Applications of Thromboelastography in Acute Ischemic Stroke Patients
Joanna Sikora* and Aleksandra Karczmarska-Wódzka
Research and Education Unit for Experimental Biotechnology, Department of Transplantology and General Surgery, Faculty of Medicine, Nicolaus Copernicus University in Toruń, Poland
Submission: June 21, 2021; Published: July 12, 2021
*Corresponding author: Joanna Sikora, Research and Education Unit for Experimental Biotechnology; Department of Transplantology and General Surgery, Faculty of Medicine, Nicolaus Copernicus University in Toruń, Collegium Medicum in Bydgoszcz, Marie Curie Skłodowskiej Street No 9, 85-094 Bydgoszcz, Poland
How to cite this article: Joanna S, Aleksandra K-W. Applications of Thromboelastography in Acute Ischemic Stroke Patients. Open Access J Neurol Neurosurg 2021; 15(4): 555919. DOI: 10.19080/OAJNN.2021.15.555919.
Abstract
Thromboelastography is a test that can be carried out beside the patient, providing valuable information on the state of coagulation. The main utility of TEG is that it allows integration of conventional coagulation tests and platelet function, achieving a larger understanding of the physiology of haemostasis. According to TEG patterns it is possible to accurately identify the underlying defect in the coagulation cascade and allow to ease patient treatment decisions. There are some reports that this technique can be used in neurological patients with acute ischemic stroke. There are some contradictory or indirect and inconclusive reports related to the applications of several tests of thromboelastography in acute ischemic stroke patients. The aim of the article is to review the available reports on the use of thromboelastography in acute ischemic stroke patients.
Keywords: Thromboelastography; stroke; Acute ischemic stroke; Coagulation; Platelets
Introduction
Thromboelastography (TEG) is a test that can be carried out beside the patient, providing valuable information on the state of coagulation [1]. TEG measures the dynamics of coagulation and it is normally used during blood transfusion therapy to begin earlier and directed towards more specific disorders such as coagulation factor deficits and/or platelet deficiency or dysfunction [2]. TEG include in vitro analysis of the relation between different components of coagulation, and thus observing interactions among platelets (PLT), fibrinogen (FIB) and coagulation proteins from a general standpoint. The main utility of TEG is that it allows integration of conventional coagulation tests and platelet function, achieving a larger understanding of the physiology of haemostasis. According to TEG patterns it is possible to accurately identify the underlying defect in the coagulation cascade and allow to ease patient treatment decisions. [3] There are limited data about TEG in Acute Ischemic Stroke (AIS). The aim of the article is to review the available reports on the use of TEG in AIS patients.
An investigation of recent published literature was conducted. Authors based on PRISMA [4]. Briefly, a database search (date of search 10.05.2021) including PubMed, CENTRAL and Google Scholar databases. The following keywords were applied thromboelastography, TEG, acute ischemic stroke. References of retrieved studies were searched manually for additional studies and reviews. Reviews were also considered by source of citations of relevant studies and interpretation of their results. Duplicate and multiple citations and reviews not containing any relevant information were excluded. Eventually, 10 original reports [A-K] directly related to the applications of TEG in AIS patients were considered eligible for inclusion in the review. Elliot et al. [5] designed a study that was aimed to providing initial TEG data in stroke patients before and after tissue plasminogen activator therapy and to providing the necessary preliminary data for further study of TEG's ability to identify clot subtype and predict response to tissue plasminogen activator therapy. AIS patients had shorter R (means the time until the first clot is detected), greater α angle (means the rate of fibrin deposition and cross-linking) and shorter K (the time from the end of the test until the clot reaches 20 mm, which is the rate of clot formation), which indicating faster clotting. Additionally, a subset formed clots with stronger platelet-fibrin matrices. Treatment with tissue plasminogen activator resulted in reduction in all indices of clot strength. TEG demonstrates that many AIS patients are hypercoaguable.
Results from another study [6] aimed to investigate the application of TEG on admission for predicting early neurological deterioration (END) in patients with AIS and its potential correlation with the evolution of ischemic lesions. Of the 246 eligible patients, END was identified in 29.3% patients. Results showed that decreased R time on admission TEG is associated with END within 3 days in patients with AIS. Yu et al. [7] suggested there are limited data on TEG as a predictive tool for hemorrhagic transformation in patients with AIS. Authors designed a study to investigate whether TEG values on admission could predict hemorrhagic transformation in patients with AIS. TEG was performed in 205 patients with AIS. The primary outcome was hemorrhagic transformation, defined as the presence of blood in brain on follow-up imaging study and secondary outcome is neurological deterioration, defined as a 2-point increase on the National Institutes of Health Stroke Scale (NIHSS) within 72 h of stroke onset. In patients with AIS, TEG value of R < 5 min can identify patients who have an increased risk of hemorrhagic transformation during hospitalization. One of the studies [8] was designed to provide initial data to compare TEG with the Conventional Coagulation Test (CCT) to analyze the coagulation function of antiplatelet drugs in such patients. The retrospective cohort study included 240 patients who received endovascular therapy. The baseline and clinical characteristics of these patients were collected with respect to TEG and CCT on day 5 after aspirin and clopidogrel post-endovascular interventions. The correlation and agreement of these 2 detecting methods were analyzed. Parameter FIB derived from CCT might be advantageous for evaluating early neurological deterioration, while parameter R detected by TEG might be superior for evaluating symptomatic intracranial hemorrhage.
Quan et al. noticed [9] that lung cancer related hypercoagulability could increase the risk of ischemic stroke. Routine coagulation tests may have limited capacity in evaluating hypercoagulability. The aim of this study was to investigate the ability of TEG in the identification of hypercoagulability in patients with Lung Cancer and Cryptogenic Ischemic Stroke (LCIS). Therefore, TEG could identify hypercoagulability in LCIS patients and healthy controls. Identification of hypercoagulability in lung cancer patients by TEG may be helpful to prevent the occurrence of LCIS. Yan et al. [10] was designed the study evaluating ticagrelor versus clopidogrel on platelet reactivity measured by TEG in patients with minor stroke or transient ischemic attack (TIA). Platelet reactivity was assessed by TEG using test to platelet mapping. Patients were divided into carriers and non-carriers according to the carrier status of CYP2C19 loss-of-function (LOF) alleles. The primary outcome was the proportion of patients with High On-Treatment Platelet Reactivity (HOPR). Ticagrelor was superior to clopidogrel in inhibiting platelet reactivity measured by TEG platelet mapping among patients with acute minor stroke or TIA, particularly in carriers of the CYP2C19 LOF alleles. Large randomised controlled trials are needed to confirm our findings.
In this case, researchers [11] used different laboratory testing in a patient with ischemic stroke and reduced prothrombin Time (PT) to identify an in-vitro effect of Lupus Anticoagulant (LA) excluding an in-vivo bleeding disorder. LA owns procoagulant properties in vivo and prolongs phospholipid-dependent clotting times in vitro. The prolonged in vitro clotting time can be misinterpreted as a bleeding disorder. The activity of various coagulation factors was evaluated both with recombinant thromboplastin Innovin (Siemens Healthcare) and reagent tissue extracted thromboplastin Thromborel® (Siemens Healthcare). Researchers conclude, that different thromboplastin reagents, plasma mixing tests and thromboplastin independent coagulation tests may be helpful to differentiate LA and in vitro changes from in vivo factor deficiency in patients with LA.
Koch et al. [12] designed a study using canine model with ischemic stroke. In this case, authors reported hemostatic parameters, including D-dimer and TEG along with clinical and imaging findings for five dogs diagnosed with ischemic stroke. A hypercoagulable state was identified in two dogs based on the results of the TEG and was suspected in the remaining three cases based on a shortened TEG clot reaction time. The demonstration of a possible hypercoagulable state, as identified by the TEG, is an interesting finding which should be explored further to help reveal predisposing hypercoagulable conditions in dogs with ischemic stroke. Wiśniewski et al. [13,14] demonstrated prospective study about hypercoagulability as measured by TEG. Authors investigated whether a hypercoagulability detected by TEG may be associated with larger size of acute ischemic infarct. In the entire group, they reported that subjects with a large ischemic focus had a higher diameter of a clot than subjects with a small ischemic focus. Stroke subjects with hypercoagulability had significantly higher probability of a larger size of acute ischemic focus compared to normal-coagulable subjects. Authors conclude that TEG, based on the parameters related to clot strength, may have clinical utility to identify the risk of the extensive ischemic infarct.
Thromboelastographic changes in patients experiencing an AIS and receiving alteplase [14,15] was investigated. It was a prospective cohort study enrolled patients who presented to the emergency department with symptoms of AIS and received intravenous alteplase. Blood samples were obtained before alteplase administration and at 30, 60, 90, 120, and 150 minutes after alteplase administration. The maximum inhibition of fibrin buildup was seen at 30 minutes after the start of alteplase infusion, and the lowest clot strength was observed at 60 minutes after initiation of alteplase. Most patients return to near baseline parameters within 150 minutes of alteplase initiation; however, two patients did not return to their baseline values within the 150-minute time frame. This study proved that TEG is a useful tool for determining changes in the coagulation system of patients whom have received recombinant tissue plasminogen activator.
Discussion
The above-mentioned studies proved the potential benefits of a comprehensive coagulation assessment for stroke evaluation. The summary are presented in table 1. Several studies have highlighted the potential usefulness of TEG coagulation monitoring and hypercoagulability detection in predicting clinical outcomes and prognosis in ischemic stroke subjects. TEG measures the dynamics of clot formation and dissolution and provides more complex and integrated measurement of venous blood coagulation properties than conventional tests. Therefore, most of presented studies of TEG may contribute to a more reliable and accurate assessment of hypercoagulability which remains the undoubted pathological cause of ischemic stroke.
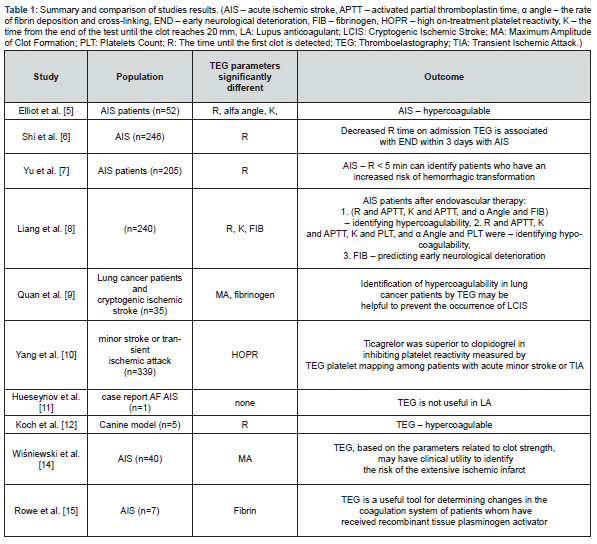
The most frequent parameter in the studies which indicated a prognostic or diagnostic value in patients with AIS was R. From a clinical point of view, it’s crucial whether a hypercoagulability that could be detected by TEG. It is a hypothesis, that an individual’s tendency to form firm and large clots in a short period of time may promote the expansion of the ischemic focus in the brain. Hypercoagulability still remains the baseline background of a higher risk of cerebrovascular events, including ischemic stroke. In one study, Quan et al. [9], hypercoagulability was also defined by the Maximum Amplitude (MA), however, there remains a broad variability in the definition of hypercoagulability as determined by MA and thus limits its predictive ability. Some studies proved, this tool may be also useful for monitoring the hemostatic processes in stroke subjects undergoing the alteplase therapy and in predicting the risk of hemorrhagic transformation after intravenous thrombolysis.
Conclusion
In conclusion, in this review we emphasized that there are some reports which indicate the use of several parameters of TEG in AIS patients. The most frequent parameter in the studies which indicated a prognostic or diagnostic value in patients with AIS was R, which may define hypercoagulability in this group of patients. Authors also point to other clinical areas among AIS patients that should be further explored. Therefore, our novel findings contribute to the expand the existing knowledge on predictive values of TEG and support its clinical usefulness in the acute phase of ischemic stroke. Nonetheless, it is crucial to further investigation in this field, including large cohort prospective studies.
References
- Hartmann Jan, Dan Mason, Hardean Achneck (2018) Thromboelastography (TEG) point-of-care diagnostic for hemostasis management. Point of Care 17(1): 15-22.
- Brazzel, Charssice (2013) Thromboelastography-guided transfusion Therapy in the trauma patient. AANA journal 81(2): 127-132.
- Othman Maha, Harmanpreet Kaur (2017) Thromboelastography (TEG). Hemostasis and Thrombosis 533-543.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 151: 264-269.
- Elliott Andrea, Jeremy Wetzel, Tiffany Roper, Evan Pivalizza, James McCarthy, et al.(2015) Thromboelastography in patients with acute ischemic stroke. Int J Stroke 10(2): 194-201.
- Shi Zhu, Wei C Zheng, Xiao L Fu, Xue W Fang, Pei S Xia, et al. (2018) Hypercoagulation on thromboelastography predicts early neurological deterioration in patients with acute ischemic stroke. Cerebrovascular Diseases 46(3-4): 123-129.
- Yu Gina, Youn-Jung Kim, Sang-Beom Jeon, Won Young Kim (2020) Thromboelastography for prediction of hemorrhagic transformation in patients with acute ischemic stroke. Am J Emerg Med 38(9): 1772-1777.
- Liang, Chunyang, (2020) Comparison between thromboelastography and the conventional coagulation test in detecting effects of antiplatelet agents after endovascular treatments in acute ischemic stroke patients: A STROBE-compliant study. Medicine (Baltimore) 99(10): e19447.
- Quan, Xuemei, Qixiong Qin, Xianting Que, Ya Chen, Yunfei Wei, Hao Chen, et al. (2020) Utility of Thromboelastography to Identify Hypercoagulability in Lung Cancer Related Ischemic Stroke Patients. Clinical and Applied Thrombosis/Hemostasis 26: 1076029620975502.
- Yang Yingying, Weiqi Chen, Yuesong Pan, Hongyi Yan, Xia Meng et al. (2020) Effect of ticagrelor versus clopidogrel on platelet reactivity measured by thrombelastography in patients with minor stroke or TIA. Aging (Albany NY) 12(20): 20085- 20094.
- Huseynov Aydin, Verena H, Maximillian K, Thomas B, Angelika A, et al. (2020) Lupus Antibody Mimicking Reduced Plasmatic Coagulation in a Patient With Atrial Fibrillation and Ischemic Stroke. Front Neurol 11: 896.
- Koch Bodil Cathrine, Bo Wiinberg, Ulrik Westrup, Annemarie Thuri Kristensen, et al. (2019) D-Dimer Concentrations and Thromboelastography in Five Dogs With Ischemic Stroke. Frontiers in veterinary science 6: 255.
- Yao Xiaoying, Quan Dong, Yeping Song, Yanqing Wang, Ye Deng, et al. Thrombelastography maximal clot strength could predict one-year functional outcome in patients with ischemic stroke. Cerebrovascular diseases 38 (3): 182-190.
- Wiśniewski, Adam, Aleksandra Karczmarska W, Joanna S, Przemysław S, Adam L, et al. Hypercoagulability as Measured by Thrombelastography May Be Associated with the Size of Acute Ischemic Infarct-A Pilot Study. Diagnostics 11(4): 712.
- Rowe A, Christal LG, Carolyn C, Roger C, Brian FW, et al. (2014) Thromboelastographic changes in patients experiencing an acute ischemic stroke and receiving alteplase. Journal of Stroke and Cerebrovascular Diseases 23(6): 1307-1311.






























