Scale for Assessment and Rating Ataxia (SARA) in Children with Ataxia: Greek Cultural Adaptation and Psychometric Properties
Alexandra Lepoura1*, Sofia Lampropoulou2, Antonis Galanos3, Marianna Papadopoulou1 and Vasiliki Sakellari1
1University of West Attica, Department of Physiotherapy, School of Health & Care Sciences, Athens, Greece
2University of Patras, Department of Physiotherapy, School of Health Rehabilitation Sciences, Patra, Greece
3National and Kapodistrian University of Athens, School of Medicine, Athens, Greece
Submission: January 26, 2023; Published: February 09, 2023
*Corresponding author: Alexandra Lepoura, University of West Attica, Department of Physiotherapy, School of Health & Care Sciences, Agiou Spiridonos 28, Egaleo, 12243, Greece
How to cite this article: Alexandra Lepoura*, Sofia Lampropoulou, Antonis Galanos, Marianna Papadopoulou and Vasiliki Sakellari. Scale for Assessment and Rating Ataxia (SARA) in Children with Ataxia: Greek Cultural Adaptation and Psychometric Properties. Open Access J Neurol Neurosurg 2024; 18(1): 555976. DOI: 10.19080/OAJNN.2024.18.555976.
Summary
Introduction: Ataxia in children is insufficiently studied and is characterized by a great heterogeinity. SARA is a well known assessment tool for ataxia clinical symptoms. The aim of the current study is the investigation of the psychometric characteristics of the adapted into Greek SARA (SARAgr) in children with cerebellar ataxia.
Methods: The performance of 30 children with progressive and non-progressive cerebellar ataxia and Gross Motor Function Classification System (GMFCS) I-V (18 boys/ 12 girls; 13.2 ± 3 years old) on SARAgr was rated twice by two pediatric physiotherapists for inter-rater, intra-rater and test-retest reliability. For convergent validity SARAgr was correlated with the Brief Ataxia Rating Scale (BARS) and the Barthel Index (BI).
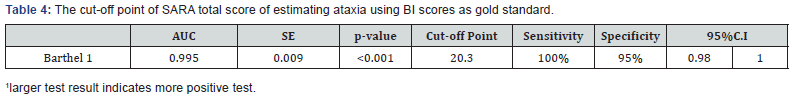
Results: SARAgr yielded an excellent test-retest (ICC OF 0.997), inter-rater (ICC of 0.996) and intra-rater (ICC of 0.999) agreement and very high correlation between total scores of SARAgr and BARS (r=0.945, p<0.0005) and Bl (r=-0.872, p<0.0005). A cut-off point at 20.3 SARAgr score was found (95%CI 0.98-1.0, p<0.001), using the BI as gold standard.
Discussion: Our findings are in accordance with previous research and indicate that the SARAgr can be used in a reliable and valid way for the assessment of cerebellar ataxia in children with different underlying pathology. The existence of SARAgr cut-off point seems decisive for the ability with which the children with ataxia function and cope independently in daily activities.
Conclusion: SARAgr can be used to assess children with cerebellar ataxia above the age of 8 years old and can identify dependency in daily activities.
Keywords: Ataxia; Children; Assessment; Scale; SARA
Introduction
Ataxia in childhood is a movement disorder that occurs in approximately 26 / 100,000 children in Europe and probably reflects a minimal prevalence worldwide [1]. In many countries, including Greece there are no official data on the prevalence of ataxia in the adult or pediatric population, while both evaluation and intervention strategies seem to have been poorly studied [2].
The underlying pathology of cerebellar ataxia in childhood is characterized by a great heterogeneity, with progressive and non progressive ataxia being the main types of its description [3]. The causes of this movement disorder can be acquired such as tumors in the cerebellum, congenital such as cerebral palsy or genetic such as Friedrich ataxia, or even as mixed situations, while in some cases, ataxia may be displayed as an acute, intermittent or recurrent condition [3]. Cerebellar ataxia related symptoms are expressed in the base of the lack of balance and coordination which is apparent in the quality and quantity of daily activities, where children interact. Ataxic gait, dysmetria, dysdiadochokinisia, tremor, nystagmus, dysarthria are some of the basic clinical features that underlie the symptomatology of ataxia, regardless its aetiology [4,5,6]. In general, lesions to any of the cerebellar input or output pathways can result in ataxia. The main responsible structures are the dorsal and ventral spinocerebellar pathways, the pontine nuclei, as well as any of the three cerebellar peduncles [7]. Those ataxia symptoms are related to the location of the lesions in the cerebellum where lateralized or diffused lesions introduce ipsilateral or generalized symptoms, respectively [7].
Clinical assessment of ataxia related symptoms can be achieved by a number of specifically designed rating scales [8]. The Scale for assessment and rating ataxia “SARA” is a short and reliable semi-quantitative scale, which was first developed for assessing and rating ataxia related symptoms for adults with spinocerebellar ataxia (SCA) [9]. It includes eight items related only to clinical ataxic symptoms manifested by cerebellar ataxia, with a total score ranging from 0-40 (no ataxia-severe ataxia). Through years, the use of SARA has been expanded and has validly used in different population with ataxia, including children and adults with “Early Onset Ataxia” [10] as well as pediatric population with cerebral tumor [11,4]. Among numerous ataxia rating scales, SARA is one of the most valid and prevalent assessment tool used for people with ataxia [8]. Just few years ago, the Childhood Ataxia and Cerebellar Group of European Pediatric Neurology Society (CACG-EPNS) validated the use of SARA in pediatric population, [12] which can be more reliably assessed in children above the age of 8 years old, as in younger children the variability of its scoring is high [12,13,4].
The objective of this study is to investigate the psychometric properties of the adapted into Greek SARA in pediatric population of ataxia with any kind of underlying pathology, as this will be of valuable assistance in both clinical and research medical and health allied settings. We anticipate that this research will provide a) a reliable and valid use of the scale for the assessment of ataxia in children and b) insights of its clinical importance through its correlation with other assessment tools and within the wide range of heterogeneity which occurs due to the different pathologies and ataxia severity.
Materials and Methods
Study Design and Setting
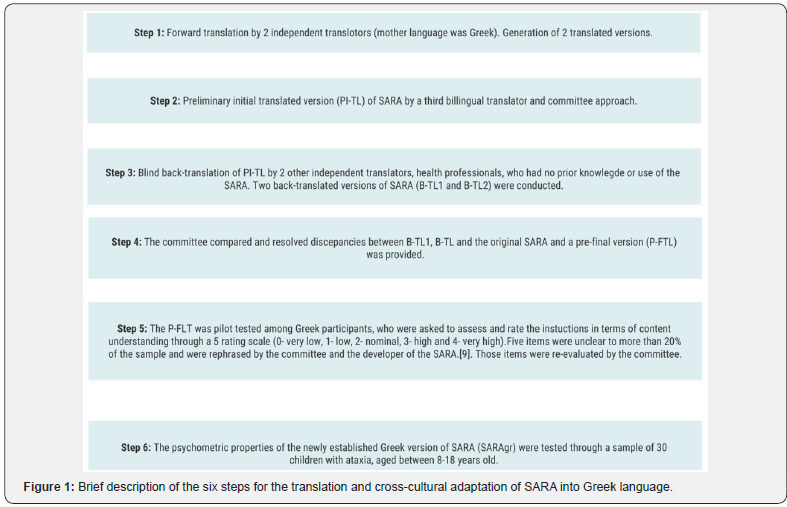
This research study describes the conduction of a 2 year cross-sectional trial for adapting the SARA in Greek language for pediatric population with ataxia. Translation and cross-cultural adaptation of SARA into Greek followed international guidelines [14], as described in Figure 1 and was processed and completed within a period of approximately 4 months. Assessment of the psychometric properties of the newly adapted into Greek scale, was conducted on site or online, due to the great distance of some participants’ location of residence from Athens, Greece, which lasted from October 2020 since July 2022.
Participants
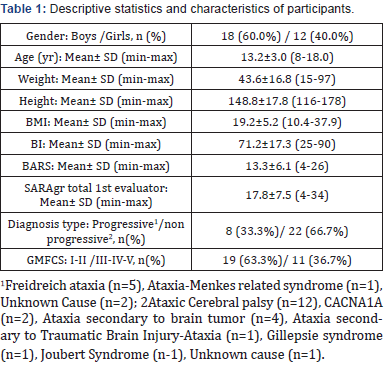
Thirty (30) children with ataxia (18 boys/ 12 girls; 13.2 ± 3 years old) (Table 1) were enrolled in this study for assessing the psychometric properties of the newly established version of SARA in Greek language. A similar sample size has been used in other studies, such as in a study upon typically developing children, [15] as well as in the studies collecting a sample of 28 children and 40 adults with ataxia, respectively [13, 10].
Recruitment was obtained through clinician invitation at public health care faculties from which approval has been achieved or through invitation and information documents, published at social media and through emails to organizations of ataxia in Greece, private pediatric physiotherapy faculties and other relative therapeutic institutes that treat children with ataxia and provide services to their families. Ethical Approval has been obtained by the Ethics Committee of the University of West Attica (study’s protocol: 14η/26-04-2021) and the “ATTIKON” General University Hospital of Athens (study’s protocol: Γ ΠΑΙΔ, ΕΒΔ 149/20-3-2020).
Inclusion criteria included (1) children, aged 8-18 years old, (2) diagnosed with cerebellar ataxia of any underlying pathology (genetic, acquired, progressive or no progressive) as their primary movement disorder, (3) with Gross Motor Function Classification System (GMFCS) I-V level and (4) the ability to understand Greek language. Exclusion criteria included the inability to understand commands and serious visual deficits.
Procedure
Parents and children were informed about the process of the study and their agreement to participate was obtained through a signed consent form for both parents and children above the age of 16 years old. A code was given to each participant for securing deidentification in the following children‘s data completion. Two pediatric physiotherapists were required to administer the SARAgr, under two testing trials, over a 2 week period (Test I and Test II).
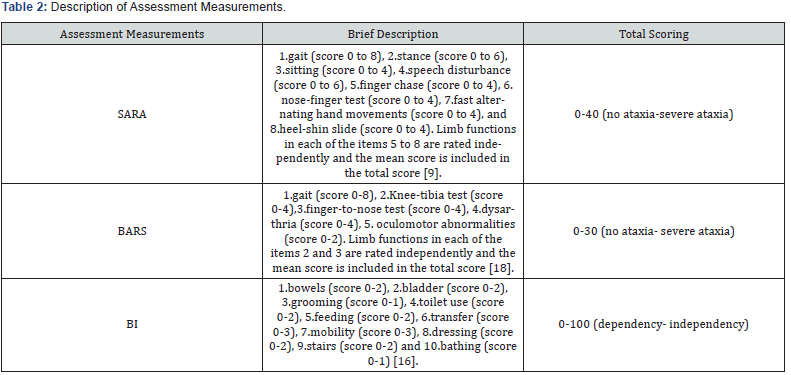
During Test I, parents were asked to complete a short form including anthropometric information and a brief medical history of their child and to fill the Barthel Index (BI) questionnaire [16] (Table 2) used in its Greek version, as this has been used in previous study [17]. Afterwards, each child was assessed with the SARAgr and the Brief Ataxia Rating Scale (BARS), [18] which lasted approximately 20 minutes (Table 2). The performance of each child during those assessments was videotaped in order to be rated by the 2 pediatric physiotherapists, who acted as independent evaluators, the evaluator 1 and 2 [13]. In Test II, the SARAgr assessment was repeated and videotaped under the same conditions as Test I for each participant. The videos were held on site or remotely with the assistance of each participant’s physiotherapist, due to the great distance of some participants’ location of residence.
Reliability of the Greek version of SARA
A similar procedure described in the literature was followed [19]. Both trials (Test I and Test II) were instructed and filmed by evaluator 1. All videos were performed with a high definition digital camera, which was set at each child’s shoulders’ height and in 1 m away from the child, while zoom function in eyes was used for the eye-movement analysis.
• For intra-rater reliability, evaluator 1 rescored the same videos, after a period of 2-6 weeks, without having reviewing the results of the first recording [19].
• For inter-raters’ reliability, both evaluator 1 and evaluator 2 scored independently all videos of the Test I trial using the SARAgr [19].
• For test-retest reliability, a second trial of SARAgr was conducted within a 2 week period and was scored by evaluator 1 [15].
Validity of the Greek version of SARA
To assess validity, the SARAgr was correlated with the BARS scale and the BI questionnaire, as already conducted in adult population with ataxia [9,20], as well as a study upon typically developing children [15]. The BI is a self-reported ordinal scale, used to measure the independent or assisted performance of individuals in ten items describing mobility and daily activities and was filled once by each child’s parent, in no longer than 5 minutes. The BARS is a five-item scale focuses on motor domains related to symptoms of ataxia (gait, upper and lower extremity coordination, speech, as well as eye movements) with a score ranging from 0-30 (no ataxia-severe ataxia) and was scored once by the evaluator 1, during the videotaped performance of each child in Test I.
Statistical Analysis
Test-retest reliability was determined by calculating Intraclass Correlation Coefficient (3.1) (ICC) between the initial assessment of the SARAgr and the reassessment within a 2 week period. The Bland Altman plot was used as a visual method for assessing stability [21]. Similarly, intra-rater and inter-rater reliability were expressed using ICC (2.1) and ICC (3.1) respectively. Internal consistency reliability of the SARAgr was determined by calculating Cronbach alpha coefficient. For clinical applications a cronbach α coefficient value of >0.8 is desirable, even though a value of 0.7 indicates sufficient reliability for research purposes [22].
Confirmatory factor analysis (CFA) was used to confirm the structure proposed by the developer of the SARA, [9] using the Analysis of Moment Structure (AMOS) Version 21.0. The sample size required for the CFA based on researchers conventions ranging for the participants ratio 3:1 to as high as 12:1 [23]. Since SARA consists of 8 items, the sample size of 30 participants covers the minimum requirements of guidelines. An acceptable model fit was indicated based on global fit indices (the chi-square-degrees of freedom (d. f.) ratio < 2.0,[24] RMSEA <0.08,[25] CFI >0.90,[25] GFI > 0.85,[26] AGFI >0.80,[26] and NFI >0.90[27]).
Construct validity of the SARAgr was assessed by establishing its correlation to BI total score and BARS total score using the Spearman’s correlation coefficient. Additionally, known groups validity of SARAgr was examined in terms of the ability of the scale to distinguish severity between two subgroups of patients, formed on the basis of their disease severity based on GMFCS level (subgroup GMFCS I-II vs subgroup GMFCS III-IV-V) and disease progress (subgroup progressive vs subgroup non progressive). Independent samples t-test was used for the statistical analysis. Content validity was assessed through item analysis of the SARAgr, by analysing the item discriminating power (corrected item correlation) and the item difficulty (item mean) depicted by the explanatory data analysis.
The variability of SARAgr’ score was further assessed by examining interpretability through floor or ceiling effects, which are considered to be present if more than 15% of respondents achieved the lowest or highest possible score, respectively. The clinical importance of SARAgr was assessed through the cut-off point of SARA total score. A receiver operating curve (ROC) analysis, [28] was conducted to obtain the cut-off level of SARAgr total score for differentiation between subgroups of patients formed on the basis of their ability of activities of daily living, calculating the respective areas under the curve (AUC). The areas under the ROC curve (AUC) with standard error and 95%CI were calculated, using the maximum likelihood estimation method. The sensitivity and specificity of different cut-off points of SARAgr total score were estimated using the BΙ score (21< severe < 60 vs 61< moderate<90) as gold standard methods of ability of activities of daily living.
All tests were two-sided, a p-value of <0.05 was used to denote statistical significance. All analyses were carried out using the statistical package SPSS vr 21.00 (IBM Corporation, Somers, NY, USA).
Results
Demographic and clinical characteristics of participants are presented in Table 1. In summary, twenty two participants were children with non-progressive type of ataxia, while most of them have been assigned in the spectrum of ataxic cerebral palsy (verified dysfunction of the cerebellum). The rest were diagnosed with genetic related dysfunctions and only one child was still under investigation of the ataxia related symptomatology. Eight participants were children with progressive type of ataxia, five of them diagnosed with Freidreich ataxia, one with a Menkesrelated syndrome and two more children with undefined cause of progressive ataxia. According to GMFCS the majority of the participants were independent in indoor settings with difficulties in outdoor activities (Level I n=2; Level II n=17). The rest children had more gross motor functional limitations with six of them needing hand-held mobility device or some assistance by a person while walking (Level III). Three children required physical assistance or powered mobility in most settings (Level V) and only one child required manual wheelchair in all settings (Level IV).
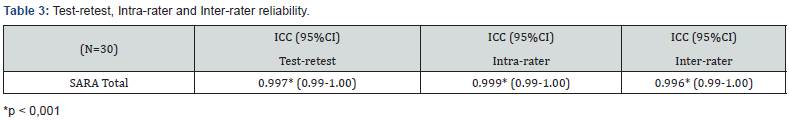
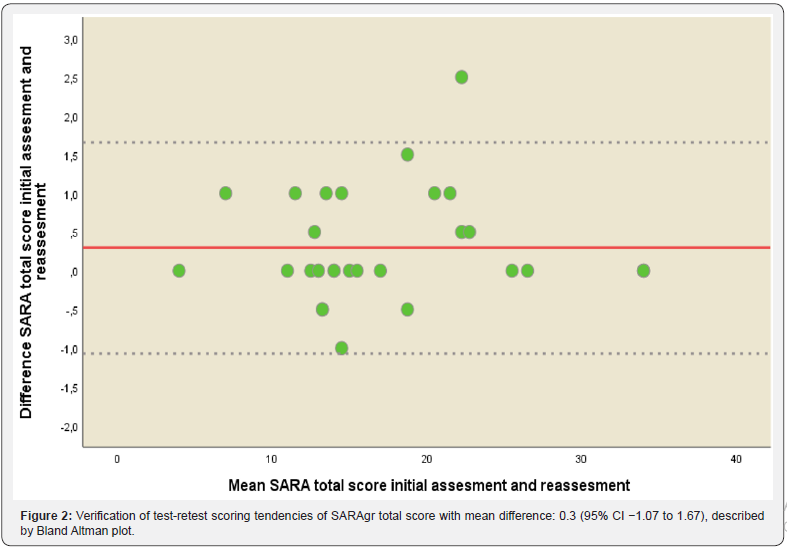
The internal consistency of the SARAgr total score was measured with Cronbach’s alpha and estimated as 0.926, indicating excellent internal consistency for total score (Table 3). Intra-rater reliability for the 1st evaluator was 0.999 (p<0.001) indicating perfect agreement. Inter-rater reliability between 1st and 2nd evaluator was 0.996 (p<0.001) indicating again perfect agreement between the 2 raters. ICC between initial assessment and reassessment (test-retest reliability) within a 2 week period of the SARAgr total score was 0.997 (p<0.001) for the 1st evaluator. Bland-Altman plot is presented in Figure 2 for SARAgr total score, inspection of scattergram showed that all differences were within mean difference ±1.96 SDs, thus confirming the agreement between the 2 assessments. The above results of stability indicated that SARAgr total score were remarkably consistent between the two occasions.
A one-factor model of SARAgr proposed by the creator was examined by confirmatory factor analysis, giving acceptable global fit indices. The resulting global fit indices X2 = 19.2, chisquare- degrees of freedom (d. f.) ratio= 0.98; RMSEA= 0.045; CFI= 0.995; NFI= 0.920; GFI= 0.887 and AGFI= 0.82 showed that the one factor solution proposed by the original scale could be retained.
There was very high correlation coefficients between SARAgr total score with BARS (r=0.945 p< 0.0005) and BI (r=-0.872 p< 0.0005) respectively. The above result indicated very high correlation between total score with both scales, which satisfied the criterion validity. The SARAgr total score was well discriminated between sub-groups, according to GMFCS level (subgroup GMFCS I-II vs subgroup GMFCS III-IV-V). SARAgr total score were higher for the subgroup GMFCS level III-IV-V compared to the subgroup GMFCS I-II (p<0.001). Moreover children with progressive type of ataxia presented higher values of SARAgr score compared with those with non-progressive type of ataxia, but the difference was not statistically significant (p=0.094). The results of the item analyses revealed difficulty indices (item mean divided by total item score) of the 8 items ranging between 0.35 and 0.63. The most difficult item was ‘item 7’ (0.53), while the easiest item was ‘item 5’ (0.35). The discriminative index is the item-to-total correlation using Pearson’s product moment correlation coefficient, with any coefficient greater than 0.28 to be considered as having a satisfactory discrimination property. The item discriminative indices of the SARAgr items ranged from 0.62 to 0.90. The most discriminative items were ‘item 2 and 5’ (r= 0.90), whilst the least discriminative item was ‘item 7’ (r =0.62).
The percentage of patients scoring at the lowest possible level of the scale and at the highest possible level was for the SARAgr total score 3.3% and 10% respectively. The critical value of 15% was not surpassed for the total score, something which proves that there is neither floor nor ceiling effect. The area under the curve (AUC) of SARAgr total score were 0.995 (95%CI 0.98-1.00, p<0.001) with cut-off point 20.3, sensitivity 100% and specificity 95% using the BI score as gold standard (Table 4).
Discussion
The translation process of the SARA was completed with conduction of minor semantic clarifications and adjustments and the new established SARAgr was applied in a sample of thirty children with different kinds of progressive and non progressive ataxia. Through that sample of children with ataxia, there was excellent internal consistency in the total scoring among the evaluators. Similarly, SARAgr yielded an excellent consistency of the measure over time (test-retest reliability), over the two evaluators (inter-rater reliability) and over the same measurement re-evaluation (intra-rater reliability). Additionally, the strong correlation found, among total scores of SARAgr with BARS and BI further support its accuracy in measuring ataxic symptoms in pediatric population above the age of 8years old. Such findings are in accordance with previous research and indicate that the SARAgr is a scale that can be used in a reliable and valid way for the assessment of ataxia in pediatric population with different underlying pathology [10,11,4].
In our research, confounding factors that have been defined in previous studies to influence rating of ataxia symptoms, such as the increased variability in SARA scoring below the age of 8 years old [15] and the presence of ataxia as a co morbid –secondary clinical feature [10] were taken into account during recruitment of the pediatric population of the current study. Among the heterogeneity of the underlying pathology of the recruited children with ataxia, the majority was found to be children with non progressive type of ataxia with ataxic type of cerebral palsy being the most prevalent, while Freidreich ataxia was found to be the most frequent type among progressive ataxia. Interestingly, there were some undiagnosed, undefined causes of both progressive and non-progressive ataxia. Even though we found different kinds of ataxia etiology (CP, CACNA1A, TBI, BT and other related to ataxia syndromes), the SARAgr was conducted with no difficulty and all children followed a similar trend with no extreme values in portion of individuals, which was verified by examination of floor and ceiling effect.
SARAgr, BARS and BI are correlated, as this has been isolated in previous studies, [10,9] Similar trend was found between SARAgr and BARS, a finding in accordance with a previous study based on the evaluation and discriminant validity of rating scales in children and adults with early onset ataxia [10]. Even though both scales are defined as reliable tools for measuring ataxia, the usage of BARS in children is not validated in terms of normative age values while the pediatric oculomotor measurement may also introduce a bias, as such function is not only influenced by cerebellar pathology, but also by other factors such as oculomotor and attention ability of the child [29]. SARAgr scores decreased in relation to the BI, a questionnaire defining daily living dependency, which has been closely correlated with the original SARA, when applied in adult population with ataxia [9]. Even though BI is not a pediatric tool, the researchers wanted to use a same assessment tool as in the original SARA and investigate its properties in relation to SARAgr in children with ataxia. Through this investigation an interesting finding was the cut-off point at 20.3 SARAgr total score in the correlation with the BI. The existence of the cut-off point delineates a threshold of minimum severity of the child’s ataxia disorder that is decisive for the ability with which the children function and cope independently in their daily activities. More specifically, children with SARAgr total score higher than 20.3 have 100% probability for severe dependency, while children with SARAgr total score less than 20.3 have 95% probability for moderate dependency. Our findings are consistent with the hypothesis that SARAgr provides a method to identify dependency in daily activities for children with ataxia. A similar analysis of cut- off point investigation of SARA has been conducted in a recent study [30] which identified a total score of 14 being critical for associated functional outcomes with the modified Rankin scale in adults with ataxia secondary to stroke. To our knowledge our study is the first to add such relevant information on functioning in pediatric population with ataxia.
As the original first developed scale [9] our results are also determined by a single factor, further supporting that the items of the newly established scale measures ataxia in pediatric population, as a common construct. The results of the item analyses revealed difficulty indices among the 8 items of the scale, with item “7” (Fast alternating hand movements) being the most difficult item to be discriminative. Item “2” (Stance) and item “5” (Finger chase) were the easiest ones, with item “5” being the most discriminative of all. The difficulty of item “7” may be accounted to the fact that 10 cycles of alternation of hand pronation and supination should be conducted with a cut point of 10 sec and parallel estimation of quality of movements. From our perspective most of the children found difficult to complete 10 cycles within 10sec, maybe due to the fact that they were trying to be accurate as much as possible. So we think that calculation of the exact time was mostly the key point for interpretation of item “7”.
Another finding in our study is that the SARAgr total score is well discriminated between sub-groups of children based on their functional motor severity, according to the GMFCS level. It seems that the SARAgr reflects the child’s motor function severity, with significantly higher total scores for children with GMFCS III, IV and V, compared to those with GMFCS I and II, as one would expect as both scales measure motor performance. Moreover, it seems that children with progressive type of ataxia present higher values of SARAgr score compared with those with non-progressive type. However, children with progressive and non-progressive type of ataxia were not equally distributed among the sample of 30 children in total, neither were the children with different GMFCS level. The small number of participants in each identified subgroup did not allow us to draw further conclusions. Future research in larger sample may identify differences not only in total score, but also in specific items of the scale.
Conclusion
The findings from the investigation of the psychometric properties of SARAgr in children with ataxia above the age of 8yeras old further support its validity and reliability in measuring ataxia related symptoms among a heterogeneity sample of progressive and non-progressive ataxia. To our knowledge this is the first study, revealing difficulty indices and correlation with functional outcomes, which further suggests that SARAgr total score may indicate the level of independency in daily activities in such population. It is important to administer the scale in larger pediatric sample sizes and investigate SARA’s item properties in relation to different underlying ataxia pathologies.
Acknowledgments
This research was supported by the volunteer participation from parents and children all over Greece and we would like to thank all of them for their availability during those difficult times with Covid pandemic. We are grateful for the support of “ATTIKON” General University Hospital of Athens and especially Dr Papaevangelou V and Dr Spanou M. We would also like to thank Dr Pyrgeli M from ELEPAP of Athens, Mrs Tsouni D and Mrs Kosma A from ELEPAP of Agrinio for promoting and communicating the research conduct. Similarly, we are more than happy for the assistance of Mrs Potamiti E, Mrs Outsika C, Dr Mouscou S, Dr Daliviga Z and Dr Pons R from the Pediatric Hospitals of Athens “Aglaia Kyriakou” and “Agia Sofia”.
Additionally we would like to thank the directors Mrs Kalamvoki S and Mr Katsogianni from private pediatric therapeutic faculties for their assistance during the conduction of the research. We are sincerely thankful for the contribution of the pediatric physiotherapists below, who helped with the videotape during the online assessments from the distant areas of Greece: Dr Chandolias K, Mrs Foti A, Mrs Kyriouvouki D, Mrs Daousi T and Mrs Charalambous L. We would like also to acknowledge Dr Gedikoglou AI and Mrs Pissa S for their contribution in the translation process.
Finally, we could not be more thankful for the valuable overall practical assistance of the therapeutic team: Mr Kellidis S, Mr Panagopoulos T, Mrs Tsiavou E and Mrs Karapatoucha V of the pediatric therapeutic faculty “Kinitro kai Kinisi”.
Conflict of Interest
The authors declare no conflict of interest.
References
- Musselman KE, Stoyanov CT, Marasigan R, Jenkins ME, Konczak J, et al. (2014) Prevalence of ataxia in children: A systematic review. Neurology 82(1): 80-89.
- Hartley H, Cassidy E, Bunn L, Kumar R, Pizer B, et al. (2019) Exercise and Physical Therapy Interventions for Children with Ataxia: A Systematic Review. Cerebellum 18(5): 951-968.
- Pavone P, Praticò AD, Pavone V, Lubrano R, Falsaperla R, et al. (2017) Ataxia in children: early recognition and clinical evaluation. Ital J Pediatr 43(1): 6.
- Panzeri D, Bettinelli MS, Biffi E, Rossi F, Pellegrini C, et al. (2020) Application of the Scale for the Assessment and Rating of Ataxia (SARA) in pediatric oncology patients: A multicenter study. Pediatr Hematol Oncol 37(8): 687-695.
- Sullivan R, Yau WY, O’Connor E, Houlden H (2019) Spinocerebellar ataxia: an update. J Neurol 266(2): 533-544.
- Steinlin M (1998) Non-progressive congenital ataxias. Brain Dev 20(4): 199-208.
- Bastian AJ (1997) Mechanisms of Ataxia. Phys Ther 77(6): 672-675.
- Perez‐Lloret S, Warrenburg B, Rossi M, Rodríguez-Blázquez C, Zesiewicz T, et al. (2021) Assessment of Ataxia Rating Scales and Cerebellar Functional Tests: Critique and RecMov Disord 36(2): 283-297.
- Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, et al. (2006) Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 66(11): 1717-1720.
- Brandsma R, Lawerman TF, Kuiper MJ, Lunsing RJ, Burger H, et al. (2017) Reliability and discriminant validity of ataxia rating scales in early onset ataxia. Dev Med Child Neurol 59(4): 427-432.
- Hartley H, Pizer B, Lane S, Sneade C, Pratt R, et al. (2015) Inter-rater reliability and validity of two ataxia rating scales in children with brain tumours. Childs Nerv Syst 31(5): 693-697.
- Brandsma R, Verschuuren-Bemelmans CC, Amrom D, Barisic N, Baxter P, et al. (2019) A clinical diagnostic algorithm for early onset cerebellar ataxia. Eur J Paediatr Neurol 23(5): 692-706.
- Lawerman TF, Brandsma R, Verbeek RJ, van der Hoeven JH, Lunsing RJ, et al. (2017) Construct Validity and Reliability of the SARA Gait and Posture Sub-scale in Early Onset Ataxia. Front Hum Neurosci 11: 605.
- Sousa VD, Rojjanasrirat W (2011) Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J Eval Clin Pract 17(2): 268-274.
- Brandsma R, Spits AH, Kuiper MJ, Lunsing RJ, Burger H, et al. (2014) Ataxia rating scales are age-dependent in healthy children. Dev Med Child Neurol 56(6): 556-563.
- Mahoney FI, Barthel DW (1965) Functional Evaluation: The Barthel Index. Md State Med J 14: 61-65.
- Lampropoulou S, Vardoulakis S, Miraka E, Gedikoglou IA, Billis E (2016) Cross Cultural Adaptation and Pilot Use of the Motor Assessment Scale (MAS) into Greek. PANR Journal, PANR e-ISSN: 2421-7824:92-110.
- Schmahmann JD, Gardner R, MacMore J, Vangel MG (2009) Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord 24(12): 1820-1828.
- Maggi FA, Braga-Neto P, Chien HF, Drumond Gama MT, Rezende Filho FM, et al. (2018) Cross-cultural adaptation and validation of the International Cooperative Ataxia Rating Scale (ICARS) to Brazilian Portuguese. Arq Neuropsiquiatr 76(10): 674-684.
- Weyer A, Abele M, Schmitz-Hübsch T, Schoch B, Frings M, et al. (2007) Reliability and validity of the scale for the assessment and rating of ataxia: A study in 64 ataxia patients. Mov Disord 22(11): 1633-1637.
- Giavarina D (2015) Understanding Bland Altman analysis. Biochem Med 25(2): 141-151.
- Higgins PA, Straub AJ (2006) Understanding the error of our ways: Mapping the concepts of validity and reliability. Nurs Outlook 54(1): 23-29.
- Guadagnoli E, Velicer WF (1988) Relation of sample size to the stability of component patterns. Psychol Bull 103(2): 265-275.
- Byrne BM (1989) The LISREL Confirmatory Factor Analytic (CFA) Model. 3-15.
- Hu LT, Bentler PM (1999) Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal 6(1): 1-55.
- McDonald RP, Marsh HW (1990) Choosing a multivariate model: Noncentrality and goodness of fit. Psychological Bulletin 107(2): 247-255.
- Bollen KA (1989) Structural Equations with Latent Variables.
- Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143(1): 29-36.
- Bürk K, Sival DA (2018) Scales for the clinical evaluation of cerebellar disorders. Handb Clin Neurol 154: 329-339.
- Yamauchi K, Kumagae K, Goto K, Hagiwara R, Uchida Y, et al. (2021) Predictive Validity of the Scale for the Assessment and Rating of Ataxia for Medium-Term Functional Status in Acute Ataxic Stroke. J Stroke Cerebrovasc Dis 30(4): 105631.






























