Role of Ultrasound in the EMG Lab
Vasudeva G Iyer*
Neurodiagnostic Center, Louisville, KY, USA
Submission: April 14, 2021; Published: May 20, 2021
*Corresponding author: Vasudeva G Iyer, Neurodiagnostic Center, Louisville, KY, USA
How to cite this article: Vasudeva G I. Role of Ultrasound in the EMG Lab. Open Access J Neurol Neurosurg 2021; 15(2): 555910. DOI: 10.19080/OAJNN.2021.15.555910.
Review
During the past 10-15 years the use of ultrasound (US) in the diagnosis of neuromuscular disorders has significantly accelerated. Traditional Electrodiagnostic Tests (EDX) provide insight into the location and pathophysiology of neuromuscular disorders. However, EDX may not provide clues to the underlying structural cause, which is more readily uncovered by imaging studies like ultrasound and MR neurography. The advantages of US are low cost, being painless and readily available at the point of care. There have been suggestions that US can replace EDX in some situations like confirming clinical diagnosis of carpal tunnel syndrome [1]. However, currently available US technology cannot provide adequate information regarding the underlying pathophysiology: demyelination, axon loss or both. EDX and US complement each other effectively in providing comprehensive data to respond to the practical question is the problem surgical or non-surgical.
While it is desirable to do US in every patient undergoing EDX, in a busy clinical EDX facility, the amount of time the electromyographer can devote to any given patient is not unlimited; hence it may not be feasible to do US in every patient undergoing EDX. This brings up the question as to how to identify situations where EDX has to be complemented with US. We have been developing guidelines in our facility reflecting where US plays a crucial role in providing valuable information for diagnosis and management. We will not be discussing the role of US in nerve block, locating nerve/muscle for biopsy, and therapeutic procedures like botulinum toxin injections in this review, but concentrate upon the role of US in complementing EDX for more precise diagnosis.
Situations where EDX fail to provide accurate localization:
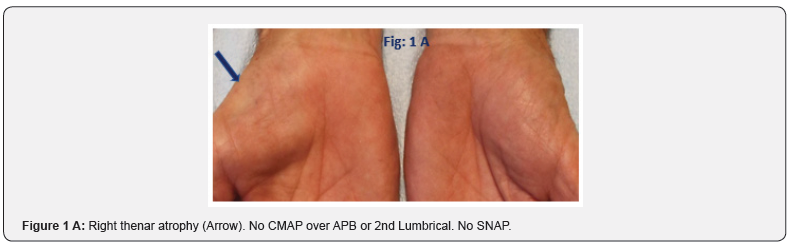
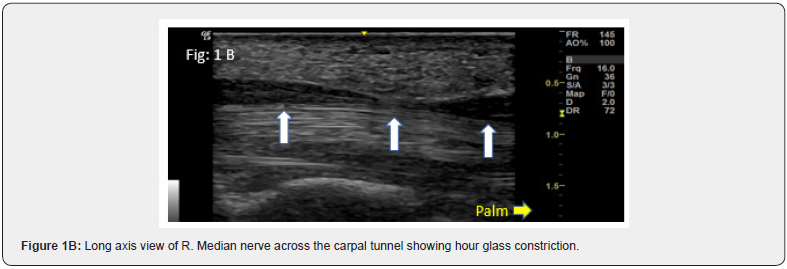
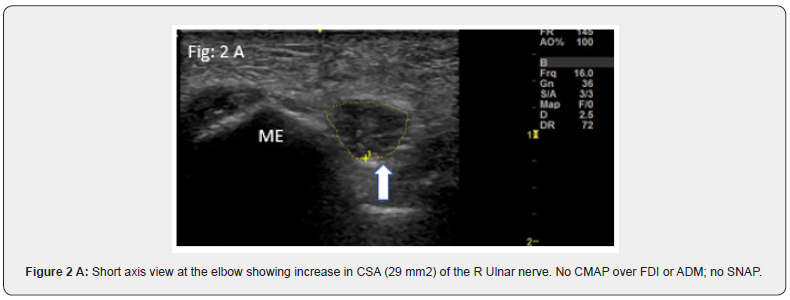
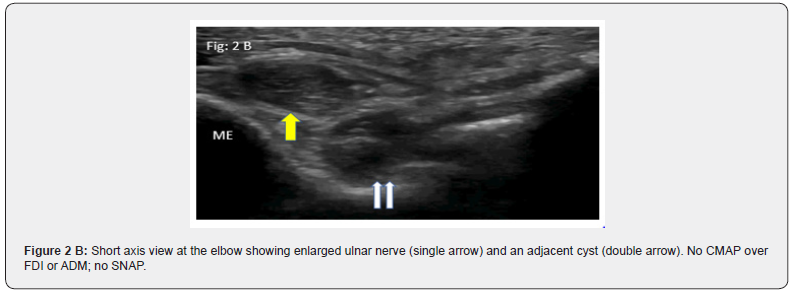
a. Significant axon loss leading to absent Compound Muscle Action Potentials (CMAP) and Sensory Nerve Action Potentials (SNAP) Typical examples are severe entrapment of median nerve at the carpal tunnel and ulnar nerve at the elbow. Documentation of increased Cross-Sectional Area (CSA) of median nerve at the carpal tunnel inlet/outlet and normal CSA at the forearm serve as reliable criteria to localize median neuropathy to the carpal tunnel [2]. A typical feature is the hour glass appearance in long axis views (Figure 1). Similarly, severe ulnar neuropathies at the elbow or wrist [3] can be confirmed by documentation of increased CSA proximal to the site of entrapment (Figure 2). Patients with severe fibular neuropathy causing loss of CMAP of Extensor Digitorum Brevis (EDB) and tibialis anterior / peroneus longus pose a similar challenge and US may provide the answer (Figure 3).
b. In cases of longstanding entrapment, in addition to focal demyelination, there may be retrograde [4] and anterograde demyelination leading to diffuse slowing of conduction; precise localization may not be possible in such cases with EDX alone. US is of great value in these situations by providing accurate localization.
c. In cases of demyelinating polyneuropathy with diffuse slowing of nerve conduction, confirming additional presence of entrapment can be challenging. In this context, US showing findings typical of entrapment can be helpful. Most common situation is patients with diabetic polyneuropathy in whom additional presence of carpal/cubital tunnel syndrome is suspected.
Negative EDX in patients with typical clinical picture of carpal/cubital tunnel syndrome:
a. Patients with symptoms of carpal tunnel syndrome rarely show normal nerve conduction studies. There are a number of publications which suggest that high frequency ultrasonography may show abnormalities in such patients [5,6].
b. US has also been reported to be positive in patients with ulnar neuropathy at the elbow, when EDX is negative [7].
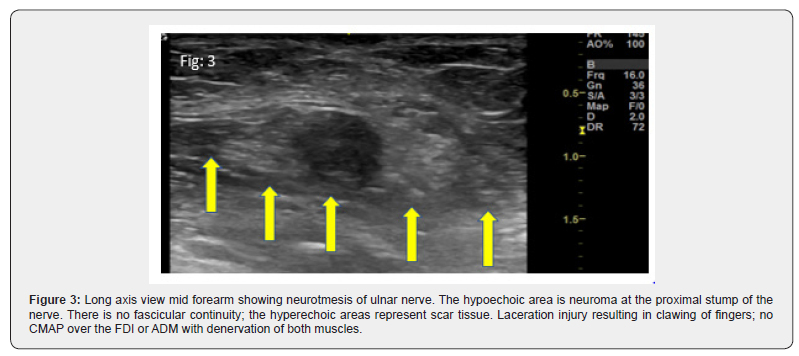
Nerve injuries: EDX does not distinguish neurotmesis from axonotmesis
Distinction between axonotmesis and neurotmesis is a crucial piece of information for planning the ideal time for surgical intervention; if neurotmesis can be confirmed, early surgical intervention is indicated. US can provide such information by revealing area of discontinuity of the nerve in neurotmesis (Figure 5).
Differentiation of chronic inflammatory demyelinating polyneuropathy (CIDP) from other polyneuropathies
This is highly important for prompt initiation of treatment with intravenous immunoglobulin in patients with CIDP. Recent studies have shown that sonographic enlargement of proximal median nerve segments in the arms and brachial plexus is a differentiating feature of CIDP [8]. In Charcot Marie Tooth (CMT) disease, the nerve enlargement is more diffuse along the entire course of the nerve specifically in type 1 A [9].
Differentiation of multifocal motor neuropathy (MMN) from amyotrophic lateral sclerosis with predominant lower motor neuron disease (ALS/LMND)
This distinction is important from prognostic and therapeutic points of view; US can be more sensitive than EDX [10]. showing multifocal ulnar and median nerve enlargement. There is also a recent report of US detecting treatment-responsive chronic neuropathies without EDX features of demyelination [11].
Role in neuralgic amyotrophy
While the clinical presentation and EDX findings are often sufficient to make a diagnosis of neuralgic amyotrophy (Parsonage Turner syndrome), MR neurography and US have shown enlargement of peripheral nerve/fascicles and features of nerve torsion or fascicular entwinement. In one study nerves with complete constriction and rotational phenomena failed to show significant reinnervation; these features, if detected by US may be an indication for surgical intervention [12].
Detection fasciculations
The needle electrode can pick up fasciculations from only a limitedl area around it; also, it can be painful to have needle inserted into structures like the tongue. US has the advantage of visualizing fasciculations from a much wider area without the pain involved in multiple needle insertions. Recent studies have documented that US is much superior to EDX in detecting fasciculations in patients with ALS [13].
Lesions in the proximity of nerves
The main question for preoperative planning are whether the lesion is actually arising from the nerve and if not, anatomically how close is it to the nerve. US provides immediate answers; it can easily identify nerve tumors like schwannomas and neurofibromas and also alert the surgeon to the potential for perioperative nerve injury while removing lesions in close proximity to the nerve.
Muscle disorders
a. Distinction between muscular dystrophies and inflammatory myopathies often made by EDX can be further substantiated using US [14].
b. While EDX suggests a myopathic disorder, US can point to a more precise diagnosis, based on the topography of muscle involvement. The classical example is sporadic inclusion body myositis (IBM) in which there is increased echogenicity of flexor digitorum profundus with sparing of flexor carpi ulnaris [15, 16].
There are many more situations where US can contribute substantially to diagnosis
a. Dynamic US is useful to document mobility of median nerve within the carpal tunnel and to document subluxation of the ulnar nerve at the elbow.
b. Another major use is imaging of diaphragm for diagnosis of phrenic nerve palsy; measurement of diaphragm thickness is being used increasingly in ALS and myopathies [17].
c. Rarely the patient may not be able to tolerate electric stimulation and needle study. This may particularly apply to pediatric patients. US may provide an alternate route to reach the diagnosis.
While it is ideal to perform ultrasound evaluation in every patient referred for EDX studies, it may not be feasible in a busy clinical EDX lab due to time constraints. It is important to have guidelines that identify situations where additional use of US can provide vital information that will help in the diagnosis and management of patients. It is likely that in the future an increasing percentage of patients seen for EDX will also undergo US, as the knowledge base of ultrasonic findings in various neuromuscular disorders increase and as US technology advances with more automated measurements.
References
- Drakapoulos D, Mitsiokapa E, Karamanis E, Vasilios K, Andreas FM (2019) Ultrasonography provides a diagnosis similar to that of nerve conduction studies for carpal tunnel syndrome. Orthopedics 42(5): e460-e464.
- Iyer V (2019) Role of ultrasonography in severe distal median nerve neuropathy. J Clin Neurophysiol 36(4): 312-315.
- Iyer V (2021) Ultrasonography in distal ulnar nerve neuropathy: Findings in 33 patients. J Clin Neurophysiol 38(2): 156-159.
- Uchida Y, Sugioka Y (1992) Electrodiagnosis of retrograde changes in carpal tunnel syndrome. Electromyography and Clinical Neurophysiology 33(1): 55-58.
- Williams J, Cartwright M (2016) Neuromuscular ultrasound in carpal tunnel syndrome with normal nerve conduction studies P4.081. Neurology 86 (16).
- Roghani RS, Holisaz MT, Norouzi AAS, Ahmad Delbari, Faeze Gohari, et al. (2018) Sensitivity of high-resolution ultrasonography in clinically diagnosed carpal tunnel syndrome patients with hand pain and normal nerve conduction studies. J Pain Res 11: 1319-1325.
- Yoon JS, Walker FO, Cartwright M (2010) Ulnar neuropathy with normal electrodiagnosis and abnormal nerve ultrasound. Arch Phys Med Rehabil 91(2): 318-320.
- Goede HS, Pol WL, Asseldonk JH, Franssen H, Nicolette CN, et al. (2017) Diagnostic value of sonography in treatment-naïve chronic inflammatory neuropathies. Neurology 88(2):143-151.
- Zanette G, Fabrizi GM, Taioli F, Matteo FL, Andrea B, et al. (2018) Nerve ultrasound findings differentiate Charcot-Marie Tooth disease (CMT) 1A from other demyelinating CMTs. Clin Neurophysiol 129(11): 2259-2267.
- Loewenbruck KF, Liesenberg J, Dittrich M, Jochen Schäfer, Beate Patzner, et al. (2016) Nerve ultrasound in the differentiation of multifocal motor neuropathy (MMN) and amyotrophic lateral sclerosis with predominant lower motor neuron disease (ALS/LMND). J Neurol 263(1): 35-44.
- Goedee HS, HerraetsIJT, Visser LH, Hessel Franssen, Jan-Thies HA, et al. (2019) Nerve ultrasound can identify treatment-responsive chronic neuropathies without electrodiagnostic features of demyelination. Muscle Nerve 60(4): 415-419.
- ArAnyi Z, Csillik A, DeVay K, Maja Rosero, PéTer Barsi, etal. (2017) Ultrasonography in neuralgic amyotrophy: Sensitivity, spectrum of findings and clinical correlations. Muscle Nerve 56(6): 1054-1062.
- Duarte ML, Lared W, Oliveria ASB, Lucas Ribeiro Dos Santos, Maria Stella Peccin, et al. (2020) Ultrasound versus electromyography for the detection of fasciculation in amyotrophic lateral sclerosis: systematic review and meta-analysis. Radiol Bras 53(2):116-121.
- Albayda J, van Alfen N (2020) Diagnostic value of muscle ultrasound for myopathies and myositis. Curr Rheumatol Rep 22(11): 82.
- Noto Y, Shiga K, Tsuji Y, Masaki Kondo, Takahiko Tokuda, et al. (2014) Contrasting echogenicity in flexor digitorum profundus-flexor carpi ulnaris: a diagnostic ultrasound pattern in sporadic inclusion body myositis. Muscle Nerve 49(5): 745-748.
- Leeuwenberg KE, van Alfen N, Stine LC, Julie J Paik, Eleni Tiniakou, et al. (2020) Ultrasound can differentiate inclusion body myositis from disease mimics. Muscle Nerve 61(6): 783-788.
- Fayssoil A, Behin A, Ogna A, Dominique Mompoint, Helge Amthor, et al. (2018) Diaphragm: Pathophysiology and ultrasound imaging in neuromuscular disorders. J Neuromuscular Dis 5(1): 1-10.






























