in ventral structures of the brainstem.
A metabolic panel should be ordered to evaluate any aberration in the sodium level, which may indicate a potential pontine myelinolysis. A history of rapid sodium correction may point to the cause of demyelination. Hyponatremia, hypophosphatemia, and hypomagnesemia may cause comas similar to a locked-in state, so such electrolytes should be closely examined. Glucose should be monitored to rule out a hypoglycemic coma. A complete blood count should be done to rule out sepsis as a cause of coma [24].
For the diagnostics of LIS, the EEG has been used systematically since the 1950ies, even before LIS was described as a nosological entity [28]. Additionally, patients may use an EEG to evaluate brain activity, sleep-wake cycles, and attention. In cases of classical and complete LIS, language comprehension and orientation may be assessed through infrared eye movement sensors or computer-modulated voice prosthetics in cases of incomplete LIS [24].
Finally, brain imaging using computer tomography (CT) or magnetic resonance imaging (MRI) is the most valuable modality to diagnose locked-in syndrome [24]. Cranial computed tomography (CCT) in LIS shows an area of low tissue density in the anterior pons, sometimes extending to the tegmentum, the mesencephalon, or the cerebellar peduncles [28]. The addition of CT or MR angiography can show vascular lesions causing the syndrome in stroke or arterial dissection cases. The addition of a contrast medium can delineate masses like tumors or abscesses and even active demyelinating lesions [24]. MRI is the most sensitive method in diagnosing structural disorders in LIS. It allows the description of the exact localization of the pontine lesion, typical for the syndrome, and additional accompanying lesions. The MRI is essential for atypical forms of injury, such as a non-vascular LIS due to mechanical trauma or intoxication. MRI not only helped diagnose the causing factors but also provided the information necessary for assessing the prognosis and the development of the LIS [28]. Cerebrospinal fluid examination (CSF) is usually necessary if no mass or vascular lesion is present on imaging. Results of the CSF analysis may reveal an infectious or autoimmune cause of the symptoms. The CSF should be sent for differential cell count, protein, glucose, staining, cultures, and CSF protein electrophoresis (IgG oligoclonal bands for MS).
Differential Diagnosis
The diagnosis of locked-in syndrome, like other nervous system disorders, is initially based on clinical suspicion through an exhaustive anamnesis, a complete neurological physical examination, and complementary diagnostic tests to rule out the most probable differential diagnoses. The anamnesis should be oriented to demonstrate the presence of risk factors such as diabetes, hypertension, atrial fibrillation, and atherosclerotic disease, which may point to the possibility of an ischemic or hemorrhagic vascular cause as a cause of brain stem injury, recent cervical trauma, fever, and headache suggesting encephalitis or meningitis, among others. Characteristically, the differential diagnoses of locked-in syndrome include Guillain-Barré Syndrome usually presents with paralysis of the extremities with or without sensory involvement and sometimes with cranial nerve involvement. Guillain-Barré Syndrome usually has a progressive presentation and, on physical examination, shows signs of involvement of the lower motor neuron as areflexia and absence of Babinsky's, unlike LIS, which can be present, and the paralysis of the extremities is global. Neuropsychiatric conditions such as akinetic mutism and catatonia share important characteristics such as the absence of language, facial expressions, and voluntary movements of the extremities, differing from LIS by the presence of brainstem reflexes, intact deep tendon reflexes, and rigidity [29,30].
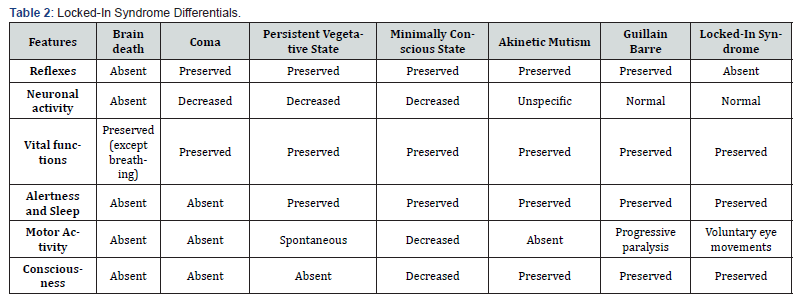
States of altered consciousness, such as the persistent vegetative state, have well-defined criteria that could simulate LIS, such as the absence of voluntary movements, the absence of understandable language, and the preservation of spontaneous eye movements with wake-sleep cycles [30]. It can be differentiated from LIS by the lack of awareness of oneself and of the environment, lack of external interaction through voluntary eye movements, and the presence of brainstem reflexes, which allow us to rule out locked-in syndrome. The minimally conscious state is characterized by limited but clear evidence of awareness of oneself and the environment with inconsistent but goal-directed behavior, being able to interact with the environment and differing from LIS by less elaboration of thought respect to their environment without the involvement of cranial nerves and with present reflexes [31]. The coma is the last of the states of consciousness, characterized by the absence of reactivity to stimuli and consciousness. In a coma, the state patient remains with closed eyes, unaware of himself or his environment. Stimulation does not produce periods of wakefulness, preserving neural activity and brainstem reflexes, unlike brain death, in which both brainstem reflexes and the wake-sleep cycle are absent due to cessation of brain activity and respiratory function [32]. The differential diagnosis of locked-in syndrome is presented in Table 2.
Prognosis & Outcomes
Locked-in syndrome (LIS) prognosis depends on several factors, one of the most crucial being the underlying cause. The overall mortality rate is estimated at 60%, with worse outcomes for vascular causes when compared with nonvascular [33]. The acute setting is correlated with higher mortality rates, with 5-year and 10-year mortality rates of 84 and 31%, respectively [33]. Younger age at onset has been associated with more prolonged survival [34]. Although the acute stage is characterized by reduced memory, attention, and cognitive endurance, chronic LIS cognitive function returns to normal, particularly in those with pure pontine lesions. Regarding motor recovery, locked-in syndrome is characterized by distal to proximal and asymmetrical limb movement improvement [34]. In a cohort study performed by Casanova et al., early intensive rehabilitation started after the morbid event led to a significant motor recovery within 3 to 6 months of onset, complete swallow recovery in 42% of the patients, and verbal communication in 28%; 42% of the patients achieved communication through devices and 35% reached effective bladder and bowel control [35].
To measure patients’ functional recovery, Patterson and Grabois developed five categories, which are regularly used today [36]:
1. No recovery: Patients with no motor return and utterly dependent on their care.
2. Minimum recovery: Patients with minimal voluntary motor return but still dependent on their care.
3. Moderate recovery: Patients with significant motor return, which allows them independence in some but not all of their daily activities.
4. Full recovery: Those who gain independence in all daily activities but still have minimal neurological deficits.
5. No neurological deficit: Patients who have no residual neurological deficit.
There is limited information regarding the quality of life in patients with LIS. Anderson et al. conducted a study using semi-structured interviews with seven long-term survivors of LIS. The results showed these patients had, on average, a worse quality of life than cancer patients but better than the terminally ill. All scored in the range of a depressive illness, and four admitted suicidal thoughts. Notoriously, they all chose to receive antibiotics in case of infections, particularly pneumonia, rejecting the opportunity to end their lives [37]. Bruno et al. performed a study on LIS members of the French Association for LIS to understand the self-perceived quality of life in chronic LIS patients. Out of 65 participants, 47 patients reported happiness, while 18 professed unhappiness. Unhappiness was associated with anxiety and dissatisfaction with mobility in the community, recreational activities, and recovery of speech production, as well as a shorter duration of LIS. On the other hand, happiness was associated with a longer time with LIS, recovery of speech production, and absence of anxiety [38].
Conclusion
This comprehensive review has explored the details surrounding locked-in syndrome (LIS), an infrequent and debilitating neurological condition. The multifaceted aspects of LIS have been studied, including its clinical manifestations and classifications, presenting a clear picture of the challenges faced, especially in its diagnosis. Epidemiologically, LIS affects males slightly more than females, with a wide age range of onset. Vascular causes are the most prevalent, often associated with comorbid conditions like hypertension and diabetes. Clinically, LIS is characterized by a distinctive combination of complete or partial paralysis of voluntary muscles while the individual maintains full consciousness. These symptoms arise from damage to the corticospinal and corticobulbar tracts in the ventral brainstem, leading to quadriplegia and anarthria, affecting speech and mobility profoundly. Diagnosing LIS can be a very complex challenge, making using advanced imaging techniques such as MRI and CT scans necessary. The prognosis of LIS is intricately linked to the underlying cause, with acute cases demonstrating higher mortality rates. However, patients with pure pontine lesions tend to exhibit better cognitive function recovery in the chronic stage. Early and intensive rehabilitation enhances motor recovery and communication capabilities in LIS patients. The extent of functional recovery spans a spectrum, ranging from no improvement to complete restoration of neurological functions.
One of the main takeaways from this review is the pressing need for early recognition and diagnosis of LIS to initiate timely and appropriate rehabilitation strategies, ultimately leading to improved patient outcomes. However, the existing gaps in knowledge make further research invaluable to explore potential therapeutic interventions that could enhance recovery and foster functional independence for these patients.
References
- Laureno R, Karp BI (1997) Myelinolysis after correction of hyponatremia. Ann Intern Med 126(1): 57-62.
- Dollfus P, Milos PL, Chapuis A, Real P, Orenstein M, et al. (1990) The locked-in syndrome: a review and presentation of two chronic cases. Paraplegia 28(1): 5-16.
- Baquis GD, Pessin MS, Scott RM (1988) Vertebrobasilar occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol 45(5): 529-533.
- Kelly JP, Kaufman HH, Kupsky WJ, et al. (1990) Pontine ischemia: clinical features, CT, and MRI. Neurology 40(7): 1083-1087.
- Wijdicks EFM, Parisi JE, Sharbrough FW (1994) Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol 35(2): 239-243.
- Schnetzer L, McCoy M, Bergmann J, Kunz A, Leis S, et al. (2023) Locked-in syndrome revisited. Ther Adv Neurol Disord 16: 17562864231160873.
- León-Carrión J, van Eeckhout P, Domínguez-Morales M Del R, Pérez-Santamaría FJ (2002) The locked-in syndrome: a syndrome looking for a therapy Brain Inj 16(7): 571-582.
- Patterson JR, Grabois M (1986) Locked-in syndrome: a review of 139 cases. Stroke17(4): 758-764.
- Halan T, Ortiz JF, Reddy D, Altamimi A, Ajibowo AO, et al. (2021) Locked-In Syndrome: A Systematic Review of Long-Term Management and Prognosis. Cureus 13(7): e16727.
- M Das J, Anosike K, Asuncion RMD (2023) Locked-in Syndrome. [Updated 2022 Dec 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Patterson JR, Grabois M (1986) Síndrome de enclaustramiento: revisión de 139 casos. Stroke 17(4): 758-764.
- Yokose M, Furuya K, Suzuki M, et al. (2018) Parálisis de la mirada vertical causada por un infarto selectivo del mesencéfalo rostral unilateral. Neurooftalmología 42: 309-311.
- Papadopoulou SL, Dionyssiotis Y, Krikonis K, Lаgopati N, Kamenov I, et al. (2019) Abordajes terapéuticos en el síndrome de enclaustramiento. Folia Med (Plovdiv) 61: 343-351.
- Laureys S, Pellas F, Van Eeckhout P, Ghorbel S, Schnakers C, et al. (2005) The locked-in syndrome: what is it like to be conscious but paralyzed and voiceless? Prog Brain Res 150: 495-511.
- Bauer G, Gerstenbrand F, Rumpl E (1979) Varieties of the locked-in syndrome. J Neurol 221(2): 77-91.
- Schnakers C, Zasler N (2007) Pain issues in the locked-in syndrome. Brain Inj 21(5): 525-530.
- Plum F, Posner JB (1980) The diagnosis of stupor and coma. F.A. Davis.
- Baquis GD, Pessin MS, Scott RM (1988) Vertebrobasilar occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol 45(5): 529-533.
- Laureys S, Pellas F, Van Eeckhout P, Ghorbel S, Schnakers C, et al. (2005) The locked-in syndrome: what is it like to be conscious but paralyzed and voiceless? Prog Brain Res 150: 495-511.
- Bauer G, Gerstenbrand F, Rumpl E (1979) Varieties of the locked-in syndrome. J Neurol 221(2): 77-91.
- Schnakers C, Zasler N (2007) Pain issues in the locked-in syndrome. Brain Inj 21(5): 525-530.
- Posner JB (1980) The diagnosis of stupor and coma. F.A. Davis.
- Pessin MS, Scott RM (1988) Vertebrobasilar occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol 45(5): 529-533.
- M Das J, Anosike K, Asuncion RMD (2023) Locked-in Syndrome. 2022 Dec 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Papadopoulou SL, Dionyssiotis Y, Krikonis K, Lаgopati N, Kamenov I, et al. (2019) Therapeutic Approaches in Locked-in Syndrome. Folia Med (Plovdiv) 61(3): 343-351.
- Locked In Syndrome (2018) National Organization of Rare Disorders (NORD).
- Smith E, Delargy M (2005) Locked-in syndrome. BMJ 330(7488): 406-409.
- Kotchoubey B, Lotze M (2013) Instrumental methods in the diagnostics of locked-in syndrome. Restor Neurol Neurosci 31(1): 25-40.
- Das JM, Anosike K, Asuncion RMD (2022) Locked-in Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Grau Veciana JM (2004) Estado vegetativo persistente Aspectos clí Medicina Intensiva, España 28(3): 108-111.
- Surdyke L, Fernandez J, Foster H, Spigel P (2017) Differential Diagnosis and Management of Incomplete Locked-In Syndrome after Traumatic Brain Injury. Case Reports in Neurological Medicine Article ID 6167052, 7 pages.
- Laureys S, Owen AM, Schiff ND (2004) Brain function in coma, vegetative state, and related disorders. The Lancet Neurology 3(9): 537-546.
- Asuncion RMD (2022) Locked-in Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing PMID: 32644452.
- Farr E, Altonji K, Harvey RL (2021) Locked-In Syndrome: Practical Rehabilitation Management. PM R 13(12): 1418-1428.
- Casanova E, Lazzari RE, Lotta S, Mazzucchi A (2003) Locked-in syndrome: improvement in the prognosis after an early intensive multidisciplinary rehabilitation. Arch Phys Med Rehabil 84(6): 862-867.
- Patterson JR, Grabois M (1986) Locked-in syndrome: a review of 139 cases. Stroke 17(4): 758-764.
- Anderson C, Dillon C, Burns R (1993) Life-sustaining treatment and locked-in syndrome. Lancet 342(8875): 867-868.
- Bruno MA, Bernheim JL, Ledoux D, Pellas F, Demertzi A, et al. (2011) A survey on self-assessed well-being in a cohort of chronic locked-in syndrome patients: happy majority, miserable minority. BMJ Open 1(1): e000039.






























