Diffuse Leptomeningeal Glioneuronal Tumour: An Intriguing Case Report of an Adult Patient with a Previous Optic Astrocytoma
Janez Ravnik1, Hojka Rowbottom1*, Uroš Smrdel2, Gorazd Bunc1, Jernej Mlakar3 and Alenka Matjašič3
1Department of Neurosurgery, University Medical Centre Maribor, Slovenia
2Institute of Oncology Ljubljana, Slovenia
3Institute of Pathology, Faculty of Medicine, University of Ljubljana, Slovenia
Submission: June 29, 2023; Published: July 12, 2023
*Corresponding author: Hojka Rowbottom, Department of Neurosurgery, University Medical Centre Maribor, Slovenia
How to cite this article: Janez R, Hojka R, Uroš S, Gorazd B, Jernej M, et al. Diffuse Leptomeningeal Glioneuronal Tumour: An Intriguing Case Report of an Adult Patient with a Previous Optic Astrocytoma. Open Access J Neurol Neurosurg 2023; 18(3): 555986.DOI: 10.19080/OAJNN.2023.18.555986.
Abstract
Diffuse leptomeningeal glioneuronal tumour (DLGT) represents a rare type of neoplasm with a limited number of cases and an ambiguous prognosis. It is mainly diagnosed in children, more often in boys. The clinical presentation depends on the area of the central nervous system involved. The diagnostic process must be extremely broad. Currently, no therapeutic guidelines exist. In this report, we present a case of a 26-year-old man diagnosed with DLGT, who at the age of 10, had a pilocytic astrocytoma (PA) that was surgically removed and was afterwards treated with radiotherapy. After 16 years, his neurological condition deteriorated and an extensive diagnostic process was conducted, including molecular genetic testing using the next generation sequencing (NGS), that led to the diagnosis of DLGT. To our knowledge, we present the first reported case of a DLGT, diagnosed in an adult, who as a child was treated for a PA, and was not a case of a malignant transformation, but rather a case of two primary tumours of the central nervous system, in whom neurofibromatosis was excluded.
Keywords: Diffuse leptomeningeal glioneuronal tumour; Molecular genetic testing; Pilocytic astrocytoma
Abbreviations: CNS: Central Nervous System; DLGT: Diffuse Leptomeningeal Glioneuronal Tumour; BRAF: Serine/Threonine Protein Kinase B-raf; CSF: Cerobrospinal Fluid; PA: Pilocytic Astrocytoma; IAM: Internal Auditory Meatus; NGS: Next Generation Sequencing; DLGT-MC-1/-2: Diffuse Leptomeningeal Glioneuronal Tumour Methylation class 1/2
Introduction
Gliomas compose the most common group of intrinsic tumours of the central nervous system (CNS) and can be divided into diffuse gliomas and “non-diffuse” gliomas, which demonstrate a more circumscribed pattern of growth. In 2016 published revised fourth edition of the WHO Classification of CNS tumours, several new pathohistological entities have been introduced in the group of “non-diffuse” gliomas and neuronal-glial tumours, such as anaplastic pleomorphic xanthoastrocytoma, RELA fusion-positive ependymoma and diffuse leptomeningeal glioneuronal tumour (DLGT) [1], which represents a rare type of tumour with sparse cases and an ambiguous prognosis [2,3]. DLGT has previously been diagnosed as disseminated oligodendroglial-like leptomeningeal tumour, dysembryoplastic neuroepithelial tumour-like neoplasm, meningeal gliomatosis, diffuse leptomeningeal neurocytoma, diffuse leptomeningeal gangliocytoma, and diffuse leptomeningeal oligodendrogliomatosis [4] and is now assigned to the category of neuronal and mixed neuronal-glial tumours [5]. DLGT is often diagnosed in children, more often boys, and has an accelerating course with a few cases occurring in adulthood [4,6]. Due to its infrequency and minor studies, different views of clinical, pathohistological and neuroradiological characteristics persist; DLGT has not yet been assigned a WHO grade [3,6]. DLGT can mimic a chronic infection, such as meningitis [7], or leptomeningeal carcinomatosis, which complicates the diagnosis [4,8]. The clinical presentation of DLGT depends on the area involved and it can manifest as paraesthesia, seizures or symptoms of hydrocephalus, such as headache and vomiting [8]. A combination of the fusion of KIAA1549 and the serine/threonine protein kinase B-raf (BRAF) and deletion of the short arm of chromosome 1 (1p) or/and the long arm of chromosome 19 (19q) is present in molecular genetic studies [6]. The most often discovered imaging features are diffuse abnormal nodular leptomeningeal growth with no evidence of a primary intraparenchymal focus, which can be seen in the basal cisterns, posterior fossa, Sylvian fissures, brainstem and the spinal cord, often associated with cystic T2 hyperintense lesions on MRI [4,8].
Pathohistological features of DLGT show a low mitotic index with a low to moderate density and cellular features that are similar to those of oligodendrogliomas [5,8], however, multiple patients often have a non-diagnostic initial biopsy due to limited tumour material [8]. Opposite to oligodendrogliomas, DLGT is negative for the presence of IDH 1 and 2 mutations [5]. DLGT demonstrates histological and immunohistochemical features of glial and neuronal differentiation with an expression of synaptophysin, OLIG2 and S-100 [9]. In the majority of cases, cytology of the cerebrospinal fluid (CSF) is negative for tumour cells, despite the extensive leptomeningeal involvement [8]; it involves mild hyperproteinaemia and lymphocyte pleocytosis [10]. Patients with a poor prognosis are often older at the time of diagnosis and have multiple site lesions and hydrocephalus, whereas those with a stable disease have a higher rate of 1p/19q codeletion [5]. Currently, no therapeutic guidelines exist, patients are treated by use of various chemotherapy regimens with or without radiation therapy and biological agents, namely carboplatin, bevacizumab, and temozolomide [9,11]. Craniospinal radiation has been proven to improve clinical outcomes and slow disease progression [11].
Case Report
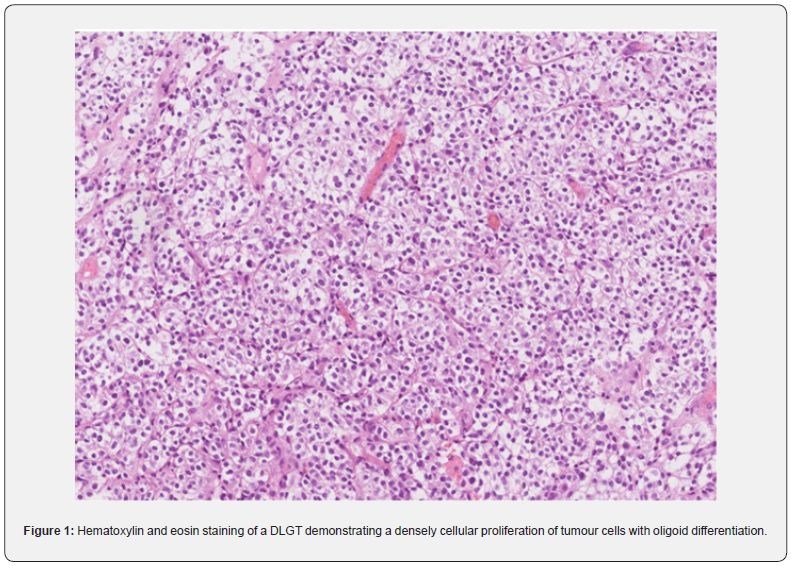
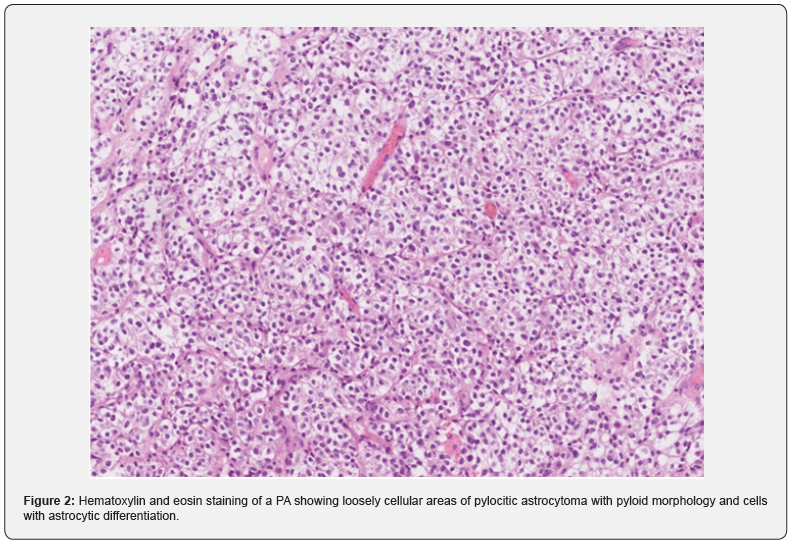
In August 2021, a 26-year-old man presented with progressive headaches to the neurosurgical outpatient clinic at the University Medical Centre in Maribor. His medical history included a pilocytic astrocytoma (PA) of his right optic nerve and tract, which was surgically removed in 2005 and left him with hemianopsia of the left eye and double vision. After the operation, he underwent radiotherapy intermittently for the next 4 years. He had been having follow-up appointments and each year an MRI of the brain was performed, and at his last check-up in June 2021, there was no change concerning the PA. In July 2021, he experienced a 3-hour-long episode of speechlessness, and an MRI of the brain was repeated, where a lesion was present in the left internal auditory meatus (IAM). After being examined by a neurosurgeon, the patient underwent a contrast-enhanced MRI of the brain and the whole neural axis, which stated the following: atrophy of the pituitary gland, thickening of the dura in the left IAM and both jugular foramina and a lesion measuring one centimetre in diameter, found in the right pontocerebellar angle, which could be a Schwannoma or meningioma. On the contrastenhanced MRI of the spine, several areas of enhanced deposits were described, particularly in the lumbar and thoracic spine. CSF, obtained through a lumbar puncture, showed a mononuclear pleocytosis with no malignant cells present. In September 2021, a laminectomy of the 5th lumbar vertebra and a biopsy of the intradural tumour and surrounding meninges were performed. Tissue was sent for histological analysis and molecular genetic testing, which confirmed that the tissue was a DLGT due to a 1p/19q codeletion, positive S100 and EMA negative, furthermore, a KIAA1549: BRAF fusion was also present. The next-generation sequencing (NGS) showed the presence of deletions of FGFR3, PTEN, ATM, FANCA, and TP53 genes as well as amplification of TSC1, CCND1, CCND2, and KRAS genes. The patient had a heterozygous deletion of loci 11q21-q24.2, 19q12-q13.32 and a partial amplification of chromosomes 7 and 12. Histology of the DLGT showed the densely cellular proliferation of tumour cells with oligoid differentiation, which is demonstrated in Figure 1. The tissue from the PA, which was surgically removed in 2005, was also reanalysed and the conclusion was there no deletion of 1p, and the morphological characteristics were different, therefore, it was not a case of recurrence, but rather a case of another primary neoplasm. However, KIAA1549: BRAF fusion was present in the tissue of both tumours. Histology of the PA demonstrated loosely cellular areas with piloid morphology, cells with astrocytic differentiation, and more densely cellular areas with oligoid differentiation, which is shown is Figure 2. Using genetic testing, neurofibromatosis types 1 and 2 were excluded. After the surgery, the patient’s neurological status remained identical; hemianopia of the left eye and nystagmus when looking to the left, with a fast component towards the left bilaterally. In October 2021, the patient received the first cycle of chemotherapy which comprised carboplatin and vincristine. Afterwards, his health deteriorated, he completely lost vision in his left eye, a right-sided internuclear ophthalmoplegia, ataxia of all four limbs and ataxia of the gait were present. A contrast-enhanced MRI of the brain demonstrated hydrocephalus and a ventriculoperitoneal shunt with a Certas™ programmable valve was inserted with the outflow resistance at level 4. The patient’s neurological condition improved; the headaches and nausea disappeared, gait ataxia subsided, and his left eye’s vision improved. A week after being discharged, he was examined by the attending oncologist to continue chemotherapy. In March 2022, the patient presented with a complete loss of vision in the left eye and visual acuity of 0.16. in the right eye. An MRI showed a thickening around the right optic nerve and scar tissue after previous operations. A pterional craniotomy with decompression of the right optic nerve was performed, which did not significantly restore the patient’s eyesight. He continued with chemotherapy and regular appointments with the attending oncologist.
Discussion
DLGT represents a low-grade CNS tumour without a definite WHO grade, which is mostly due to its rarity [3,6]. The 2021 updated WHO classification of CNS tumours, has subtyped DLGT into 3 entities according to their molecular characteristics: DLGT with 1q gain, DLGT with methylation class 1 and DLGT with methylation class 2 [12]. DLGT methylation class 1 (DLGTMC- 1) is characterised by 1p deletion and DLGT methylation class 2 (DLGNT-MC-2) by 1q duplication with the first group characterised by lower age at diagnosis and a less aggressive course [5], whereas the latter is connected to more aggressive behaviour and shorter survival [10]. The clinical course and prognosis of DLGT with methylation class 1 are similar to WHO grade II CNS tumours, whereas DLGT with 1q gain or DLGT with methylation class 2 behave more as a CNS WHO grade III tumour [12]. The patient in our case report had no gain of 1q, but rather a 1p/19q codeletion, however, methylation analysis of the tumour tissue was not conducted. Molecular and genetic testing, utilising NGS, is becoming an extremely important part of the diagnostic process, since there have been cases of DLGT without diffuse leptomeningeal involvement; a characteristic of the majority of DLGT [13]. Children represent the majority of cases with DLGT [3,4,12,14], however, the patient in this case report was 26 years old at the onset of the disease. The diagnosis of DLGT is often challenging, since patients present with non-specific symptoms of raised intracranial pressure with CSF analysis negative for tumour cells [14], which was also present in our instance In cases of DGLT, a high protein concentration in the CSF can be found, which is consistent with diffuse pial glial neuronal tumours and is due to tumour cells infiltration of the meninges, chemical stimulation of tumour metabolites, destruction of the bloodbrain barrier and increased vascular permeability [11]. Biopsy, histological analysis, and molecular genetic testing are crucial for diagnosing DLGT, as imaging findings are not specific enough and cases of DLGT have been misdiagnosed as tuberculosis [14,15]. The imaging features indicating a case of DLGT are a prominent leptomeningeal enhancement with or without communicating hydrocephalus [15], which is due to the pronounced development of the tumour within the subarachnoid space [16]. The immunohistochemistry of DLGT cells shows that they are positive for OLIG 2 and S100 and negative for GFAP, cytokeratin and EMA [16]; our patient was negative for EMA and positive for S100, and the others were not tested. Utilising the molecular genetic testing and pathohistological analysis, we were able to deduce that ours was not a case of a malignant transformation, but rather a case of another primary CNS tumour. Neurofibromatosis was also excluded. In the literature, however, we found a case of a patient with a thoracic PA, which after 10 years of multiple recurrences and resections, transformed into a DLGT with highgrade characteristics [17]. The main aim of the therapy, at the present moment, is not a total resection of the tumour, as that is not feasible, but rather a reduction and when required, insertion of a ventriculoperitoneal shunt, if hydrocephalus occurs [12], as was present in this case. At present, no standardised treatment protocol has been developed for patients with DLGT, the majority are treated with a combination of surgery, chemotherapy and radiotherapy [14], however, targeted therapy and immunotherapy are also an option [11]. The majority of patients, who are treated with chemotherapy (systemic or intrathecal), receive a combination of temozolomide, carboplatin and vincristine [16]; the patient in our case report was administered carboplatin and vincristine systemically. The main purpose of the current surgical treatment is to insert a ventriculoperitoneal shunt in cases of hydrocephalus or to insert an Ommaya sac under the scalp for intraventricular chemotherapy [11]. Craniospinal radiation therapy is not used as the first line of management, due to its significant side effects and is utilised in cases with the progression of the tumours [12]; so far, the patient in our case report has not undergone radiotherapy, but only surgery and chemotherapy. Chemotherapy appears to halt the progression, whereas radiotherapy is used in cases of disease progression [14]. Targeted therapy comprises EGFR tyrosine kinase inhibitors, ALK inhibitors, HER2 monoclonal antibodies, and vascular endothelial growth factor monoclonal antibodies [11]. The median survival time of patients with DLGT, according to a systematic review of the literature performed by Jiang and colleagues, was 173 months, but the survival curve fell significantly before 72 months. Patients treated with chemotherapy and those with a KIAF-BRAF fusion exhibited a better clinical course with overall survival of 60 months. There was no difference in survival of patients treated by surgical resection, radiotherapy or ventriculoperitoneal shunting [18].
Conflict of Interest
None to declare.
References
- Wesseling P, Capper D (2018) WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol 44(2): 139-150.
- Sáez-Alegre M, Saceda Gutiérrez JM, Utrilla Contreras C, Aracil Santos FJ, García-Feijoo P, et al. (2021) Diffuse leptomeningeal glioneuronal tumour: where to biopsy? Case report and literature review. Childs Nerv Syst 37(7): 2405-2408.
- Chen W, Kong Z, Fu J, Zhao D, Wang R, et al. (2020) Diffuse leptomeningeal glioneuronal tumour (DLGNT) with hydrocephalus as an initial symptom: a case-based update. Childs Nerv Syst 36(3): 459-468.
- Lakhani DA, Mankad K, Chhabda S, Feizi P, Patel R, et al. (2020) Diffuse Leptomeningeal Glioneuronal Tumor of Childhood. AJNR Am J Neuroradiol 41(11): 2155-2159.
- Stapińska-Syniec A, Gogol A, Acewicz A, Sobstyl M, Wierzba-Bobrowicz T, et al. (2022) Atypical clinical presentation mimicking stroke in an adult patient caused by a rare diffuse leptomeningeal glioneuronal tumour. Pol J Pathol 73(4): 371-375.
- Sarkinaite M, Devyziene I, Makstiene J, Matukevicius A, Gleizniene R (2022) Diffuse Leptomeningeal Glioneuronal Tumour with 9-Year Follow-Up: Case Report and Review of the Literature. Diagnostics 12(2): 342.
- Nandi D, Sahu A (2020) Know the less known: Diffuse leptomeningeal glioneuronal tumour. Indian J Med Res 152(Suppl 1): S148-S149.
- Manoharan N, Ajuyah P, Senapati A, Wong M, Mullins A, et al. (2021) Diffuse leptomeningeal glioneuronal tumour (DLGNT) in children: the emerging role of genomic analysis. Acta Neuropathol Commun 9(1): 147.
- Rebella G, Milanaccio C, Morana G, Tortora D, Verrico A, et al. (2022) Calcifications in diffuse leptomeningeal glioneuronal tumors: a case series. Quant Imaging Med Surg 12(5): 2985-2994.
- Jensen MP, Lim E, Dixon L, Quilichini B, Jenkins H, et al. (2022) Cerebrospinal fluid cytology findings in a case of diffuse leptomeningeal glioneuronal tumour. Cytopathology 33(6): 738-741.
- Bao J, Sweeney JF, Liu Y, Genovese FL, Adamo MA, et al. (2023) Rapidly progressive diffuse leptomeningeal glioneuronal tumor in an adult female: illustrative case. J Neurosurg Case Lessons 5(5): CASE22502.
- Patankar AP, Vaghela P, Nasit J, Gohil R (2022) Diffuse Leptomeningeal Glioneuronal Tumor: A Rare Case Report with Review of Literature. Asian J Neurosurg 17(3): 532-535.
- Madsen PJ, Hollawell ML, Santi M, Surrey LF, Vossough A, et al. (2023) Diffuse leptomeningeal glioneuronal tumor in a child masquerading as an intramedullary spinal pilocytic astrocytoma. Neurooncol Adv 5(1): vdad049.
- Teh YG, Azizan N, Mohd Naim NA, Ng CY, Wong KJ, et al. (2021) Case Report: Unusual High-Grade Diffuse Leptomeningeal Glioneuronal Tumor Mimicking Tuberculous Meningitis in a Child from an Endemic Region. Front Pediatr 9: 767614.
- Lee J, Ko H, Choi J, Lee Y, Son B (2018) A Case of Diffuse Leptomeningeal Glioneuronal Tumor Misdiagnosed as Chronic Tuberculous Meningitis without Brain Biopsy. Case Rep Neurol Med 2018: 1391943.
- Karimzadeh P, Nilipour Y, Khalili M, Nikkhah A, Taghavijelodar M, et al. (2023) A case of diffuse leptomeningeal glioneuronal tumor in a 10‐year‐old boy: First report from Iran. Clin Case Rep 9(12) e05199.
- Farimani PL, Rebchuk AD, Chang SJ, Yip S, Hawkins C, et al. (2023) Malignant transformation of adult-onset pilocytic astrocytoma to diffuse leptomeningeal glioneuronal tumor within the thoracic spine: a case report and review of the literature. Acta Neurochir (Wien).
- Jiang H, Qiu L, Song J, Xu D, Sun L, et al. (2022) Clinical progression, pathological characteristics, and radiological findings in children with diffuse leptomeningeal glioneuronal tumors: A systematic review. Front Oncol 12: 970076.






























